Abstract
Phosphaturic mesenchymal tumor (PMT) has been elucidated as a cause of tumor-induced osteomalacia (TIO) associated with mesenchymal neoplasm. TIO is associated with the production of phosphatonins, such as fibroblast growth factor 23 (FGF23), which participate in phosphate homeostasis. Fibroblast growth factor receptor 1 (FGFR1) is a known receptor of FGF23, and it was recently found that the fibronectin 1 (FN1)-FGFR1 fusion gene is present in 60% of PMT cases. Immunohistochemical evaluation of FGFR1 expression in PMT has not been reported till date. We analyzed 11 cases of PMT in this study and found that 36% of cases (4/11 cases) exhibited cytoplasmic and membranous staining with strong intensity, and 64% of cases (7/11 cases) exhibited cytoplasmic dot-like staining with moderate to weak intensity. The aforementioned 36% of cases may reflect the presence of the FN1-FGFR1 fusion gene, as the FN1 promoter enhances FGFR1 expression. Although FGFR1 signaling increases FGF23 expression in an autocrine/paracrine loop, FGF23 serum level does not correlate with FGFR1 membranous expression (staining with strong intensity). Thus, we speculate that important factors other than FGFR1 are involved in the tumor biology of PMTs overexpressing FGF23.
Keywords: Phosphaturic mesenchymal tumor, immunohistochemistry, FGF23, FGFR1
Introduction
Tumor-induced osteomalacia (TIO) has been described as a minor cause of osteomalacia [1]. The majority of TIO cases are associated with mesenchymal neoplasms, while rare epithelial neoplasms are documented specifically in prostate carcinoma [2]. Various mesenchymal neoplasms have been thought to cause TIO, however, it was recently shown that a histologically uniform neoplasm, phosphaturic mesenchymal tumor (PMT), accounts for most mesenchymal neoplasm-associated cases of TIO [3,4].
PMTs often occur at various sites in the body, including soft tissues, bone, and the sinonasal region [3]. PMTs are typically composed of bland, spindled to stellate cells, with osteoclast-like giant cells sometimes observed. PMTs also exhibit a particularly well developed capillary network, with the larger vessels presenting as a staghorn pattern, in some cases [3]. The PMT matrix typically exhibits grungy or flocculent calcification [3].
TIO is triggered by the production of phosphatonins, hormones that disrupt phosphate reuptake in the kidney [5]. Among the phosphatonins, fibroblast growth factor 23 (FGF23) is the most common; it is secreted chiefly by osteocytes, acts primarily on proximal renal tubular cells, and participates in phosphate homeostasis [6]. Unlike most other FGF family members, FGF23 binding to FGF receptors (FGFRs) is often accompanied by a transmembrane co-receptor; however, high levels of FGF23 may activate FGFRs without a co-receptor [7]. FGFR1 is a well-established receptor of FGF23, and is involved in regulating cell proliferation, survival, migration, and differentiation [8].
Genetic alterations at FGFR1 loci are associated with various neoplastic and non-neoplastic disorders [9,10]. In 2015, Lee et al. identified fibronectin 1 (FN1)-FGFR1 fusion genes in 60% (9/15) of PMTs by next-generation sequencing of the tumor transcriptome and fluorescence in situ hybridization (FISH) [11].
FGFR1 appears to be related to PMT development because it is a receptor of FGF23, which is overexpressed in tumors, and because it is involved in fusion gene formation; both these factors likely confer a growth advantage for PMTs. However, immunohistochemical evaluation of FGFR1 expression in PMT has not been reported; hence, in this study, we attempted to bridge this knowledge gap.
Materials and methods
Patient samples
First, the samples used in this study were the same as those used in another paper which we were reporting regarding the relationship between CD56 expression and somatostatin receptor 2A expression in PMT. Briefly, The computerized pathological database of The University of Tokyo Hospital was searched to identify cases of PMT between 2000 and 2015 and formalin-fixed, paraffin-embedded blocks of 14 cases were found to be available. Due to the scarcity of the sample, 3 cases were not used in this study; finally, 11 cases were examined. Clinical information for these 11 cases was obtained from the computerized medical record system. Curettage was performed for cases that had developed in the bone, and enucleation was performed for cases that had developed in soft tissue.
Enzyme-linked immunosorbent assay
Serum FGF23 concentration was measured using an enzyme-linked immunosorbent assay (ELISA) that detects only full-length FGF23 (Intact FGF23 ELISA, Kainos Laboratories, Tokyo, Japan). Based on this measurement, the normal level of serum FGF23 ranges from 10 to 50 pg/ml. Preoperative and postoperative serum FGF23 levels were measured.
Histopathological evaluation
Each surgically obtained specimen was fixed in 10% buffered-formalin and then embedded in paraffin. Specimens obtained from bone had been subjected to decalcification using an ethylenediaminetetraacetic acid (EDTA) (10% EDTA 2Na; MUTO PURE CHEMICALS, Tokyo, Japan)-based reagent for the duration of approximately one week before paraffin-embedding. Four-micrometer thick sections were obtained from paraffin-embedded blocks in all 11 cases, and stained with hematoxylin and eosin.
Immunohistochemistry
Four-micrometer thick sections were obtained from paraffin-embedded blocks. Immunohistochemistry was performed using a primary antibody against FGFR1 (ab10646, 1:100; Epitomics, Burlingame, CA). Immunohistochemistry was conducted using a BenchMark XT Autoimmune Stainer (Roche Ventana Medical Systems Inc., Tokyo, Japan) and an I-View DAB detection kit (Roche Ventana Medical Systems Inc.). The proportion of immunopositive tumor cells was evaluated. Intensity of immunostaining was assigned one of four scores as follows: If immunopositivity of tumor cells was strong in low-power view (40x), the intensity was scored as 3+ (strong intensity); if weak in low-power view, but obviously stained in high-power view (400x), the intensity was scored as 1+ (weak intensity); a score of 2+ (moderate intensity) was given for moderate intensity immunostaining between 1+ and 3+; if tumor cells were not immunostained, the intensity was scored as 0.
Ethics
This study was approved by the ethics committees and governance boards of The University of Tokyo (10461-2).
Results
Clinical findings
The summary of the clinicopathological findings of the cases are presented in Table 1. The median patient age was 61 years old (ranging from 40 to 77 years old), and the male-to-female ratio was 7:4. Eight cases (73%) arose from bone and three cases (27%) arose from soft tissue. Mean preoperative level of serum FGF23 was 225.3 pg/ml (ranging from 98.9 pg/ml to 9165 pg/ml). Postoperatively, serum FGF23 levels in all cases dropped variably. No case was clinically malignant.
Table 1.
The summary of the clinicopathological findings of the cases
| Case* | FGFR1 | Decalcification | Age/Sex | FGF23 (pg/ml) | Location | Histological Findings | |||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Inten-sity | Propor-tion | Staghorn vessels | Osteoclast-like cells | Calcification | |||||
| 6 | 3+ | 90% | + | 58y/M | 3066 | Right femur | + | + | + |
| 8 | 3+ | 90% | + | 68y/M | 446 | Right humerous | + | + | - |
| 9 | 3+ | 90% | + | 64y/F | 111 | Right ilium | + | + | + |
| 11 | 3+ | 90% | - | 77y/M | 242 | Left parotid gland | + | + | + |
| 1 | 2+ | 80% | + | 68y/F | 2236 | Right sciatic bone | + | + | - |
| 2 | 2+ | 90% | + | 63y/M | 9165 | 1st lumbar vertebra | + | + | - |
| 3 | 2+ | 90% | - | 61y/M | 225 | Dura | - | + | + |
| 4 | 2+ | 90% | + | 40y/F | 162 | Right knee | + | + | + |
| 7 | 2+ | 90% | + | 58y/M | 98.9 | Left femur | + | + | + |
| 10 | 2+ | 90% | + | 41y/F | 423 | Sphenoid bone | + | + | + |
| 5 | 1+ | 70% | - | 51y/M | 482 | Left sole | - | + | - |
M: male, F: female, y: years old.
Case numbers were the same as those of previous report documenting the relationship between CD56 expression and somatostatin receptor 2A expression in phosphaturic mesenchymal tumors.
Histological findings
All cases exhibited monotonous proliferation of tumor cells within a background of prominent microvessels; staghorn-like vessels were observed in nine cases (82%). Osteoclast-like giant cells were scattered. Tumor cells exhibited a spindled shape and had scant eosinophilic cytoplasm and bland nuclei without distinct nucleoli (Figure 1A). Grungy or flocculent calcification was patchily observed in seven cases (64%; Figure 1B). Cases with histological atypia indicating malignancy were not present.
Figure 1.
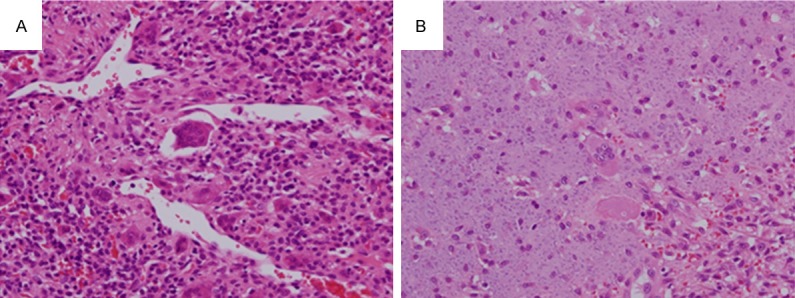
Histological findings. A. Case 3. Monotonous proliferation of tumor cells is present within a background of prominent microvessels; staghorn-like vessels were also observed. Osteoclast-like giant cells are scattered. Tumor cells are spindled in shape with scant eosinophilic cytoplasm and bland nuclei without distinct nucleoli (400×). B. Case 3. Grungy or flocculent calcification is observed (400×).
Immunohistochemical findings
Immunohistochemistry was performed for FGFR1; the results are incorporated in Table 1. EDTA-based decalcification did not affect the quality of immunostaining. FGFR1 was immunostained in 90% of tumor cells in nine cases (82%), with an intensity ranging from 2+ (cases 2, 3, 4, 7, and 10) to 3+ (cases 6, 8, 9, and 11) (case 11; Figure 2A). The other two cases were immunopositive for FGFR1 in 80% of tumor cells with an intensity of 2+ (case 1; Figure 2B) and in 70% of tumor cells with an intensity of 1+ (case 5; Figure 2C). In the cases with an intensity of 3+ (4 cases, 36%), both the cytoplasm and membrane of tumor cells were stained. Other cases with an intensity of 2+ or 1+ (7 cases, 64%) exhibited cytoplasmic dot-like staining; membranes were not apparently stained.
Figure 2.
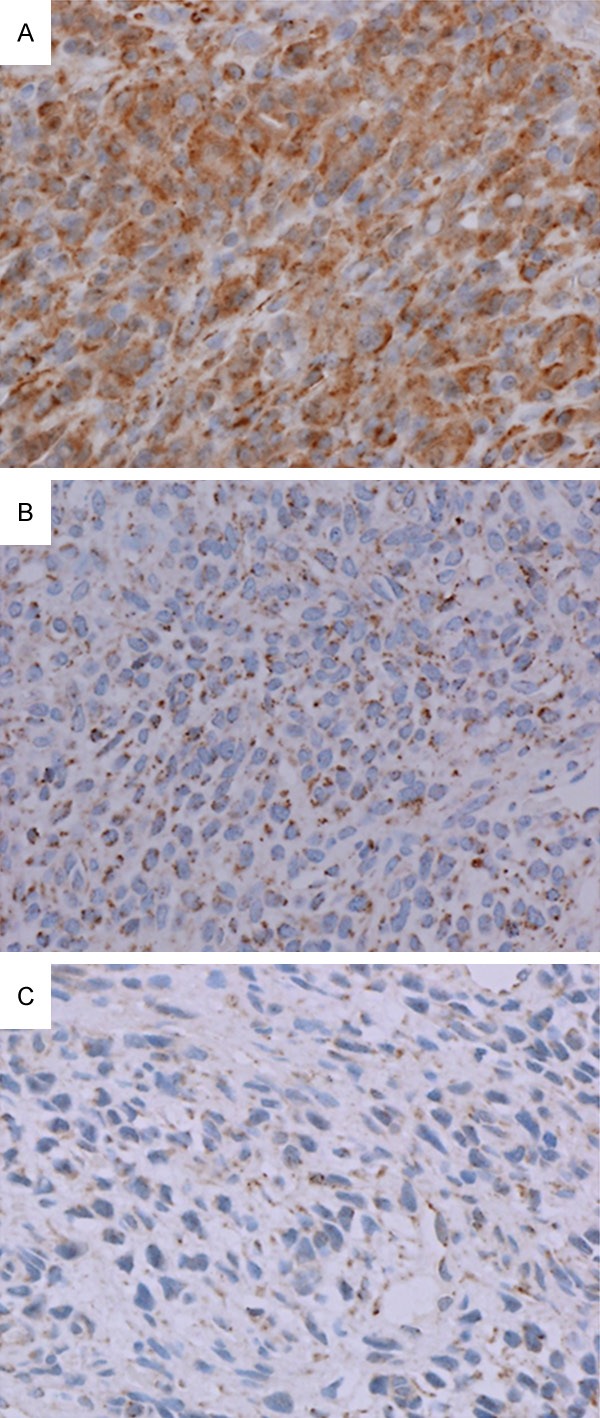
Immunohistochemical findings for fibroblast growth factor receptor 1 (FGFR1). A. Case 11. Non-decalcified specimen. Approximately 90% of tumor cells stained with an intensity of 3+ (400×). B. Case 1. Decalcified specimen. Approximately 80% of tumor cells stained with an intensity of 2+ (400×). C. Case 5. Non-decalcified specimen. Approximately 70% of tumor cells stained with an intensity of 1+ (400×).
Discussion
In 2004, Folpe et al. demonstrated that most TIO-related tumors exhibit relatively uniform histological features, distinguishing PMT as a distinct tumor entity [3]. Several distinct tumor types are often encompassed within the differential diagnosis of PMT, as demonstrated by the various diagnoses provided for PMT in the Folpe et al. review [3]. Solitary fibrous tumor (SFT) is the most difficult tumor entity to distinguish from PMT, particularly when grungy or flocculent calcification and osteoclast-like giant cells are inconspicuous; PMT exhibits small vessel proliferation that is sometimes accompanied by staghorn vessels, reminiscent of SFT [3]. Another tumor entity difficult to differentiate from PMT is chondromyxoid fibroma; at least one third of chondromyxoid fibromas show calcification of the chondromyxoid matrix; however, chondromyxoid fibromas do not display the grungy or flocculent calcification characteristic of PMT [12]. Tumor entities consisting of tumor cells with spindled or ovoid morphology and containing osteoclast-like giant cells are also included in the differential diagnosis of PMT; examples of these entities include non-ossifying fibroma and tenosynovial giant cell tumor [13,14]. Another tumor type resembling PMT is schwannoma, two cases of which were identified in the Folpe et al. review [3]. All the cases in this study were preoperatively diagnosed as PMT based on clinical information; thus, preoperative serum FGF23 was measured in every case. All the cases of PMT in this study were easy to diagnose as such, because each one exhibited one or more histological characteristics of PMT in addition to elevated serum FGF23 levels.
Immunohistochemical expression pattern of FGFR1 was largely divided into two types: membrane and cytoplasmic staining, and dot-like cytoplasmic staining. As FGFR1 is a receptor tyrosine kinase (RTK), membranous staining is expected regardless of cytoplasmic staining. However, in only 36% (4/11 cases), FGFR1 was immunostained in the membrane with an intensity score of 3+; other cases exhibited non-membranous FGFR1 expression with intensity scores of 2+ or 1+. This may reflect the fact that the FN1-FGFR1 fusion gene is not consistently present in PMT [15]. Overexpression (score 3+) of FGFR1 is speculated to be induced by the FN1 gene promoter, resulting from formation of the FN1-FGFR1 fusion gene [11]. If FN1-FGFR1 fusion-positive PMTs and fusion-negative PMTs are compared, the fusion-positive PMTs are expected to exhibit higher membranous expression of FGFR1 than the fusion-negative. In this study, the FN1-FGFR1 fusion gene was not examined, but 36% of cases exhibiting membranous expression of FGFR1 may reflect the proportion of FN1-FGFR1 fusion gene-positive cases present in this study; this percentage would be consistent with the 60% of FN1-FGFR1 fusion gene-positive cases of PMT reported by Lee et al. [11]. If the population examined by Lee et al. contained a relatively high proportion of FN1-FGFR1 fusion gene-positive cases and our patient population contained a relatively low proportion of FN1-FGFR1 fusion gene-positive cases, the difference in percentages, 60% and 36%, is not beyond expectations.
Fibronectin polymerizes to form superfibronectin, which facilitates FGFR1 transphosphorylation and activation by placing multiple FGFR1 molecules in close proximity, as a result of the formation of FN1-FGFR1 fusion protein [11]. As another mechanism of FGFR1 transphosphorylation and activation, its ligand, FGF23, is an important factor, especially when FGFR1 is present as a form of FN1-FGFR1 fusion protein, since loss of the first Ig-like domain of FGFR1 via genetic fusion or alternative splicing in the fusion protein could accelerate FGF23 binding affinity to FGFR1 [16]. FGFR1 signaling enhances FGF23 expression; thus, the autocrine/paracrine loop promoted by the increase in FGFR1 binding affinity to FGF23 and upregulation of FGF23 expression via the FGFR1 signaling pathway may be involved in PMT tumorigenesis [11].
The degree of immunohistochemical FGFR1 expression does not appear to correlate with serum FGF23 levels in this study; also, FGFR1 expression does not seem to have any relationship with characteristic histological findings. Because of the aforementioned autocrine/paracrine loop, it is expected that higher serum FGF23 levels should enhance the FGFR1 signaling pathway and promote FGF23 production; FGF23-induced functional activation of FGFR1 does not necessarily augment expression of FGFR1, as suggested by the results of this study. Of the three cases (cases 1, 2, and 6) with serum FGF23 levels over 1000 pg/ml, only one case (case 6) exhibited membranous expression of FGFR1; however, in case 9 with a serum FGF23 level of only 111 pg/ml, FGFR1 also exhibited membranous expression. These data reinforce the notion that FGFR1 expression is not correlated with serum FGF23 levels. Therefore, we speculate that factors other than FGFR1 expression status influence FGF23 serum levels.
In conclusion, 36% of the PMT cases (4/11) in this study exhibited membranous expression of FGFR1; this may reflect the presence of the FN1-FGFR1 fusion gene, as the FN1 promoter enhances expression of FGFR1. Because FGF23 serum level does not appear to correlate with FGFR1 membranous expression, we speculate that important factors other than FGFR1 are likely involved in the tumor biology of PMTs overexpressing FGF23.
Disclosure of conflict of interest
None.
References
- 1.Chong WH, Molinolo AA, Chen CC, Collins MT. Tumor-induced osteomalacia. Endocr Relat Cancer. 2011;18:R53–77. doi: 10.1530/ERC-11-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakahama H, Nakanishi T, Uno H, Takaoka T, Taji N, Uyama O, Kitada O, Sugita M, Miyauchi A, Sugishita T. Prostate cancer-induced oncogenic hypophosphatemic osteomalacia. Urol Int. 1995;55:38–40. doi: 10.1159/000282746. [DOI] [PubMed] [Google Scholar]
- 3.Folpe AL, Fanburg-Smith JC, Billings SD, Bisceglia M, Bertoni F, Cho JY, Econs MJ, Inwards CY, Jan de Beur SM, Mentzel T, Montgomery E, Michal M, Miettinen M, Mills SE, Reith JD, O’Connell JX, Rosenberg AE, Rubin BP, Sweet DE, Vinh TN, Wold LE, Wehrli BM, White KE, Zaino RJ, Weiss SW. Most osteomalacia-associated mesenchymal tumors are a single histopathologic entity: an analysis of 32 cases and a comprehensive review of the literature. Am J Surg Pathol. 2004;28:1–30. doi: 10.1097/00000478-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Folpe AL. phosphaturic mesenchymal tumour. Lyon: IARC; 2013. [Google Scholar]
- 5.Fukumoto S, Yamashita T. FGF23 is a hormone-regulating phosphate metabolism--unique biological characteristics of FGF23. Bone. 2007;40:1190–1195. doi: 10.1016/j.bone.2006.12.062. [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharyya N, Chong WH, Gafni RI, Collins MT. Fibroblast growth factor 23: state of the field and future directions. Trends Endocrinol Metab. 2012;23:610–618. doi: 10.1016/j.tem.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro OM, Kusek JW, Keane MG, Wolf M. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 9.White KE, Cabral JM, Davis SI, Fishburn T, Evans WE, Ichikawa S, Fields J, Yu X, Shaw NJ, McLellan NJ, McKeown C, Fitzpatrick D, Yu K, Ornitz DM, Econs MJ. Mutations that cause osteoglophonic dysplasia define novel roles for FGFR1 in bone elongation. Am J Hum Genet. 2005;76:361–367. doi: 10.1086/427956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker BC, Engels M, Annala M, Zhang W. Emergence of FGFR family gene fusions as therapeutic targets in a wide spectrum of solid tumours. J Pathol. 2014;232:4–15. doi: 10.1002/path.4297. [DOI] [PubMed] [Google Scholar]
- 11.Lee JC, Jeng YM, Su SY, Wu CT, Tsai KS, Lee CH, Lin CY, Carter JM, Huang JW, Chen SH, Shih SR, Marino-Enriquez A, Chen CC, Folpe AL, Chang YL, Liang CW. Identification of a novel FN1-FGFR1 genetic fusion as a frequent event in phosphaturic mesenchymal tumour. J Pathol. 2015;235:539–545. doi: 10.1002/path.4465. [DOI] [PubMed] [Google Scholar]
- 12.Wu CT, Inwards CY, O’Laughlin S, Rock MG, Beabout JW, Unni KK. Chondromyxoid fibroma of bone: a clinicopathologic review of 278 cases. Hum Pathol. 1998;29:438–446. doi: 10.1016/s0046-8177(98)90058-2. [DOI] [PubMed] [Google Scholar]
- 13.Marks KE, Bauer TW. Fibrous tumors of bone. Orthop Clin North Am. 1989;20:377–393. [PubMed] [Google Scholar]
- 14.Sciot R, Rosai J, Dal Cin P, de Wever I, Fletcher CD, Mandahl N, Mertens F, Mitelman F, Rydholm A, Tallini G, van den Berghe H, Vanni R, Willen H. Analysis of 35 cases of localized and diffuse tenosynovial giant cell tumor: a report from the Chromosomes and Morphology (CHAMP) study group. Mod Pathol. 1999;12:576–579. [PubMed] [Google Scholar]
- 15.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Shi E, Kan M, Xu J, Wang F, Hou J, McKeehan WL. Control of fibroblast growth factor receptor kinase signal transduction by heterodimerization of combinatorial splice variants. Mol Cell Biol. 1993;13:3907–3918. doi: 10.1128/mcb.13.7.3907. [DOI] [PMC free article] [PubMed] [Google Scholar]


