Abstract
Background: Early prostate cancer antigen 2 (EPCA-2), a kind of nuclear matrix protein, may relate to prostate cancer. However, the association of EPCA-2 level in serum with prostate diseases has not been clarified in Chinese Han population. Methods: EPCA-2 and prostate-specific antigen (PSA) levels in serum were detected by enzyme linked immunosorbent assay in 116 patients with prostate cancer (PCa), 342 patients with benign prostatic hyperplasia (BPH), and 174 healthy controls (Control) in Chinese population. Associations of serum EPCA-2 and PSA level with prostate diseases were analyzed by ANOVA. Comparison of diagnostic effect for prostate cancer between EPCA-2 and PSA was evaluated by Receiver Operator Curve, Chi-square test, and others. Results: Serum EPCA-2 and PSA levels in PCa group were significantly higher than BPH and Control group (EPCA-2: F=200.05, P<0.01; PSA: F=210.65, P<0.01). However, EPCA-2 levels in the prostate cancers with different pathological grade were no significant difference. Furthermore, for detection of prostate cancer, EPCA-2 had a sensitivity of 81.9% and a specificity of 87.6%. Conclusions: Serum EPCA-2 could be used as a potential serological marker to diagnose prostate cancer in Chinese Han population, which was more specific than PSA and did not associate with pathological grades of prostate cancer.
Keywords: EPCA-2, PSA, prostate cancer, serum marker, Chinese
Introduction
Prostate cancer has become one of the most common nonskin malignancies among men with an incidence of approximately 0.01% worldwide, whose mortality has also dramatically increased in China [1]. Early diagnosis is beneficial to improve the therapeutic effect and reduce the mortality of prostate cancer. At present digital rectal examination and serum prostate specific antigen (PSA) level assay are the most important techniques to diagnose prostate cancer initially. However, the confirmed diagnosis relies on the pathobiology resulted from aspiration biopsy or surgical operation of prostate.
The total PSA, introduced in the late 1980s, is now the most widely used test to screen prostate cancer [2]. However, its level may also be seen increased in some benign diseases such as benign prostate hyperplasia and prostatitis [3]. Komatsu et al [4] reported that PSA could also elevate in physiological fluctuation. Therefore, it is a controversial topic that the prostate cancer diagnosis or prediction only uses the elevation of serum PSA level [5]. A more sensitive and specific biological marker for prostate cancer diagnosis is in need of exploration.
Nuclear matrix protein is a main component of nuclear matrix, which is the insoluble structural framework inside cell nucleus to determine nuclear shape and higher order DNA organization [6]. While the function and structure changes of nuclear matrix protein have been found in the carcinogenesis of cells [7]. Alan et al have shown the characteristic differences of nuclear matrix protein patterns among a series of prostate tumors and the specificity in rat sex-accessory tissues [8].
Early prostate cancer antigen (EPCA) is one of the nuclear matrix protein associated with prostate cancer. Dhir et al [9] had identified that EPCA expressed only in the prostate of patients with prostate cancer, not in others without the disease. Furthermore, previous studies had shown that EPCA could accurately diagnose for prostate cancer even at the earlier stage of carcinogenesis for prostate cancer [9,10]. Two EPCA subtypes have been identified, the first one also designated as EPCA, and the second as EPCA-2 [10]. The serum EPCA level was firstly analyzed by Paul et al [7] in a series of 46 individuals, and found significantly higher in prostate cancer patients than other populations. Furthermore, the study also revealed that the serum EPCA, at a cutoff of 1.7, had a sensitivity of 92% for prostate cancer patients, a specificity of 100% for healthy controls [7].
However, the association of serum EPCA-2 level with prostate diseases, and the diagnosis values of EPCA-2 for prostate cancer were unclarified. In the present study, we identified the association of serum EPCA-2 level with prostate diseases and the differentiation of prostate cancers in Chinese samples. Furthermore, we also examined the potential uses of EPCA-2 in blood samples for the clinical management of prostate cancer.
Materials and methods
Subjects
One hundred and sixteen patients with localized prostate cancer (PCa), three hundred and forty-two patients with benign prostatic hyperplasia (BPH, diagnosis referred the AUA guideline [11]) were recruited from Xinxiang Central Hospital and Chinese PLA General Hospital during October 2011 to September 2014. The patients with prostate cancer were further stratified by Gleason Grading System [12] (2-4 scores as well differentiated prostate cancer, WDPCa, N=34; 5-7 scores as moderately differentiated prostate cancer, MDPCa, N=42; 8-10 scores as poorly differentiated prostate cancer, PDPCa, N=40). All pathological diagnosis and Gleason score were evaluated independently by at least two pathologists and clinicians in Chinese PLA General Hospital or Xinxiang Central Hospital.
One hundred and seventy-four healthy controls (Control) without prostate diseases history and complex disorders including hypertension, immunological mediated disease, diabetes, and tumors were selected from Beijing and Henan Province. All participators in the study were Chinese Han. The mean age of the PCa group was 71.2±6.3 (WDPCa: 71.4±6.5; MDPCa: 71.4±6.5; PDPCa: 71.0±6.2), BHP was 71.2±4.9, and Control was 71.0±5.5. No significant difference was existed in the age among each group.
All subjects consented to participate in the study after reviewing the informed consent. The study was approved by both the Institutional Review Board of the Xinxiang Central Hospital and Chinese PLA General Hospital.
Blood collection and preparation
Venous blood was collected from patients and healthy controls which had not been treated by catheterization, digital rectal examination, transrectal ultrasonography, cystoscopy, and prostate massage in a week, prostate biopsy in a month. Peripheral blood samples (5 mL for each) from each subject were drawn into vacutainer tubes containing the anticoagulant ethylenediaminetetraacetic acid (EDTA) between 6:00 and 7:00 AM to avoid circadian fluctuation of the measured parameters [13]. Serum was obtained through centrifugation at 3000 rpm for 20 min, and was then used to evaluate the indirect enzyme linked immunosorbent assay (ELISA) for EPCA-2 and PSA, or stored at -70°C until required.
Assessment of serum EPCA-2 and PSA levels
Serum levels of EPCA-2 and PSA were evaluated using a Human EPCA-2 ELISA kit (CK-E11846, RapidBio, Inc., USA) and Human PSA ELISA kit (KB11690, Jianglai, Ltd., PRC). Microtiter strips coated with a monoclonal antibody against EPCA-2 and PSA were used. Samples, including 50 μl standards of known EPCA-2 or PSA concentrations, or 50 μl EPCA-2 or PSA antigens with diluent were added to the wells. The strips were incubated for 30 min at room temperature, followed by washed in 200 μl of wash buffer for 3 times. A horseradish peroxidase (HRP) conjugated monoclonal antibody specific for EPCA-2 or PSA was then added and the strips was incubated for 1 h at room temperature. Subsequently, the strips were washed to remove all unbound enzyme, and 50 μl of color reagents A and B mixed together for incubation within 15 min. The intensity of the color was measured at 450 nm by Microplate Reader (PHOmo, Autobio, Ltd., PRC), that directly indicated the concentration of EPCA-2 or PSA presented in the samples.
Data analysis
Statistical analyses were performed using the SPSS 18.0 statistical package. The correlations among age and the serum levels of EPCA-2 and PSA were assessed by Spearman’s rho correlation coefficient [14]. The differences of serum EPCA-2 and PSA levels among PCa, BPH, and Control, even among WDPCa, MDPCa, and PDPCa were compared using analysis of variance (ANOVA). The diagnosing accuracies of EPCA-2 and PSA were summarized using receiver operating characteristic curves, sensitivity, specificity, positive/negative likelihood ratio, positive/negative predicted value, and coincidence rate [15,16]. Furthermore, the comparison of diagnosis effects between EPCA and PSA for prostate cancer was performed with Chi-square test [17]. Data including age and serum EPCA-2 and PSA levels were expressed as mean ± SD. All of the criterions for significance was set at P<0.05.
Results
To evaluate the correlations of age with serum EPCA-2 and PSA levels, Spearman rho correlation coefficient was used and revealed that age was not significantly related to EPCA-2 and PSA (r=0.025 and 0.008, P=0.537 and 0.843, respectively). But the correlation analysis also suggested that serum EPCA-2 level was moderately related to PSA (r=0.320, P<0.01).
The serum EPCA-2 and PSA levels were higher in patients with prostate cancer (41.60±18.59 and 22.78±23.33 ng/mL) than patients with benign prostatic hyperplasia (16.42±9.34 and 5.54±4.70 ng/mL) and healthy controls (12.42±7.28 and 1.96±2.41 ng/mL), and the difference was significant (F=200.05 and 210.65, respectively, both P<0.01, Table 1). A total of 116 patients with prostate cancer including 34 well, 42 moderate, and 40 poor differentiation of pathology enrolled in the present study. However, none of the serum EPCA-2 and PSA levels was significant difference among the patients with well (43.54±18.11 and 17.06±20.99 ng/mL), moderate (40.93±19.22 and 20.42±19.84 ng/mL), and poor (40.67±18.67 and 30.12±27.00 ng/mL) differentiated prostate cancer (F=2.01 and 5.44, P=0.37 and 0.07, respectively, Table 2).
Table 1.
Association analysis of serum EPCA and PSA levels with prostatic diseases (Mean ± SD)
| Group | N | Age | EPCA-2 (ng/mL) | PSA (ng/mL) |
|---|---|---|---|---|
| PCa | 116 | 71.2±6.3 | 41.60±18.59 | 22.78±23.33 |
| BPH | 342 | 71.2±4.9 | 16.42±9.34 | 5.54±4.70 |
| Control | 174 | 71.0±5.5 | 12.42±7.28 | 1.96±2.41 |
| F | 0.58 | 200.05 | 210.65 | |
| P | 0.75 | <0.01 | <0.01 |
Table 2.
Association analysis of serum EPCA and PSA levels with differentiations of prostate cancer (Mean ± SD)
| Differentiation | N | Age | EPCA-2 (ng/mL) | PSA (ng/mL) |
|---|---|---|---|---|
| Well | 34 | 71.4±6.5 | 43.54±18.11 | 17.06±20.99 |
| Moderate | 42 | 71.4±6.3 | 40.93±19.22 | 20.42±19.84 |
| Poor | 40 | 71. 0±6.2 | 40.67±18.67 | 30.12±27.00 |
| F | 0.11 | 2.01 | 5.44 | |
| P | 0.94 | 0.37 | 0.07 |
The receiver operating characteristic curves revealed a remarkable rise of the area under the curve (AUC) for EPCA-2 (0.903, 95% CI: 0.877-0.925) compared with PSA (0.838, 95% CI: 0.807-0.866) as shown in Figure 1. The most outlying point closest to the top left corner (the maximum Youden) was selected to be the optimal cutoff value. Then 24.44 ng/mL was determined as the cutoff value for detecting prostate cancer of EPCA-2 established with these pilot sets of Chinese Han population. In addition, the cutoff value of PSA in the present study was set at 2.5 or 4.0 ng/mL followed the National Comprehensive Cancer Network (NCCN) Practice Guidelines for Prostate Cancer (v.1.2001). The serum EPCA-2 level distributions of 632 samples were shown in Figure 2, and the red line represented the cut off value.
Figure 1.
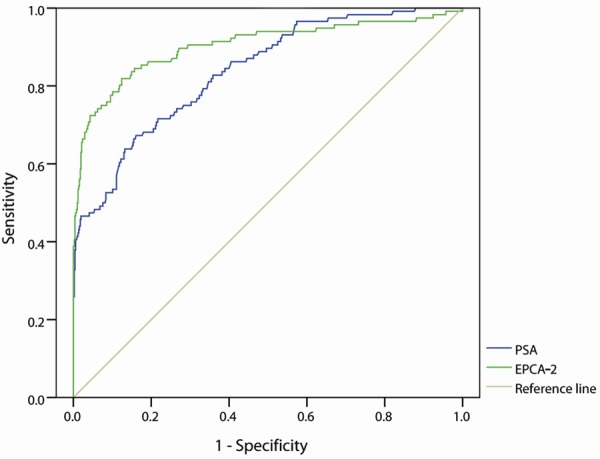
Receiver operator characteristic (ROC) curves for EPCA-2 and PSA in 632 samples. The area under ROC curve of EPCA-2 and PSA is 0.903 (95% CI: 0.877-0.925) and 0.838 (95% CI: 0.807-0.866), respectively.
Figure 2.
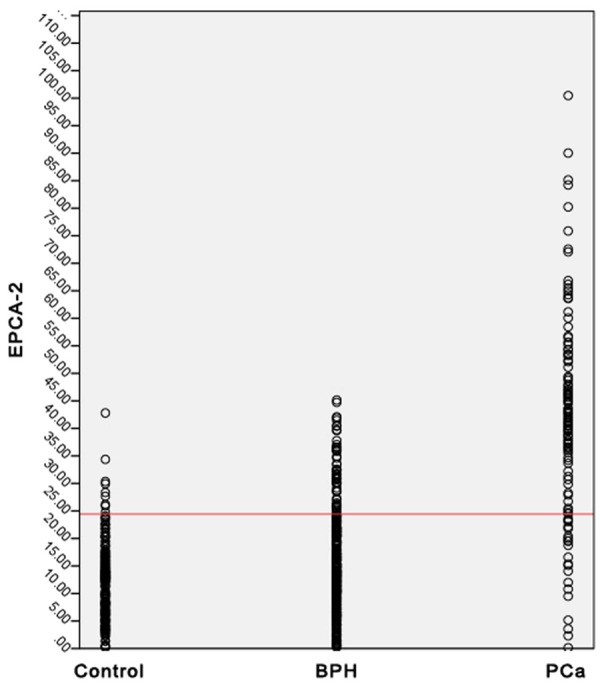
Distribution of serum EPCA-2 levels in 632 samples. The samples consist of 174 healthy control (first column), 342 patients with benign prostatic hyperplasia (second column), and 116 patients with prostate cancer (third column) are presented. Red line represents the cutoff value of 24.44 ng/mL for EPCA-2.
After establishing the cut off value of EPCA-2, 95 of 116 patients with prostate cancer and 64 of 516 samples without prostate cancer were evaluated to be serum EPCA-2 positive samples. However, 106 or 100 of 116 patients with prostate cancer and 275 or 215 of 516 samples without prostate cancer were respectively evaluated to be serum PSA positive sample when the cutoff value of PSA set at 2.5 or 4.0 ng/mL (Tables 3 and 4). Therefore, serum EPCA-2 level at a cutoff of 24.44 ng/mL had a sensitivity of 81.9% and specificity of 87.6% compared with a sensitivity of 91.4% or 86.2 and specificity of 46.7% or 58.3% for PSA with cut off value of 2.5 or 4.0 ng/mL (Table 5). Chi-square tests indicated that the specificity of EPCA-2 for detecting prostate cancer was significantly higher than PSA (Cutoff value of 2.5 ng/mL: chi-square value =181.5, P<0.01; 4.0 ng/mL: chi-square value =107.7, P<0.01; Tables 3, 4 and 5). But the sensitivities of EPCA-2 and PSA were no significant difference (Cut off value of 2.5 ng/mL: chi-square value =3.226, P=0.07; 4.0 ng/mL: chi-square value =0.485, P=0.49; Tables 3, 4 and 5). In addition, the positive/negative likelihood ratio, positive/negative predicted value, and coincidence rate of EPCA-2 and PSA were showed in Table 5.
Table 3.
Comparison of the positive rate for prostate cancer detection between EPCA-2 and PSA (Cut off: 2.5 ng/mL)
| Chi-square test | PCa | Non-PCa | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| PSA + | PSA - | Total | PSA + | PSA - | Total | |
| EPCA-2 + | 85 | 10 | 95 | 48 | 16 | 64 |
| EPCA-2 - | 21 | 0 | 21 | 227 | 225 | 452 |
| Total | 106 | 10 | 116 | 275 | 241 | 516 |
Table 4.
Comparison of the positive rate for prostate cancer detection between EPCA-2 and PSA (Cut off: 4.0 ng/mL)
| Chi-square test | PCa | Non-PCa | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| PSA + | PSA - | Total | PSA + | PSA - | Total | |
| EPCA-2 + | 81 | 14 | 95 | 35 | 29 | 64 |
| EPCA-2 - | 19 | 2 | 21 | 180 | 272 | 452 |
| Total | 100 | 16 | 116 | 215 | 301 | 516 |
Table 5.
Comparison of the diagnostic indicators between EPCA-2 and PSA for prostate cancer detection
| Diagnostic Indicators | PSA (2.5 ng/mL) | PSA (4.0 ng/mL) | EPCA-2 (24.44 ng/mL) |
|---|---|---|---|
| Sensitivity (%) | 91.4 | 86.2 | 81.9#,## |
| Specificity (%) | 46.7 | 58.3 | 87.6*,** |
| Positive Likelihood Ratio | 1.71 | 2.08 | 6.60 |
| Negative Likelihood Ratio | 0.18 | 0.24 | 0.21 |
| Positive Predicted Value (%) | 27.8 | 31.8 | 59.8 |
| Negative Predicted Value (%) | 96.0 | 95.0 | 95.6 |
| Coincidence Rate (%) | 42.7 | 46.4 | 69.1 |
Comparison between EPCA-2 and PSA (2.5 ng/mL), preponderant chi-square value =3.226, P=0.07;
Comparison between EPCA-2 and PSA (4.0 ng/mL), preponderant chi-square value =0.485, P=0.49;
Comparison between EPCA-2 and PSA (2.5 ng/mL), preponderant chi-square value =181.5, P<0.01;
Comparison between EPCA-2 and PSA (4.0 ng/mL), preponderant chi-square value =107.7, P<0.01.
Discussion
In the present study, we focused on EPCA-2, a potential serum tumor marker in prostate cancer, and systematically investigated its concentration in 632 serum samples from 116 patients with prostate cancer, 342 patients with benign prostatic hyperplasia, and 174 healthy controls. We first determined that the serum EPCA-2 and PSA levels were not affected by age. Furthermore, we observed that the serum EPCA-2 and PSA levels were associated with type of prostatic diseases. EPCA-2 had a higher specificity for detecting prostate cancer than PSA. All of these findings are entirely novel understanding for EPCA-2 in Chinese Han population.
Prostate specific antigen, a biomarker of prostate cancer, has been widely applied in clinical diagnosis and efficacy evaluation for prostate cancer [18]. However, the positive rate hovered around approximately 20% due to the increased PSA levels resulted by inflammation or benign hyperplasia of prostate [19,20]. Furthermore, PSA could be detected expression in blood only at the time that the barrier of prostate epithelial cell-vascular had been invaded and destroyed by carcinomas [18,21]. To improve the positive rate of detection prostate cancer, several novel predicting biomarkers such as beta1,4-GalNAc-disialyl-Lc4 antigen (RM2) [22], prostate specific antigen density (PSAD) [23], and prostate specific antigen velocity (PSAV) [24] were introduced, but most of these predictors are also based on PSA.
The present study had revealed that the specificity of PSA for detecting prostate cancer was only approximately 50%, while EPCA-2 was 87.6% that may be more accurately for cancer detection. The EPCA-2 antigen was determined to structurally and functionally belong to the nuclear matrix proteins, which plays an essential role of regulators in DNA replication, transcription and gene expression [25]. Numerous studies, including both animal experiments and clinical studies, have demonstrated that the nuclear matrix proteins are specifically related to carcinogenesis of various organizations especially of prostate [26,27]. Furthermore, another kind of nuclear matrix protein, EPCA, has been verified to be a potential predictor for detecting prostate cancer due to its high sensitivity and specificity [8,28]. Taking together, we infer that EPCA-2 may be at least a potential biomarker for prostate cancer screening in Chinese Han samples. However, the associations of EPCA with EPCA-2, and the advantages of EPCA-2 compared with EPCA needs further explorations.
In summary, we presented the association of EPCA-2 with prostatic diseases, and revealed the potential values of EPCA-2 for prostate cancer detection in Chinese Han population, but limitations also existed. Firstly, the results of the present study were not in accordance with Leman’s due to the different ethnic samples selection, ELISA kits, and other laboratorial conditions which would obviously affect the experimental results. Secondly, we only analyzed the parameter of diagnosis for prostate cancer, not including the treatment effects and prognosis, and further studies should be done to analyze more variables. Thirdly, because of the limitations from quality of cases and time-consuming, our sample size was inadequate that could easily led to false positive or negative results; hence other multi-center clinical studies were needed. Finally, whether EPCA-2 would be considered as a biomarker to determine the risk and prognosis of prostate cancer needs further explorations.
Acknowledgements
This study was funded by the Technology Development Program of Science & Technology Department of Henan (No. 122102310013).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Catalona WJ, Smith DS, Ratliff TL, Dodds KM, Coplen DE, Yuan JJ, Petros JA, Andriole GL. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324:1156–1161. doi: 10.1056/NEJM199104253241702. [DOI] [PubMed] [Google Scholar]
- 3.Saribacak A, Yilmaz H, Ciftci S, Ustuner M, Ozkan L, Ozkan TA, Dillioglugil O. The role of empiric antibiotic treatment in preventing unnecessary prostate biopsies in asymptomatic patients with PSA levels between 4 and 10 ng/ml. Int J Clin Exp Med. 2014;7:2230–2235. [PMC free article] [PubMed] [Google Scholar]
- 4.Komatsu K, Wehner N, Prestigiacomo AE, Chen Z, Stamey TA. Physiologic (intraindividual) variation of serum prostate-specific antigen in 814 men from a screening population. Urology. 1996;47:343–346. doi: 10.1016/s0090-4295(99)80450-6. [DOI] [PubMed] [Google Scholar]
- 5.Li J, White N, Zhang Z, Rosenzweig J, Mangold LA, Partin AW, Chan DW. Detection of prostate cancer using serum proteomics pattern in a histologically confirmed population. J Urol. 2004;171:1782–1787. doi: 10.1097/01.ju.0000119823.86393.49. [DOI] [PubMed] [Google Scholar]
- 6.Xu Y, Gan ES, He Y, Ito T. Flowering and genome integrity control by a nuclear matrix protein in Arabidopsis. Nucleus. 2013;4:274–276. doi: 10.4161/nucl.25612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paul B, Dhir R, Landsittel D, Hitchens MR, Getzenberg RH. Detection of prostate cancer with a blood-based assay for early prostate cancer antigen. Cancer Res. 2005;65:4097–4100. doi: 10.1158/0008-5472.CAN-04-4523. [DOI] [PubMed] [Google Scholar]
- 8.Partin AW, Getzenberg RH, CarMichael MJ, Vindivich D, Yoo J, Epstein JI, Coffey DS. Nuclear matrix protein patterns in human benign prostatic hyperplasia and prostate cancer. Cancer Res. 1993;53:744–746. [PubMed] [Google Scholar]
- 9.Dhir R, Vietmeier B, Arlotti J, Acquafondata M, Landsittel D, Masterson R, Getzenberg RH. Early identification of individuals with prostate cancer in negative biopsies. J Urol. 2004;171:1419–1423. doi: 10.1097/01.ju.0000116545.94813.27. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Z, Ma W, Zeng G, Qi D, Ou L, Liang Y. Serum early prostate cancer antigen (EPCA) level and its association with disease progression in prostate cancer in a Chinese population. PLoS One. 2011;6:e19284. doi: 10.1371/journal.pone.0019284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McVary KT, Roehrborn CG, Avins AL, Barry MJ, Bruskewitz RC, Donnell RF, Foster HE Jr, Gonzalez CM, Kaplan SA, Penson DF, Ulchaker JC, Wei JT. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol. 2011;185:1793–1803. doi: 10.1016/j.juro.2011.01.074. [DOI] [PubMed] [Google Scholar]
- 12.Divatia M, Shen S, Ro J. Gleason grading system. Advances in Surgical Pathology Prostate Cancer. Philadelphia. 2012:142–156. [Google Scholar]
- 13.Ding M, Song X, Zhao J, Gao J, Li X, Yang G, Wang X, Harrington A, Fan X, Lv L. Activation of Th17 cells in drug naïve, first episode schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:78–82. doi: 10.1016/j.pnpbp.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Al-Ayadhi LY, Mostafa GA. Elevated serum levels of interleukin-17A in children with autism. J Neuroinflammation. 2012;9:158. doi: 10.1186/1742-2094-9-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005;47:458–472. doi: 10.1002/bimj.200410135. [DOI] [PubMed] [Google Scholar]
- 16.Lotan Y, Roehrborn CG. Sensitivity and specificity of commonly available bladder tumor markers versus cytology: results of a comprehensive literature review and meta-analyses. Urology. 2003;61:109–118. doi: 10.1016/s0090-4295(02)02136-2. [DOI] [PubMed] [Google Scholar]
- 17.Eliasziw M, Donner A. Application of the McNemar test to non-independent matched pair data. Stat Med. 1991;10:1981–1991. doi: 10.1002/sim.4780101211. [DOI] [PubMed] [Google Scholar]
- 18.Rehman I, Azzouzi A, Catto J, Allen S, Cross SS, Feeley K, Meuth M, Hamdy FC. Proteomic analysis of voided urine after prostatic massage from patients with prostate cancer: a pilot study. Urology. 2004;64:1238–1243. doi: 10.1016/j.urology.2004.06.063. [DOI] [PubMed] [Google Scholar]
- 19.Inahara M, Suzuki H, Kojima S, Komiya A, Fukasawa S, Imamoto T, Naya Y, Ichikawa T. Improved prostate cancer detection using systematic 14-core biopsy for large prostate glands with normal digital rectal examination findings. Urology. 2006;68:815–819. doi: 10.1016/j.urology.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Zhao R, Huang Y, Cheng G, Liu J, Shao P, Qin C, Hua L, Yin C. Developing a follow-up strategy for patients with PSA ranging from 4 to 10 ng/ml via a new model to reduce unnecessary prostate biopsies. PLoS One. 2014;9:e106933. doi: 10.1371/journal.pone.0106933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, Minasian LM, Ford LG, Lippman SM, Crawford ED, Crowley JJ, Coltman CA Jr. Prevalence of prostate cancer among men with a prostate-specific antigen level ≤4.0 ng per milliliter. N Engl J Med. 2004;350:2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 22.Hasegawa Y, Oyama N, Nagase K, Fujibayashi Y, Furukawa T, Murayama Y, Arai Y, Saito S, Welch MJ, Yokoyama O. Monoclonal antibody RM2 as a potential ligand for a new immunotracer for prostate cancer imaging. Nucl Med Biol. 2012;39:944–947. doi: 10.1016/j.nucmedbio.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Benson MC, Whang I, Pantuck A, Ring K, Kaplan SA, Olsson CA, Cooner WH. Prostate specific antigen density: a means of distinguishing benign prostatic hypertrophy and prostate cancer. J Urol. 1992;147:815–816. doi: 10.1016/s0022-5347(17)37393-7. [DOI] [PubMed] [Google Scholar]
- 24.Bazinet M, Meshref AW, Trudel C, Aronson S, Péloquin F, Nachabe M, Bégin LR, Elhilali MM. Prospective evaluation of prostate-specificantigen density and systematic biopsies for early detection of prosttic carcinoma. Urology. 1994;43:44–51. doi: 10.1016/s0090-4295(94)80260-2. [DOI] [PubMed] [Google Scholar]
- 25.Leman ES, Getzenberg RH. Nuclear structure as a source of cancer specific biomarkers. J Cell Biochem. 2008;104:1988–1993. doi: 10.1002/jcb.21363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brünagel G, Schoen RE, Bauer AJ, Vietmeier BN, Getzenberg RH. Nuclear matrix protein alterations associated with colon cancer metastasis to the liver. Clin Cancer Res. 2002;8:3039–45. [PubMed] [Google Scholar]
- 27.Getzenberg RH, Pienta KJ, Huang EY, Coffey DS. Identification of nuclear matrix proteins in the cancer and normal rat prostate. Cancer Res. 1991;51:6514–6520. [PubMed] [Google Scholar]
- 28.Uetsuki H, Tsunemori H, Taoka R, Haba R, Ishikawa M, Kakehi Y. Expression of a novel biomarker, EPCA, in adenocarcinomas and precancerous lesions in the prostate. J Urol. 2005;174:514–518. doi: 10.1097/01.ju.0000165154.41159.b1. [DOI] [PubMed] [Google Scholar]


