Abstract
B7-H6, a newly identified B7 family member molecule, binds to its receptor on NK cells, NKp30, and then triggers the anti-tumor NK cell cytotoxicity and leads to the cytokine secretion. As of now, numerous studies have demonstrated that the higher B7-H6 expression could be found in certain human cancers and have important clinical significance. In our present study, we carried out the tissue microarray and the immunohistochemistry assay to investigate the clinical significance of B7-H6 expression in human ovarian cancer. Our results showed that the positive B7-H6 staining was predominantly observed on the membrane and in the cytoplasm of the ovarian cancer cells. In order to further investigate the correlation between clinical parameters and the B7-H6 protein levels in the ovarian tissues, we categorized all the 110 patients into two major subgroups according to the intensity of B7-H6 immunohistochemical staining, i.e., the lower B7-H6 expression group, 34 cases (0 ≤ H-score < 100), and the higher B7-H6 expression group, 76 cases (H-score ≥ 100), and we found that B7-H6 expression in the ovarian cancer tissues is significantly correlated with distant metastasis status (P = 0.028) and FIGO stage (P = 0.031), whereas it is not correlated with patient’s age, tumor size, tumor location, pathological stage or nodal metastasis. The survival analysis demonstrated that the overall survival rate of the subgroup with lower B7-H6 expression is significantly better than that of the subgroup with higher B7-H6 expression (P = 0.0456, Hazard Ratio: 1.707, 95% CI, 1.010-2.885). Thus, our present data revealed that higher B7-H6 expression in ovarian cancer tissues was positively correlated with tumor metastasis and cancer progression, and supports the notion that B7-H6 expression is involved in the progression of human ovarian cancer, the detailed mechanism merits further investigation.
Keywords: B7-H6, ovarian cancer, immunohistochemistry, tissue microarray, prognosis
Introduction
Ovarian cancer, one of the most lethal gynecological malignancies, has been shown to be represented with increasing estimated new cases and new deaths in recent decades [1]. It has been suggested that the ovarian cancer patients have poor prognoses, and most of them were usually diagnosed at advanced stages [2]. Although the current conventional therapeutic modalities, such as the surgical resection and the chemotherapy, could lead to cancer remission in more than half of all patients, but the overall 5-year survival of ovarian cancer patients still remains lower than 25 percents [3]. Thus, it’s important for us to develop novel biomarkers that can sufficiently contribute to the diagnosis and the prognostic prediction of this malignancy.
It has been demonstrated that the immune regulation in tumor microenvironment of ovarian cancer significantly associated with the cancer progression and the patient’s prognosis in various clinical retrospective studies, providing evidence for the immune editing hypothesis of this malignancy [4,5]. In recent years, many studies focused on the subtypes, the functions as well as the regulation of infiltrating immune cells in human ovarian cancer tissues with important prognostic values [3,6]. Moreover, a cohort of co-stimulatory molecules was shown to play an essential role in the cell-mediated anti-tumor immunity [7]. Many studies indicated that, some of the B7 family members could be aberrantly expressed in certain human cancer tissues, and were confirmed to impair the antitumor immunity, lead to the immune evasion of the tumor cells, and finally engage in the oncogenesis and associate with the tumor progression [8,9]. For instance, B7-H1, B7-H3 and B7-H4 could be highly expressed by the tumor cells, and have important diagnostic values and immunotherapeutic implications in ovarian cancer [10-13].
B7-H6 was a newly identified B7 family member, which was shown to represent with a high level of similarity to other B7 family members, could be widely expressed on hematopoietic and non-hematopoietic tumor cells [14]. Some studies have revealed that abnormal B7-H6 expression in certain human cancers, such as lung cancer and gastric cancer, significantly associated with tumor cell differentiation [15,16]. As of now, no reports have studied the clinical significance of B7-H6 expression in human ovarian cancer. In the present study, we used the tissue microarray and the immunohistochemistry method to investigate the B7-H6 expression in human ovarian cancer tissues, and to analyze its clinical implications.
Materials and methods
Patient characteristics
Formalin-fixed, paraffin-embedded ovarian cancer tissue samples were collected from 110 patients who underwent surgical resection between February 2005 and December 2009 in our hospital, and the median age at diagnosis was 56 years. No patients received pre-operative chemotherapy or radiotherapy. All tumor tissues were confirmed as the serous ovarian cancer by using hematoxylin and eosin (H&E) staining after surgical resection. In addition, 10 benign ovarian tissues were also collected and used as controls. The clinical parameters of the patients are shown in Table 1. The survival data of all the 110 ovarian cancer patients were available, and the protocols for the present study were approved by the ethics committee of the hospital.
Table 1.
Correlation between clinical parameters and B7-H6 expression
| Clinical parameters | Cases | B7-H6 immunostaining score | P-value | ||
|---|---|---|---|---|---|
|
| |||||
| H-score < 100 | H-score ≥ 100 | χ 2 | |||
| Age | |||||
| < 60 | 76 | 26 | 50 | 1.661 | 0.198 |
| ≥ 60 | 34 | 8 | 26 | ||
| Tumor size (cm) | |||||
| < 7 | 50 | 16 | 34 | 0.051 | 0.821 |
| ≥ 7 | 60 | 18 | 42 | ||
| Tumor location | |||||
| Left | 23 | 10 | 13 | 1.961 | 0.375 |
| Right | 24 | 8 | 16 | ||
| Bilateral | 65 | 18 | 47 | ||
| Pathological stage | |||||
| T1 | 58 | 17 | 41 | 0.325 | 0.569 |
| T2 | 39 | 12 | 27 | ||
| T3 | 13 | 5 | 8 | ||
| Nodal metastasis | |||||
| Yes | 55 | 12 | 43 | 2.740 | 0.098 |
| No | 49 | 18 | 3 | ||
| Distant metastasis | |||||
| Yes | 82 | 21 | 61 | 4.807 | 0.028* |
| No | 27 | 13 | 14 | ||
| FIGO stage | |||||
| I-II | 33 | 15 | 18 | 4.671 | 0.031* |
| III-IV | 77 | 19 | 58 | ||
a. Values in bold signify P < 0.05; b. Chi square test for trend.
Tissue microarray construction
The construction of tissue microarray was performed as described in our previous study [17]. In brief, H&E-stained standard slides were reviewed from each section of ovarian cancer tissues, and a representative tumor region and the corresponding formalin-fixed paraffin-embedded tissue block were selected for use in the tissue microarray. The viable invasive cancer tissue (epithelial cells) and surrounding tumor stroma from central parts within the tumors were carefully selected and marked on the H&E slides, and then were sampled for the tissue microarray block which was assembled by using a tissue-arraying instrument (Beecher Instruments, Silver Springs, MD, USA).
Antibodies and major regents
Rabbit anti-human B7-H6 polyclonal antibody (ab121794) was purchased from Abcam (Cambridge, MA, USA), the horseradish peroxidase (HRP)-labeled goat anti-mouse/rabbit secondary antibody used in immunohistochemistry was purchased from Dako (Glostrup, Denmark). The EDTA solution (pH 9.0) used in antigen retrieval was purchased from Maxim Biotechnology Limited Corporation (Fuzhou, P. R. China).
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue microarray was cut into 3-μm-thick section, and was dewaxed in xylene, rehydrated and graded ethanol solutions. Antigen retrieval was done by heating the tissue section at 100°C for 30 min in EDTA (pH 9.0) solution. Then, the sections were immersed in a 0.3% hydrogen peroxide solution for 30 min to block endogenous peroxidase activity, rinsed in phosphate buffered saline (PBS) for 5 min, blocked with 3% BSA at room temperature for 30 min, and incubated with rabbit anti-human B7-H6 polyclonal antibody (diluted in 1:150) at 4°C overnight. A negative control was performed by omitting the primary antibodies. The horseradish peroxidase (HRP)-labeled goat anti-mouse/rabbit secondary antibody used in immunohistochemistry was purchased from Dako (Glostrup, Denmark). Diaminobenzene was used as the chromogen and hematoxylin as the nuclear counterstain. Sections were dehydrated, cleared and mounted.
Evaluation of immunohistochemical staining
The ovarian cancer tissue microarray was examined independently by two senior pathologists who were not informed of patients’ clinicopathological characteristics. The B7-H6 immunostaining densities were assessed according to the H-score method which has been described in our previous reports [18,19]: H-score = (% tumor cells unstained × 0) + (% tumor cells stained weak × 1) + (% tumor cells stained moderate × 2) + (% tumor cells stained strong × 3). The H-scores ranged from 0 (100% negative tumor cells) to 300 (100% strong staining tumor cells). Results from the two pathologists were averaged and used in the statistical analysis.
Statistical analyses
Statistical analyses were performed by using the GraphPad Prism 5.0 software package (GraphPad Software, Inc., San Diego, USA). The paired student’s t-test, the Chi-square test or the survival analysis were used where appropriate. A P-value of < 0.05 was deemed significant.
Results
B7-H6 expression in human ovarian tissues
Our immunohistochemistry results showed that the positive B7-H6 immunohistochemical staining was predominantly observed on the membrane and in cytoplasm of the tumor cells (Figure 1C), while weak staining of B7-H6 was found in ovarian cystadenoma (Figure 1F). In order to further investigate the correlation between clinical parameters and the B7-H6 protein expression levels in the ovarian tissues, we categorized all the 110 patients into two major subgroups according to the intensity of B7-H6 immunohistochemical staining, i.e., the lower B7-H6 expression group, 34 cases (0 ≤ H-score < 100), and the higher B7-H6 expression group, 76 cases (H-score ≥ 100).
Figure 1.
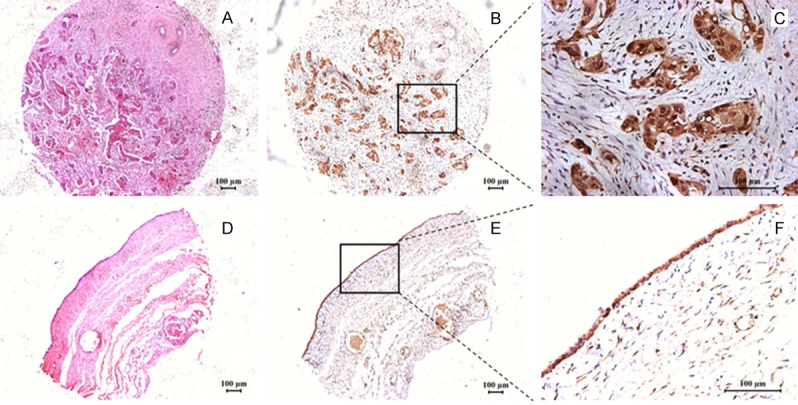
Immunohistochemical staining of B7-H6 in ovarian tissues. A. H&E staining of ovarian cancer tissue. B, C. Immunohistochemical staining of B7-H6 in ovarian cancer tissue. The positive B7-H6 immunostaining was predominantly observed on the membrane and in cytoplasm of the tumor cells. D. H&E staining of ovarian cystadenoma. E, F. Immunohistochemical staining of B7-H6 in ovarian cystadenoma tissue.
B7-H6 expression in relation to patients’ clinical parameters
The correlation between patients’ clinical parameters and tumor cell B7-H6 expression is shown in Table 1. It demonstrates that B7-H6 expression in the ovarian cancer tissues is significantly correlated with distant metastasis status (χ2 = 4.827, P = 0.028) and FIGO stage (χ2 = 4.671, P = 0.031), whereas it is not correlated with patient’s age, tumor size, tumor location, pathological stage and nodal metastasis. Thus, our data suggests that higher B7-H6 expression in ovarian cancer tissues is positively correlated with tumor metastasis and cancer progression, suggesting that B7-H6 expression is involved in the progression of human ovarian cancer.
B7-H6 expression in relation to patients’ postoperative prognoses
In order to further investigate the prognostic value of B7-H6 expression in human ovarian cancer, we performed the log-rank survival analysis according to the B7-H6 expression level in cancer tissues and collected survival data. The minimum P-value seek was conducted according to the method from the literatures and our previous study by using different H-score values as cut-offs [18-20], and when we selected H-score = 140 as the cut-off value, the survival analysis demonstrates that the overall survival rate of the subgroup with lower B7-H6 expression (0 ≤ H-score ≤ 140) is significantly better than that the subgroup with higher B7-H6 expression (H-score > 140) (P = 0.0456, Hazard Ratio: 1.707, 95% CI, 1.010-2.885, Figure 2).
Figure 2.
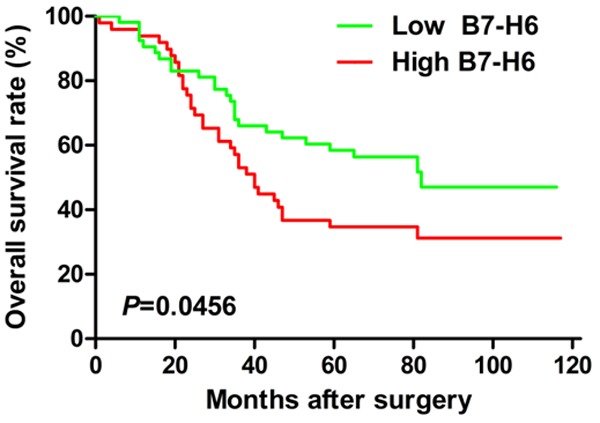
B7-H6 expression in relation to patients’ postoperative prognoses. When we selected H-score = 140 as cut-off value, the survival analysis demonstrates that the overall survival rate of the subgroup with lower B7-H6 expression (0 ≤ H-score ≤ 140) is significantly better than that the subgroup with higher B7-H6 expression (H-score > 140) (P = 0.0456, Hazard Ratio: 1.707, 95% CI, 1.010-2.885).
Discussion
B7 family members, also known as the co-stimulatory molecules, have been demonstrated to role importantly in the T-cell mediated anti-tumor immune response, and has essential implications in human cancer progression [7,8]. For instance, numerous studies have shown that the inhibitory B7 family ligands, such as B7-H1, B7-H3 and B7-H4, are over-expressed in a series of human cancers, and their expression levels significantly associated with cancer progression and patients’ prognoses [8,9]. As of now, the field of co-signaling research has been advanced by the understanding of underlying mechanisms of the immune modulation by the newly characterized co-stimulatory molecules, called immune checkpoints, and the successful preclinical and clinical trials targeting these molecules in the treatment of human cancers, such as PD-1/B7-H1, CTLA-4, etc. [21-23].
As we know, the natural killer cells are an important immune effector cell subset that could mediate the anti-tumor responses, and meanwhile, the cancer cells could also escape the NK-cell-mediated recognition in tumor microenvironment. B7-H6 could bind to the activating receptor NKp30 on natural killer cells, and alerts innate immunity to cellular transformation [14]. B7-H6 mRNA as well as protein expression, could not be detected in normal tissues, but could be found mainly on certain tumor cells, however, the detailed B7-H6 expression pattern in human cancers still remains limited due to the stress-induced self-recognition by natural killer cells [24]. Fiegler et al. reported that B7-H6 down-regulation was associated with decreased B7-H6 reporter activity and reduced histone acetylation at the B7-H6 promoter, suggesting the novel immuno-therapeutic strategy combining targeting B7-H6 with histone deacetylase inhibitors [25]. Wu et al. also reported that B7-H6-specific chimeric antigen receptors could lead to the tumor elimination and enhance the host antitumor immunity [26].
The clinical implication of B7-H6 expression in human cancer still remains elusive. It has been demonstrated that B7-H6 expression could be found in human lung cancer tissues, and its expression level was significantly associated with the degree of differentiation, whereas it was not correlated with other clinical parameters as well as patients’ prognoses [16]. In human gastric cancer, the B7-H6 expression was also significantly associated with the differentiation of tumor cells [15]. Semeraro et al. reported that the interaction between NKp30 and B7-H6 may contribute to the clinical outcome of high-risk neuroblastoma patients, and the expression of NKp30 isoforms on circulating NK cells, as well as the concentration of soluble B7-H6 in the serum of the patients, could be used as biomarkers for risk stratification of this malignancy [27]. In our present study, we demonstrated that the expression level of B7-H6 was significantly associated with distant metastasis, tumor stage and post-operative prognosis, suggesting this co-signaling ligand for NKp30, could be expressed by ovarian cancer cells, involved in the cancer progression of this malignancy, and have potential prognostic value in clinical use.
Acknowledgements
We thank senior pathologists Jun Xie and Dachuan Zhang (Department of Pathology, the Third Affiliated Hospital of Soochow University, Changzhou, Jiangsu, China) for their expert suggestions and technical assistances. This work was supported by grants from the National Natural Science Foundation of China (No. 81301960), and the Innovative Talents Training Project of Changzhou Health Bureau.
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Barnett B, Kryczek I, Cheng P, Zou W, Curiel TJ. Regulatory T cells in ovarian cancer: biology and therapeutic potential. Am J Reprod Immunol. 2005;54:369–377. doi: 10.1111/j.1600-0897.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 4.Goyne HE, Cannon MJ. Dendritic cell vaccination, immune regulation, and clinical outcomes in ovarian cancer. Front Immunol. 2013;4:382. doi: 10.3389/fimmu.2013.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lavoue V, Thedrez A, Leveque J, Foucher F, Henno S, Jauffret V, Belaud-Rotureau MA, Catros V, Cabillic F. Immunity of human epithelial ovarian carcinoma: the paradigm of immune suppression in cancer. J Transl Med. 2013;11:147. doi: 10.1186/1479-5876-11-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goode EL, DeRycke M, Kalli KR, Oberg AL, Cunningham JM, Maurer MJ, Fridley BL, Armasu SM, Serie DJ, Ramar P, Goergen K, Vierkant RA, Rider DN, Sicotte H, Wang C, Winterhoff B, Phelan CM, Schildkraut JM, Weber RP, Iversen E, Berchuck A, Sutphen R, Birrer MJ, Hampras S, Preus L, Gayther SA, Ramus SJ, Wentzensen N, Yang HP, Garcia-Closas M, Song H, Tyrer J, Pharoah PP, Konecny G, Sellers TA, Ness RB, Sucheston LE, Odunsi K, Hartmann LC, Moysich KB, Knutson KL. Inherited variants in regulatory T cell genes and outcome of ovarian cancer. PLoS One. 2013;8:e53903. doi: 10.1371/journal.pone.0053903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu B, Chen L, Liu L, Zhu Y, Wu C, Jiang J, Zhang X. T-cell-mediated tumor immune surveillance and expression of B7 co-inhibitory molecules in cancers of the upper gastrointestinal tract. Immunol Res. 2011;50:269–275. doi: 10.1007/s12026-011-8227-9. [DOI] [PubMed] [Google Scholar]
- 8.Seliger B, Quandt D. The expression, function, and clinical relevance of B7 family members in cancer. Cancer Immunol Immunother. 2012;61:1327–1341. doi: 10.1007/s00262-012-1293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 10.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, Honjo T, Fujii S. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zang X, Sullivan PS, Soslow RA, Waitz R, Reuter VE, Wilton A, Thaler HT, Arul M, Slovin SF, Wei J, Spriggs DR, Dupont J, Allison JP. Tumor associated endothelial expression of B7-H3 predicts survival in ovarian carcinomas. Mod Pathol. 2010;23:1104–1112. doi: 10.1038/modpathol.2010.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kryczek I, Wei S, Zhu G, Myers L, Mottram P, Cheng P, Chen L, Coukos G, Zou W. Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Res. 2007;67:8900–8905. doi: 10.1158/0008-5472.CAN-07-1866. [DOI] [PubMed] [Google Scholar]
- 13.Simon I, Katsaros D, Rigault de la Longrais I, Massobrio M, Scorilas A, Kim NW, Sarno MJ, Wolfert RL, Diamandis EP. B7-H4 is over-expressed in early-stage ovarian cancer and is independent of CA125 expression. Gynecol Oncol. 2007;106:334–341. doi: 10.1016/j.ygyno.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 14.Brandt CS, Baratin M, Yi EC, Kennedy J, Gao Z, Fox B, Haldeman B, Ostrander CD, Kaifu T, Chabannon C, Moretta A, West R, Xu W, Vivier E, Levin SD. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med. 2009;206:1495–1503. doi: 10.1084/jem.20090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen XJ, Shen J, Zhang GB, Chen WC. B7-H6 protein expression has no prognostic significance in human gastric carcinoma. Pathol Oncol Res. 2014;20:203–207. doi: 10.1007/s12253-013-9686-1. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Zhang G, Qin Y, Bai R, Huang J. B7-H6 expression in non-small cell lung cancers. Int J Clin Exp Pathol. 2014;7:6936–6942. [PMC free article] [PubMed] [Google Scholar]
- 17.Chen L, Deng H, Lu M, Xu B, Wang Q, Jiang J, Wu C. B7-H1 expression associates with tumor invasion and predicts patient’s survival in human esophageal cancer. Int J Clin Exp Pathol. 2014;7:6015–6023. [PMC free article] [PubMed] [Google Scholar]
- 18.Chen LJ, Sun J, Wu HY, Zhou SM, Tan Y, Tan M, Shan BE, Lu BF, Zhang XG. B7-H4 expression associates with cancer progression and predicts patient’s survival in human esophageal squamous cell carcinoma. Cancer Immunol Immunother. 2011;60:1047–1055. doi: 10.1007/s00262-011-1017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L, Di D, Luo G, Zheng L, Tan Y, Zhang X, Xu N. Immunochemical staining of MT2-MMP correlates positively to angiogenesis of human esophageal cancer. Anticancer Res. 2010;30:4363–4368. [PubMed] [Google Scholar]
- 20.Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang W, Xiong YQ, Wu WZ, Wang L, Tang ZY, Sun HC. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J. Clin. Oncol. 2008;26:2707–2716. doi: 10.1200/JCO.2007.15.6521. [DOI] [PubMed] [Google Scholar]
- 21.Jung K, Choi I. Emerging Co-signaling Networks in T Cell Immune Regulation. Immune Netw. 2013;13:184–193. doi: 10.4110/in.2013.13.5.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibnody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li G, Yang Q, Zhu Y, Wang HR, Chen X, Zhang X, Lu B. T-Bet and Eomes Regulate the Balance between the Effector/Central Memory T Cells versus Memory Stem Like T Cells. PLoS One. 2013;8:e67401. doi: 10.1371/journal.pone.0067401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fiegler N, Textor S, Arnold A, Rolle A, Oehme I, Breuhahn K, Moldenhauer G, Witzens-Harig M, Cerwenka A. Downregulation of the activating NKp30 ligand B7-H6 by HDAC inhibitors impairs tumor cell recognition by NK cells. Blood. 2013;122:684–693. doi: 10.1182/blood-2013-02-482513. [DOI] [PubMed] [Google Scholar]
- 26.Wu MR, Zhang T, DeMars LR, Sentman CL. B7H6-specific chimeric antigen receptors lead to tumor elimination and host antitumor immunity. Gene Ther. 2015;22:675–684. doi: 10.1038/gt.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Semeraro M, Rusakiewicz S, Minard-Colin V, Delahaye NF, Enot D, Vely F, Marabelle A, Papoular B, Piperoglou C, Ponzoni M, Perri P, Tchirkov A, Matta J, Lapierre V, Shekarian T, Valsesia-Wittmann S, Commo F, Prada N, Poirier-Colame V, Bressac B, Cotteret S, Brugieres L, Farace F, Chaput N, Kroemer G, Valteau-Couanet D, Zitvogel L. Clinical impact of the NKp30/B7-H6 axis in high-risk neuroblastoma patients. Sci Transl Med. 2015;7:283ra255. doi: 10.1126/scitranslmed.aaa2327. [DOI] [PubMed] [Google Scholar]


