Abstract
Recent data indicate that the tight junction proteins are abnormally regulated in several human cancers and the expression of these proteins is involved in the etiology and progression of cancer. To explore the expression distinction of the tight junction proteins claudin-5, -7, -8 and -9 in the adjacent non-neoplastic tissues and cervical carcinoma tissues, 72 cervical carcinoma tissues and the samples of non-neoplastic tissues adjacent to the tumors were examined for expression of claudin-5, -7, -8 and -9 by streptavidin-perosidase immunohistochemical staining method. The positive expression rates of claudin-5 in cervical carcinoma tissues and adjacent non-neoplastic tissues were 31.9% (23/72) and 51.4% (37/72) respectively (P < 0.05). The positive expression rates of claudin-7 in cervical carcinoma tissues and adjacent non-neoplastic tissues were 47.2% and 50.0% respectively (P = 1.000). The positive expression rates of claudin-8 in cervical carcinoma tissues and adjacent non-neoplastic tissues were 54.2 % and 27.8% respectively (P < 0.01). The positive expression rates of claudin-9 in cervical carcinoma tissues and adjacent non-neoplastic tissues were 38.9% and 56.9% respectively (P < 0.05). Thus in our study, the expression of claudin-5 and claudin-9 was down-regulated while the expression of claudin-8 was up-regulated in cervical carcinoma tissues compared with adjacent non-neoplastic tissues. The expression of claudin-7 has no obviously difference between cervical carcinoma tissues and adjacent non-neoplastic tissues. In addition, correlations between claudin-5, -8 and -9 expression with lymphatic metastasis were observed. Our study reveals that the expression of claudin-5, -8 and -9 altered between in cervical carcinoma tissues and adjacent non-neoplastic tissues.
Keywords: Tight junction, claudin-5, claudin-7, claudin-8, claudin-9, cervical carcinoma
Introduction
The formation of adhesive contacts between cells is essential for the function of many tissues [1]. This is particularly true for epithelial cells, which adhere tightly to one another to form epithelial sheets that line organ cavities and act as barriers between body compartments [2]. Tight junctions, adherens junctions, gap junctions, and desmosomes are the known cell membrane structures that participate in cell-to-cell adhesion [3]. The tight junction is one type of specialized intercellular junctional complex that mediates adhesion between epithelial cells [4]. The tight junction (TJ) is a specialized membrane domain at the most apical region of polarized epithelial and endothelial cells that not only creates a primary barrier to prevent paracellular transport of solutes but also restricts the lateral diffusion of membrane lipids and proteins to maintain the cellular polarity [5]. In epithelial cells the TJ functions in an adhesive manner and can prevent cell dissociation [6]. An important step in the formation of cancer metastases is interaction and penetration of the vascular endothelium by dissociated cancer cells [7]. TJ are therefore the first barrier that cancer cells must overcome in order to metastasize [8]. Early studies have demonstrated a correlation between the reduction of TJ and tumor differentiation and experimental evidence has emerged to place TJ in the frontline as the structure that cancer cells must overcome in order to metastasize [9,10].
Recently, more than 40 different proteins have been discovered to be located at the TJs of epithelia, endothelia and myelinated cells [11]. The tight junction structure can be separated into three regions of molecules: (a) Transmembrane region includes those molecules including occludin, claudins (claudins) and junctional adhesion molecules (JAM) that mechanically confer adhesiveness to the cell by homotypic bonding to the same molecule on adjacent cells. (b) The plaque region of the tight junction includes those molecules that anchor the transmembrane molecules to the tight junction structure and link them to the cell cytoskeleton and signaling pathways; essentially controlling the tight junction structure and function, including the zonula occludens (ZO) family of MAGUK proteins. (c) The third region is composed of associated molecules, that is, those molecules that often have other roles in the cell, but are known associates of the tight junction, where they function as part of the signaling mechanism for the structure (such as α-catenin and ponsin) [12,13]. The barrier and fence functions of tight junctions have been well appreciated [14,15]. However, it is only recently that the tight junction has come to be recognized as a complex, multiprotein structure that also plays a role in other diverse cellular processes, including the regulation of cell polarity, proliferation, and differentiation [16,17]. In addition to integral membrane proteins that mediate direct contact between cells, the tight junction also contains a large number of cytoplasmic proteins associated with the transmembrane proteins in a dense, cytoplasmic plaque [18,19]. Some of these cytoplasmic proteins serve as adaptors that link the integral membrane proteins to the cell’s actin cytoskeleton, thus stabilizing the tight junction structure [20,21]. Other cytoplasmic proteins play roles in transcription, cell polarity, or other signaling functions [22,23]. A great many of these tight junction-associated proteins contain postsynaptic density/discs large/zonula occludens (PDZ) domains and act as scaffolds to recruit other proteins into the tight junction plaque [24,25]. This unprecedented expansion of information has changed our view of TJs from merely a paracellular barrier to a complex structure involved in signaling cascades that control cell growth and differentiation.
The claudin protein family which located at transmembrane region have a crucial role in formation of tight junctions (TJs), and consists of approximately 27 members, most of which can strongly attract PDZ-containing proteins [26,27]. Hence, claudin proteins are organized into multimolecular complexes, and the activation of signaling pathways [28,29]. Recently, the abnormal expression of members of the claudin protein family has been reported to participate in tumorigenesis. the alteration of claudins proteins in cancer has been interpreted as a mechanism for the loss of cell adhesion and an important step in the progression of cancer to metastasis [30,31]. Although a considerable body of work exists on claudins and their role in a number of diseases, it is only in the last few years that their possible role in tumorigenesis has been studied. An early study in the field showed that the expression of occludin, a transmembrane proteins that contribute to formation of tight junctions, has been found to be often down-regulated in gastrointestinal tumors [32]. Similarly, it also has been demonstrated that loss of claudin-7 correlates with histological grade in both ductal carcinoma and invasive ductal carcinoma of the breast, suggesting that the loss of claudin-7 may play a important role in tumor cell dissemination and augment the cell’s metastatic potential [33]. These reports of decreased claudin proteins expression in cancer are consistent with the generally accepted idea that tumorigenesis is accompanied by a disruption of tight junctions, a process that may play an important role in the loss of cohesion, invasiveness, and lack of differentiation observed in cancer cells. Paradoxically, other studies have shown that certain claudin proteins are up-regulated in cancer. Over-expression of claudin-3 and -4 has been shown in ovarian carcinoma, prostate cancer and pancreatic cancers [34]. Such limited evidence, along with work carried out on other types of cancer, indicates that tight junctions may play a key role in cancer cell tumorigenesis. In this report, we have examined the expression of tight junction molecules claudin-5, -7, -8 and -9 in cervical carcinoma tissues and adjacent non-neoplastic tissues, and examined the correlations between levels of claudin-5, -7, -8, -9 and patient clinicopathological characteristics in primary cervical carcinoma.
Materials and methods
Patients
A total of 72 tissue samples with pathologically confirmed primary cervical squamous cell carcinoma were collected from patients being treated at the Yantai Yuhuangding Hospital during the period between June 2012 and April 2014. Adjacent normal cervical tissues were obtained from regions outside the tumor margin (> 5 cm) at the same time. All tissues were macro-dissected and confirmed by sequential pathological analysis of paraffin sections. The patients’ medical records were reviewed to determine their age and gender. Sections of the primary tumor were analyzed to identify the histological grade, and the presence or absence of regional lymph node metastasis. For the use of these clinical materials for research purposes, prior patient’s consent and approval from the Institute Research Ethics Committee was obtained. All the cancer cases were classified and staged according to the International Federation of Gynecology and Obstetrics (FIGO) staging system for cervical carcinoma.
Reagents
Rabbit polyclonal anti-human claudin-5 antibody (sc-28670), Rabbit polyclonal to claudin-7 antibody (sc-33532), Rabbit polyclonal to claudin-8 antibody (sc-66834), Goat polyclonal to claudin-9 antibody (sc-17672) were purchased from Santa Cruz Biotechnology (USA) and an streptavidin-perosidase immunohistochemistry reagent kit were purchased from Maixin Biology (Fujian, China).
Immunohistochemistry
The sections were dewaxed by heating at 55°C for 30 min and subjected to two 15 min washes with xylene. Then, the sections were rehydrated by a series of 5 min washes in ethanol. The sections were placed into an enamel cylinder containing 10 mmol/L sodium citrate (pH 6.0), heated by gas cooker at 95°C for 5 min for antigen unmasking, and then were treated with 3% hydrogen peroxide for 30 min to inactivate endogenous peroxidase activity. After being incubated with fetal bovine serum for 30 min and sections were then incubated at 4°C overnight with rabbit anti-human claudin-5 antibody, rabbit anti-human claudin-7 anti-body, rabbit anti-human claudin-8 anti-body, or goat anti-human claudin-9 antibody diluted 1:300, 1:400, 1:400 and 1:300 respectively. The sections were then washed with PBS and incubated for 30 min with biotinylated secondary antibody at 37°C. The substrate, 3’3-diaminobenzidine tetrachloride, dissolved in steamed water, was added to visualize the positive expression. Negative control sections were immunostained as described above, but incubated with PBS instead of a primary antibody.
Criteria for the positive claudin-5, -7, -8 and -9 expression in tissue
The cells positively expressing claudin-5, -7, -8 and -9 were identified by brown staining of cell membrane. The claudin-5, -7, -8 and -9 positive tissues were quantified based on the percentage of positive cells. Scoring was performed as follows: negative (-), < 10% positive tumor cells; positive (+), ≥ 10% positive tumor cells. Positive or negative reactions were determined in five random fields of each sample with image processing software Image-Pro Plus 6.0.
Statistical analysis
The Chi-square test/Chi-Square Goodness-of-Fit Test was used to determine the prognostic significance value for disease progression of each factor alone, using a P-value < 0.05 for statistically significant associations. All the data were analyzed using SPSS 12.0 statistical software.
Results
Expression of claudin-5 and claudin-9 was down-regulated in cervical carcinoma
In our study, claudin-5 expression was evaluated in the membranes of 72 cervical carcinomas tissues and 72 specimens containing cervical tissue adjacent to the carcinoma. Positive expression of protein was found in 31.9% (23/72) of cervical carcinoma tissues and in 51.4% (37/72) of adjacent tissues (Table 1). The expression of claudin-5 in cervical carcinoma tissues was significantly lower than in adjacent tissues (The Chi-square test/Chi-Square Goodness-of-Fit Test, P = 0.036 < 0.05) (Figure 1A, 1B). As shown in Table 1 the expression of claudin-5 was not correlated with age (P = 1.000), sex (P = 0.786), expression of Ki67 (P = 0.464) and histological grade (P = 1.000) but correlated with lymph node metastasis (P < 0.01).
Table 1.
Expression of claudin-5 and claudin-9 and clinic pathological characteristics in cervical carcinoma patients
| Item | n | claudin-5 (+) | claudin-5 (-) | P | n | claudin-9 (+) | claudin-9 (-) | P |
|---|---|---|---|---|---|---|---|---|
| Cervical carcinoma tissue | 72 | 23 | 49 | < 0.05 | 72 | 28 | 44 | < 0.05 |
| Adjacent tissue | 72 | 37 | 35 | 72 | 41 | 31 | ||
| Gender | ||||||||
| Male | 40 | 14 | 26 | 0.786* | 40 | 14 | 26 | 0.314* |
| Female | 32 | 9 | 23 | 32 | 14 | 18 | ||
| Age (year) | ||||||||
| ≤ 60 | 34 | 12 | 22 | 1.000* | 34 | 13 | 21 | 1.000* |
| > 60 | 38 | 11 | 27 | 38 | 15 | 23 | ||
| Histological grade | ||||||||
| Well-differentiated | 21 | 6 | 15 | 1.000* | 21 | 8 | 13 | 0.149* |
| Moderately and poor differentiated | 51 | 17 | 34 | 51 | 20 | 31 | ||
| Lymph node metastasis | ||||||||
| + | 35 | 7 | 22 | < 0.01 | 35 | 6 | 29 | < 0.01 |
| - | 37 | 16 | 21 | 37 | 22 | 15 | ||
| Ki67 | ||||||||
| + | 29 | 7 | 22 | 0.464* | 29 | 8 | 21 | 0.296* |
| - | 43 | 16 | 27 | 43 | 20 | 23 |
No statistical significance.
Figure 1.
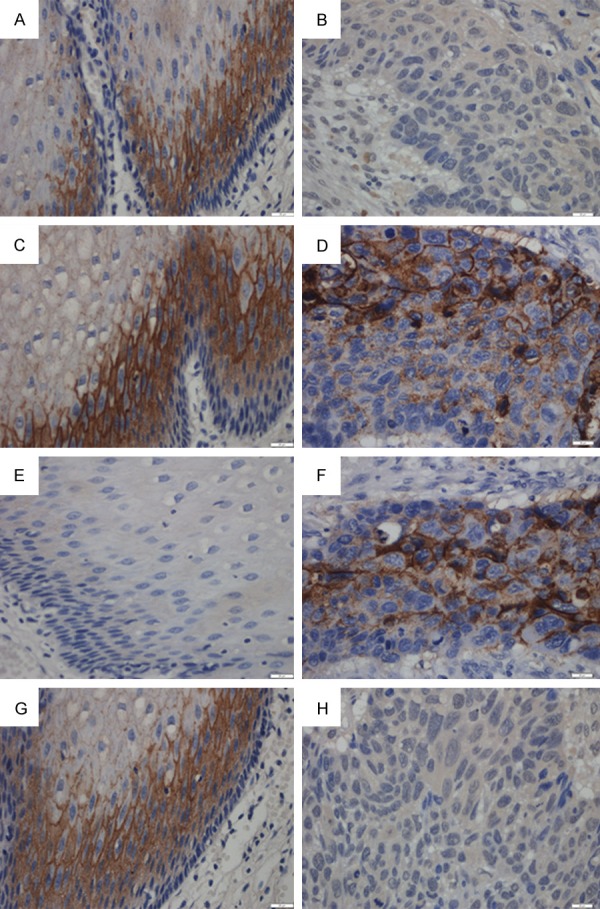
Immunohistochemical demonstration of claudins protein and expression in human cervical carcinoma and adjacent tissue. Claudins and were expressed in the cell membrane. (A) High claudin-5 expression was detected in tissue adjacent to human cervical carcinoma compared with low expression in human cervical carcinoma tissue (B) (400×). (C) Claudin-7 was highly expressed in epithelial cells adjacent to cervical carcinoma and cancer tissue itself (D). (E) The low expression of claudin-8 in tissue adjacent to human cervical carcinoma compared to strong expression of claudin-8 in human cervical carcinoma tissue (F). (G) High claudin-9 expression was detected in tissue adjacent to human cervical carcinoma compared with low claudin-9 expression in human cervical carcinoma tissue (H) (400×).
Positive expression of claudin-9 protein was found in 38.9% (28/72) of cervical carcinoma tissues and in 56.9% (41/72) of adjacent tissues (Table 1). The expression of claudin-9 in cervical carcinoma tissues was significantly lower than in adjacent tissues (The Chi-square test/Chi-Square Goodness-of-Fit Test, P = 0.017 < 0.05) (Figure 1G, 1H). As shown in Table 1 the expression of claudin-9 was not correlated with age (P = 1.000), sex (P = 0.314), histological grade (P = 0.149), expression of Ki67 (P = 0.296) but correlated with lymph node metastasis (P < 0.01).
Expression of claudin-7 in cervical carcinoma has no obvious difference with adjacent non-neoplastic tissue
The membrane staining of claudin-7 was strong in cervical carcinoma tissues and adjacent tissues. Claudin-7 was expressed in 47.2% (34/72) of cervical carcinoma tissues. Cells were positive for claudin-7 in 50.0% (36/72) of tissues adjacent to the cancer. We conclude that claudin-7 expression in cervical carcinoma samples has no obvious difference with histologically normal cervical tissues (Figure 1C, 1D) (The Chi-square test/Chi-Square Goodness-of-Fit Test, P = 1.000). As shown in Table 2 the expression of claudin-7 was not correlated with age (P = 0.242), sex (P = 0.464), histological grade (P = 1.000), expression of Ki67 (P = 0.175) and lymph node metastasis (P = 0.276).
Table 2.
Expression of claudin-7 and claudin-8 and clinic pathological characteristics in cervical carcinoma patients
| Item | n | claudin-7 (+) | claudin-7 (-) | P | n | claudin-8 (+) | claudin-8 (-) | P |
|---|---|---|---|---|---|---|---|---|
| Cervical carcinoma tissue | 72 | 34 | 38 | 1.000* | 72 | 39 | 33 | < 0.01 |
| Adjacent tissue | 72 | 36 | 36 | 72 | 20 | 52 | ||
| Gender | ||||||||
| Male | 40 | 18 | 22 | 0.464* | 40 | 22 | 18 | 0.645* |
| Female | 32 | 16 | 16 | 32 | 17 | 15 | ||
| Age (year) | ||||||||
| ≤ 60 | 34 | 14 | 20 | 0.242* | 34 | 20 | 14 | 0.146* |
| > 60 | 38 | 20 | 18 | 38 | 19 | 19 | ||
| Histological grade | ||||||||
| Well-differentiated | 21 | 10 | 11 | 1.000* | 21 | 15 | 6 | 0.124* |
| Moderately and poor differentiated | 51 | 24 | 27 | 51 | 24 | 27 | ||
| Lymph node metastasis | ||||||||
| + | 35 | 15 | 20 | 0.276* | 35 | 23 | 12 | < 0.05 |
| - | 37 | 19 | 16 | 37 | 16 | 21 | ||
| Ki67 | ||||||||
| + | 29 | 15 | 14 | 0.175* | 29 | 20 | 9 | < 0.01 |
| - | 43 | 19 | 24 | 43 | 19 | 24 |
No statistical significance.
Expression of claudin-8 was increased in cervical carcinoma
The membrane staining of claudin-8 was strong in cervical carcinoma tissues and weak in adjacent tissues. Claudin-8 was expressed in 54.2% (39/72) of cervical carcinoma tissues. Cells were positive for claudin-8 in 27.8% (20/72) of tissues adjacent to the cancer. We conclude that claudin-8 expression is significantly higher (Figure 1E, 1F) in cervical carcinoma samples than in histologically normal cervical tissue. (The Chi-square test/Chi-Square Goodness-of-Fit Test, P < 0.01). As shown in Table 2 the expression of claudin-8 was not correlated with age (P = 0.146), sex (P = 0.645), histological grade (P = 0.124), but correlated with expression of Ki67 (P < 0.01) and lymph node metastasis (P < 0.05).
Claudin-5 and claudin-9 were concurrently expressed in cervical tissues adjacent to the carcinoma
We investigated the correlation between claudin-5 and claudin-9 expression using The Chi-square test/Chi-Square Goodness-of-Fit Test, and then we find a correlation between claudin-5 and claudin-9 in cervical tissues adjacent to the carcinoma (Table 3).
Table 3.
Correlation between the expression of claudin-5 and claudin-9 in cervical tissues adjacent to the carcinoma
| Item | claudin-9 (+) | claudin-9 (-) | φ* | P |
|---|---|---|---|---|
| claudin-5 (+) | 25 | 12 | 0.716 | < 0.01 |
| claudin-5 (-) | 16 | 19 |
Discussion
Tight junctions are present in epithelial and endothelial cell membranes, forming a component of intercellular junctional complexes and playing important roles in barrier function, cell polarity, and cell signaling pathways [35]. Claudins which expressed in a tissue-specific pattern are major tight-junction constituents and display four transmembrane domains [36]. The exact role and function of individual claudins and other TJ proteins in different epithelia are still not clear. Most stratified epithelia show numerous plasma membrane sites positive for several of the TJ proteins, the distribution of which is different in various types of epithelia [37]. A dynamic interaction as well as changes in different types of claudins in TJs occur in several diseases, including tumors [38]. For example, claudin-1 is present in high-resistance, and usually absent in “leaky” epithelia [39] and its involvement in the barrier function of TJs has been shown experimentally [40]. Down-regulation of claudin-1 has been demonstrated in breast cancers compared with normal breast epithelia, and claudin-1 mRNA expression has been found to be lost or down-regulated in most breast cancer lines [41].
During the invasion process, loss of intercellular adhesion is one of the early critical steps toward metastasis [42]. Currently, it is revealed that the alternation of claudins expression is one of the mechanisms responsible for loss of cell adhesion, altered polarity, poor differentiation and enhanced invasive potential of neoplastic cells [43]. In tumor cells derived from rat mammary carcinoma, tight junctions were observed between weakly metastatic tumor cells and normal fibroblasts [7]. In pancreatic carcinoma, claudin-4 is overexpressed and this is associated with decreased invasiveness both in vitro and in vivo [44]. These findings, as well as the present results, suggest that reduced cell-to-cell adhesions formed by tight junctions lead to the dissociation of cancer cells from the original tumor, thus facilitating tumor invasiveness and metastatic potential [8]. However, little data are available on the functional association between cervical carcinogenesis and claudins at present. In this study, expression of claudin-5, -7, -8 and -9 was examined in 72 cases of cervical carcinoma and adjacent non-neoplastic tissues. We showed that the claudin-5, and claudin-9 was downregulated while the claudin-8 was up-regulated in cervical carcinoma, compared with adjacent non-neoplastic tissues. The most prominent expression of claudins was seen for claudins-8, where about 54.2% of cervical carcinoma cases showed positivity, whereas expression was weaker for claudin-5, which showed 31.9% of cervical carcinoma cases positive, and for claudin-7 and claudins-9, which showed 47.2%, 38.9% of cervical carcinoma cases positive respectively. Considering the specificity of claudin expression patterns in cancer, it has been suggested that claudins may represent useful molecular markers for many different cancers. For example, a set of four markers, including claudin-3, was found to be sufficient to accurately identify all 158 ovarian cancers tested, including eight early-stage serous cancers [45]. In addition, claudin expression may be used as a prognostic indicator, It is reported that low claudin-1 expression has been shown to be associated with a poor prognosis in stage II colon cancer [46]. It is also showed that claudin-10 expression is an independent prognostic factor for hepatocellular carcinoma recurrence after curative hepatectomy [47]. In conclusion, the present work infers that the expression of claudin-5, -8 and -9 altered between human cervical carcinomas and adjacent non-neoplastic tissues, and correlated with lymph node metastasis. In addition, claudin-5 and claudin-9 were concurrently expressed in cervical adjacent non-neoplastic tissues. However, the specific mechanism responsible for these observations needs to be addressed in the future.
Disclosure of conflict of interest
None.
References
- 1.Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 2.Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- 3.Dejana E, Corada M, Lampugnani MG. Endothelial cell-to-cell junctions. FASEB J. 1995;9:910–918. [PubMed] [Google Scholar]
- 4.Gumbiner B, Stevenson B, Grimaldi A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol. 1988;107:1575–1587. doi: 10.1083/jcb.107.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elkouby-Naor L, Ben-Yosef T. Functions of claudin tight junction proteins and their complex interactions in various physiological systems. Int Rev Cell Mol Biol. 2010;279:1–32. doi: 10.1016/S1937-6448(10)79001-8. [DOI] [PubMed] [Google Scholar]
- 6.Behrens J, Mareel MM, Van Roy FM, Birchmeier W. Dissecting tumor cell invasion: epithelial cells acquire invasive properties after the loss of uvomorulin-mediated cell-cell adhesion. J Cell Biol. 1989;108:2435–2447. doi: 10.1083/jcb.108.6.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin T, Jiang W. Tight junctions and their role in cancer metastasis. Histol Histopathol. 2001;16:1183–1195. doi: 10.14670/HH-16.1183. [DOI] [PubMed] [Google Scholar]
- 8.Martin TA, Jiang WG. Loss of tight junction barrier function and its role in cancer metastasis. Biochimica Biophys Acta. 2009;1788:872–891. doi: 10.1016/j.bbamem.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Hoover KB, Liao SY, Bryant PJ. Loss of the tight junction MAGUK ZO-1 in breast cancer: relationship to glandular differentiation and loss of heterozygosity. Am J Pathol. 1998;153:1767–1773. doi: 10.1016/S0002-9440(10)65691-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin TA, Watkins G, Mansel RE, Jiang WG. Loss of tight junction plaque molecules in breast cancer tissues is associated with a poor prognosis in patients with breast cancer. Eur J Cancer. 2004;40:2717–2725. doi: 10.1016/j.ejca.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Mariscal L, Betanzos A, Nava P, Jaramillo B. Tight junction proteins. Pro Biophysi Mol Biol. 2003;81:1–44. doi: 10.1016/s0079-6107(02)00037-8. [DOI] [PubMed] [Google Scholar]
- 12.Van Itallie CM, Anderson JM. The molecular physiology of tight junction pores. Physiology. 2004;19:331–338. doi: 10.1152/physiol.00027.2004. [DOI] [PubMed] [Google Scholar]
- 13.Tsukita S, Yamazaki Y, Katsuno T, Tamura A. Tight junction-based epithelial microenvironment and cell proliferation. Oncogene. 2008;27:6930–6938. doi: 10.1038/onc.2008.344. [DOI] [PubMed] [Google Scholar]
- 14.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 15.Heiskala M, Peterson PA, Yang Y. The roles of claudin superfamily proteins in paracellular transport. Traffic. 2001;2:92–98. doi: 10.1034/j.1600-0854.2001.020203.x. [DOI] [PubMed] [Google Scholar]
- 16.Matter K, Aijaz S, Tsapara A, Balda MS. Mammalian tight junctions in the regulation of epithelial differentiation and proliferation. Curr Opin Cell Biol. 2005;17:453–458. doi: 10.1016/j.ceb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Matter K, Balda MS. Signalling to and from tight junctions. Nat Rev Mol Cell Biol. 2003;4:225–237. doi: 10.1038/nrm1055. [DOI] [PubMed] [Google Scholar]
- 18.Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–C1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 19.Balda MS, Garrett MD, Matter K. The ZO-1-associated Y-box factor ZONAB regulates epithelial cell proliferation and cell density. J Cell Biol. 2003;160:423–432. doi: 10.1083/jcb.200210020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balda MS, Matter K. Tight junctions at a glance. J Cell Sci. 2008;121:3677–3682. doi: 10.1242/jcs.023887. [DOI] [PubMed] [Google Scholar]
- 21.Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- 22.Anderson JM. Cell signalling: MAGUK magic. Curr Biol. 1996;6:382–384. doi: 10.1016/s0960-9822(02)00501-8. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto T, Harada N, Kano K, Taya S, Canaani E, Matsuura Y, Mizoguchi A, Ide C, Kaibuchi K. The Ras target AF-6 interacts with ZO-1 and serves as a peripheral component of tight junctions in epithelial cells. J Cell Biol. 1997;139:785–795. doi: 10.1083/jcb.139.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fanning AS, Anderson JM. PDZ domains: fundamental building blocks in the organization of protein complexes at the plasma membrane. J Clin Invest. 1999;103:767–72. doi: 10.1172/JCI6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147:1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lal-Nag M, Morin PJ. The claudins. Genome Biol. 2009;10:235. doi: 10.1186/gb-2009-10-8-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krause G, Winkler L, Piehl C, Blasig I, Piontek J, Müller SL. Structure and function of extracellular claudin domains. Ann N Y Acad Sci. 2009;1165:34–43. doi: 10.1111/j.1749-6632.2009.04057.x. [DOI] [PubMed] [Google Scholar]
- 28.Morita K, Furuse M, Fujimoto K, Tsukita S. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci U S A. 1999;96:511–516. doi: 10.1073/pnas.96.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh AB, Sharma A, Dhawan P. Claudin family of proteins and cancer: an overview. J Oncol. 2010;2010:541957. doi: 10.1155/2010/541957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hewitt KJ, Agarwal R, Morin PJ. The claudin gene family: expression in normal and neoplastic tissues. BMC Cancer. 2006;6:186. doi: 10.1186/1471-2407-6-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morin PJ. Claudin proteins in human cancer: promising new targets for diagnosis and therapy. Cancer Res. 2005;65:9603–9606. doi: 10.1158/0008-5472.CAN-05-2782. [DOI] [PubMed] [Google Scholar]
- 32.Katoh M. Epithelial-mesenchymal transition in gastric cancer (Review) Int J Oncol. 2005;27:1677–1683. [PubMed] [Google Scholar]
- 33.Kominsky SL, Argani P, Korz D, Evron E, Raman V, Garrett E, Rein A, Sauter G, Kallioniemi OP, Sukumar S. Loss of the tight junction protein claudin-7 correlates with histological grade in both ductal carcinoma in situ and invasive ductal carcinoma of the breast. Oncogene. 2003;22:2021–2033. doi: 10.1038/sj.onc.1206199. [DOI] [PubMed] [Google Scholar]
- 34.Rangel LB, Agarwal R, D’Souza T, Pizer ES, Alò PL, Lancaster WD, Gregoire L, Schwartz DR, Cho KR, Morin PJ. Tight junction proteins claudin-3 and claudin-4 are frequently overexpressed in ovarian cancer but not in ovarian cystadenomas. Clin Cancer Res. 2003;9:2567–2575. [PubMed] [Google Scholar]
- 35.Niessen CM. Tight junctions/adherens junctions: basic structure and function. J Invest Dermatol. 2007;127:2525–2532. doi: 10.1038/sj.jid.5700865. [DOI] [PubMed] [Google Scholar]
- 36.Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE. Structure and function of claudins. Biochim Biophys Acta. 2008;1778:631–645. doi: 10.1016/j.bbamem.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 37.Furuse M, Sasaki H, Tsukita S. Manner of interaction of heterogeneous claudin species within and between tight junction strands. J Cell Biol. 1999;147:891–903. doi: 10.1083/jcb.147.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sawada N, Murata M, Kikuchi K, Osanai M, Tobioka H, Kojima T, Chiba H. Tight junctions and human diseases. Medical Electron Microscopy. 2003;36:147–156. doi: 10.1007/s00795-003-0219-y. [DOI] [PubMed] [Google Scholar]
- 39.Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol. 2006;68:403–429. doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- 40.Inai T, Kobayashi J, Shibata Y. Claudin-1 contributes to the epithelial barrier function in MDCK cells. Eur J Cell Biol. 1999;78:849–855. doi: 10.1016/S0171-9335(99)80086-7. [DOI] [PubMed] [Google Scholar]
- 41.Krämer F, White K, Kubbies M, Swisshelm K, Weber BH. Genomic organization of claudin-1 and its assessment in hereditary and sporadic breast cancer. Human Genet. 2000;107:249–256. doi: 10.1007/s004390000375. [DOI] [PubMed] [Google Scholar]
- 42.Geiger TR, Peeper DS. Metastasis mechanisms. Biochim Biophys Acta. 2009;1796:293–308. doi: 10.1016/j.bbcan.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 43.Anderson JM. Molecular structure of tight junctions and their role in epithelial transport. Physiology. 2001;16:126–130. doi: 10.1152/physiologyonline.2001.16.3.126. [DOI] [PubMed] [Google Scholar]
- 44.Michl P, Barth C, Buchholz M, Lerch MM, Rolke M, Holzmann KH, Menke A, Fensterer H, Giehl K, Löhr M. Claudin-4 expression decreases invasiveness and metastatic potential of pancreatic cancer. Cancer Res. 2003;63:6265–6271. [PubMed] [Google Scholar]
- 45.Zhang B, Cai FF, Zhong XY. An overview of biomarkers for the ovarian cancer diagnosis. Eur J Obstet Gynecol Reprod Biol. 2011;158:119–123. doi: 10.1016/j.ejogrb.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 46.Resnick MB, Konkin T, Routhier J, Sabo E, Pricolo VE. Claudin-1 is a strong prognostic indicator in stage II colonic cancer: a tissue microarray study. Mod Pathol. 2005;18:511–518. doi: 10.1038/modpathol.3800301. [DOI] [PubMed] [Google Scholar]
- 47.Cheung ST, Leung KL, Ip YC, Chen X, Fong DY, Ng IO, Fan ST, So S. Claudin-10 expression level is associated with recurrence of primary hepatocellular carcinoma. Clin Cancer Res. 2005;11:551–556. [PubMed] [Google Scholar]


