Abstract
Heat shock protein 90 (HSP90), a molecular chaperone, plays important roles in cellular protection against various stressful stimuli and in the regulation of cellular growth and apoptosis. HSP90 has 4 different types of human isoforms; HSP90α, HSP90β, glucose related protein 94 (GRP94) and tumor necrosis factor (TNF) receptor-associated protein 1 (TRAP1). We assessed the differential expression of these HSP90 isoforms in small-cell lung cancer (SCLC) and the correlation of their expression levels with clinicopathological factors and patient survival rates. This study included 117 SCLCs, comprised of 108 primary and 9 metastatic tumor tissues. We performed immunohistochemical staining for HSP90α, HSP90β, GRP94 and TRAP1 in 117 tumors and found that HSP90α and HSP90β were positive in 11 (9%) and 61 tumors (52%), respectively, most of which showed weak expression, whereas GRP94 and TRAP1 were positive in 115 (98%) and 117 tumors (100%), respectively, the majority of which showed moderate or strong expression. None of the HSP90 isoforms showed significant associations with clinicopathological factors or survival status in patients with SCLC. Our results indicate that GRP94 and TRAP1 might contribute more to the carcinogenesis or biology of SCLC than HSP90α and HSP90β, and that isoform selectivity should be considered when HSP90 inhibitors are studied or utilized for the treatment of SCLC.
Keywords: Small cell lung cancer, HSP90, GRP94, TRAP1, isoform, immunohistochemistry
Introduction
Small-cell lung cancer (SCLC) is an aggressive cancer that accounts for approximately 15-20% of all lung cancers [1]. Only 15% of patients with SCLC have the disease locally at the time of diagnosis, whereas more than 55% have metastasized. The five-year survival rate for the metastasized stage of the disease is 1-2% [2]. Therefore, new therapeutic strategies and helpful predictive biomarkers are an urgent priority. Recent advances in understanding the molecular mechanisms of carcinogenesis and tumor progression have led to the development of novel molecular therapeutic strategies and selective targeted agents that can improve the prognosis of cancer patients. Over the last decade, epidermal growth factor receptor (EGFR), tyrosine kinase inhibitors and anaplastic lymphoma kinase (ALK) inhibitors have improved the survival rate of patients with non-SCLC (NSCLC), especially adenocarcinoma. In the case of SCLC, several candidate molecular targets, including the PI3K/Akt/mTOR pathway, fibroblast growth factor receptor (FGFR), cell cycle checkpoint regulators, histone modifiers, DNA repair mediators, neural cell migration mediators, and mediators of stem cell characteristics, have all been studied [3,4]. However, effective targeted therapies and predictive biomarkers for SCLC have not yet been established.
Heat shock proteins (HSPs) have attracted attention as targets for novel anticancer strategies [5-7]. They are highly conserved proteins found in all types of cells, both prokaryotic and eukaryotic, and are induced by various environmental and pathophysiological stressors [8,9]. HSPs act as molecular chaperones that promote folding of newly translated proteins or the refolding of denatured proteins to maintain cellular integrity and to protect cells from stressful conditions [9,10]. In addition, they play important roles in the regulation of cellular growth and apoptosis [11]. Overexpression of HSPs has been reported in various cancers and appears to be related to tumor growth, differentiation, resistance to treatment, and prognosis [12-14].
HSPs are classified into several families according to their molecular mass [5]. Ninety kilodalton HSP (HSP90) is a representative family that is the most widely studied target for cancer therapy and includes different types of human isoforms such as cytoplasmic isoforms HSP90α (inducible) and HSP90β (constitutional), an endoplasmic reticulum isoform, glucose related protein 94 (GRP94), and a mitochondrial isoform, tumor necrosis factor (TNF) receptor-associated protein 1 (TRAP1) [5]. Previous studies have suggested that there is a significant relationship between HSP90 expression and various clinicopathological parameters in diverse types of cancers [15-21]. However, studies on HSP90 expression in SCLC tumor tissues have been very limited [20,21]. Moreover, to date, the differential expression of HSP90 isoforms in SCLC have not yet been reported.
In this study, we examined the differential expression of HSP90 isoforms in SCLC and the association between their expression levels and a variety of clinicopathological factors, including overall patient survival, to assess the potential of HSP90 expression as a predictive biomarker for HSP90 inhibitor therapy or as a prognostic biomarker of SCLC.
Materials and methods
Patients and tissue samples
We retrieved the pathology archives from 183 SCLC patients with primary or metastatic tumor specimens obtained by small biopsy or surgical resection at Samsung Changwon Hospital between January 2002 and December 2009. We excluded patients who had a previous diagnosis of any other cancer, received chemotherapy or radiotherapy prior to biopsy or surgical resection, and those for whom accurate medical records were not available. In addition, we excluded specimens obtained from recurrent tumors and those that were inadequate for immunohistochemical staining. Ultimately, 117 SCLC patients were included in this study. Clinicopathological data such as age, sex, smoking status, performance status, tumor size, lymph node metastasis, initial stage, and survival data were obtained from medical records. Tumor size, nodal status, and initial stage were clinically determined based on imaging studies, including computed tomography (CT) and positron emission tomography (PET), because all but one tumor specimen were obtained by small biopsy, not by surgical resection, and provided only limited pathological information. The duration of overall survival (OS) was defined as the time interval between the date of diagnosis and the date of last follow-up or death. This study was approved by the institutional review board at our medical institution.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue samples were cut into 4-μm sections for immunohistochemical staining. Immunohistochemical staining was performed using the Ventana Benchmark XT (Roche-Ventana, Tucson, AZ, USA). All sections were deparaffinized and subjected to pretreatment with CC1 (Roche-ventana) for 60 minutes at 100°C. Sections were then washed with reaction buffer followed by incubation with primary antibodies for 32 or 60 minutes at 37°C. The applied primary antibodies were HSP90α (clone D7a, 1:100, Abcam, Cambridge, UK), HSP90β (clone E296, 1:50, Epitomics, Burlingame, CA, USA), GRP94 (clone EPR3988, 1:100, Epitomics, Burlingame, CA, USA) and TRAP1 (clone EPR5381, 1:100, Epitomics, Burlingame, CA, USA). An ul-traView Universal DAB kit (Roche-ventana, Tucson, AZ, USA) was used in accordance with the manufacturer’s recommendations to detect the location of the primary antibody followed by counterstaining with hematoxylin (Roche-ventana, Tucson, AZ, USA). A negative control stain without primary antibody was also employed.
Immunostained slides were evaluated by two independent pathologists (HWL and EHL) blinded to clinicopathological data. Discrepant cases were reviewed on a multihead microscope until consensus was reached. For each HSP90 isoform, cases were considered positive when 10% or more of the tumor cells expressed the protein. The staining intensity of the positive cases was scored as 1 (weak), 2 (moderate), or 3 (strong).
Statistical analysis
Statistical analyses were performed with SPSS ver. 18 (SPSS Inc., Chicago, IL, USA). To evaluate relationships between immunohistochemical results and clinicopathological parameters, we used either Fisher’s exact test for categorical variables, the Mann-Whitney test for ordinal variables or independent sample t-test for continuous variables. The impact of parameters on overall survival (OS) was analyzed by the Kaplan-Meier method, and differences were compared using the log-rank test. A P-value < 0.05 was considered statistically significant.
Results
Clinicopathological characteristics
Of the 117 SCLC patients studied, which included 89 males and 28 females, the median age at diagnosis was 68 years (range, 43 to 93 years). Eighty-nine patients (76%) were current or past smokers, while 28 (24%) had never smoked. The performance status, as determined by Eastern Cooperative Oncology Group (ECOG) score, was 0-1 for 62 patients (53%) and ECOG score 2-4 for 55 patients (47%). Thirteen tumors (11%) had a maximum diameter of 3 cm or less, whereas 104 tumors (89%) were larger than 3 cm. Nodal metastasis was detected in 101 (88%) cases. Seventy-seven tumors (66%) were categorized as extended disease and 40 (34%) were limited disease. One hundred and eight tissue samples (92%) were obtained from primary lung tumors; the other 9 (8%) were obtained from metastatic sites comprising 7 lymph nodes and 2 brain metastases. These clinicopathological characteristics are summarized in Table 1.
Table 1.
Clinicopathological characteristics of 117 patients with small cell lung cancer
| Characteristics | N | (%) |
|---|---|---|
| Median age (range) | 68 (43-93) | |
| Sex | ||
| Male | 89 | (76) |
| Female | 28 | (24) |
| Smoking status | ||
| Never smoker | 28 | (24) |
| Past smoker | 48 | (41) |
| Current smoker | 41 | (35) |
| Performance status (ECOG score) | ||
| 0 | 23 | (20) |
| 1 | 39 | (33) |
| 2 | 25 | (21) |
| 3 | 20 | (17) |
| 4 | 10 | (9) |
| Tumor size (cm) | ||
| ≤ 3 | 13 | (11) |
| > 3 | 104 | (89) |
| Lymph node metastasis | ||
| Absent | 16 | (12) |
| Present | 101 | (88) |
| Stage | ||
| Limited | 40 | (34) |
| Extended | 77 | (66) |
| Biopsy site | ||
| Primary | 108 | (92) |
| Metastatic | 9 | (8) |
Differential expression of HSP90 isoforms
HSP90α, HSP90β and GRP94 showed diffuse or patch-like cytoplasmic expression with variable intensity, while TRAP1 showed strong cytoplasmic and nuclear expression in most cases (Figure 1). HSP90α was weakly expressed in 11 tumors (9%), while HSP90β was expressed in 61 tumors (52%). Of those 61 tumors, 56 were weakly positive and the other 5 were moderately positive for HSP90β. One hundred and fifteen tumors (98%) were positive for GRP94, and its expression was weak in 47 tumors, moderate in 35, and strong in 33. TRAP1 was positive in all tumors, strongly expressed in 113, moderately in 2, and weakly in 2 (Table 2). We examined whether there is any correlation among the expression levels of HSP90 isoforms, but we could not find any significant correlation (data not shown).
Figure 1.
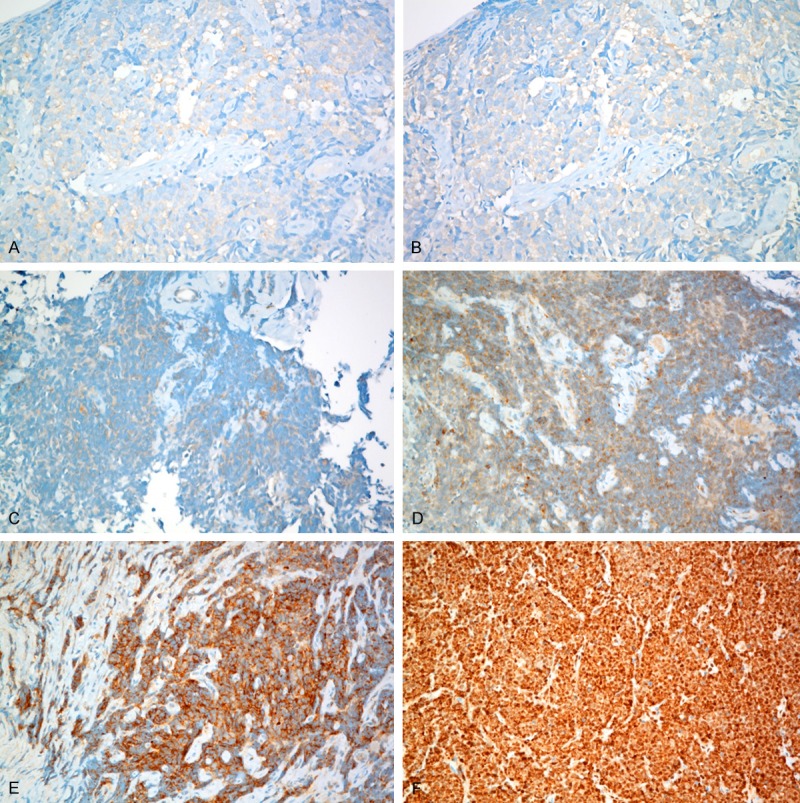
Immunohistochemical staining for HSP90 isoforms in small cell lung cancer: weakly positive expression for HSP90α (A) and HSP90β (B); weakly (C), moderately (D) and strongly (E) positive expression of GRP94; strongly positive expression of TRAP1 (F).
Table 2.
Differential expression of HSP90 isoforms in small cell lung cancer
| Expression intensity | HSP90 isoforms, N (%) | |||
|---|---|---|---|---|
|
| ||||
| HSP90α | HSP90β | GRP94 | TRAP1 | |
| Negative | 106 (91) | 56 (48) | 2 (2) | 0 (0) |
| Weakly positive | 11 (9) | 56 (48) | 47 (40) | 2 (2) |
| Moderately positive | 0 (0) | 5 (4) | 35 (30) | 2 (2) |
| Strongly positive | 0 (0) | 0 | 33 (28) | 113 (96) |
Correlation between the expression levels of HSP90 isoforms and clinicopathological characteristics
We investigated the correlation between the expression levels of HSP90 isoforms and clinicopathological characteristics. HSP90α and TRAP1 were excluded from this analysis because the HSP90α-positive group was too small compared with the negative group and TRAP1 was strongly expressed in almost all cases. With regard to GRP94, for statistical analysis, negative or weakly positive cases were reclassified as the low expression group, whereas the moderately or strongly positive cases were classified as the high expression group. Correlation results between the expression levels of HSP90β and GRP94 and clinicopathological factors are shown in Table 3. In this analysis, we could not find any significant association between the two HSP90 isoforms and clinicopathological factors. HSP90β expression had a slight association with nodal metastasis, but it was not statistically significant (P = 0.099).
Table 3.
Correlation between the expression levels of HSP90β and GRP94 and clinicopathological factors in 117 patients with small cell lung cancer
| Variables | HSP90β | GRP94 | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Negative (%) | Positive (%) | P | Low (%) | High (%) | P | |
| Total | 56 (48) | 61 (52) | 49 (42) | 68 (58) | ||
| Age (years, mean ± SD) | 68 ± 8 | 68 ± 9 | 0.916 | 69 ± 9 | 68 ± 8 | 0.375 |
| Sex | ||||||
| Male | 42 (47) | 47 (53) | 0.831 | 35 (39) | 54 (61) | 0.382 |
| Female | 14 (50) | 14 (50) | 14 (50) | 14 (50) | ||
| Smoking status | ||||||
| Never smoker | 14 (50) | 14 (50) | 0.312 | 13 (46) | 15 (54) | 0.983 |
| Past smoker | 20 (42) | 28 (58) | 18 (37) | 30 (63) | ||
| Current smoker | 22 (54) | 19 (46) | 18 (44) | 23 (56) | ||
| Performance status (ECOG score) | ||||||
| 0-1 | 32 (52) | 30 (48) | 0.459 | 25 (40) | 37 (60) | 0.851 |
| 2-4 | 24 (44) | 31 (56) | 24 (44) | 31 (56) | ||
| Tumor size (cm) | ||||||
| ≤ 3 | 6 (46) | 7 (54) | 1.000 | 6 (46) | 7 (54) | 0.772 |
| > 3 | 50 (48) | 54 (52) | 43 (41) | 61 (59) | ||
| Lymph node metastasis | ||||||
| Absent | 11 (69) | 5 (31) | 0.099 | 5 (31) | 11 (69) | 0.267 |
| Present | 45 (45) | 56 (55) | 44 (44) | 57 (56) | ||
| Stage | ||||||
| Limited | 19 (48) | 21 (52) | 1.000 | 20 (50) | 20 (50) | 0.252 |
| Extended | 37 (48) | 40 (52) | 29 (38) | 48 (62) | ||
| Biopsy site | ||||||
| Primary | 54 (50) | 54 (50) | 0.166 | 46 (43) | 62 (57) | 0.733 |
| Metastatic | 2 (22) | 7 (78) | 3 (33) | 6 (67) | ||
Correlation between the expression levels of HSP90 isoforms and patient survival
The median follow-up period was 70 days (range, 4 to 3304 days). During follow-up, 115 (98%) of the 117 patients died from disease progression. The median time from diagnosis to death was 6 months. The 2-year cumulative survival rate was 8%. HSP90β and GRP94 did not appear to have any significant impact on the OS of patients with SCLC. Of the clinicopathological factors that were measured, higher ECOG score (P < 0.001), larger tumor size (P = 0.010), lymph node metastasis (P < 0.001), and extended stage (P = 0.005) were all significantly correlated with poorer OS.
Discussion
The main functions of HSP90 as a molecular chaperone are to promote the proper folding of unfolded or misfolded proteins and to suppress their aggregation. These functions contribute to the important roles of HSP90 in the protection and maintenance of cellular viability against environmentally and pathophysiologically stressful stimuli [9,10]. In addition, HSP90 has been implicated in the regulation of cell signaling, protein trafficking and apoptosis [6,11]. Based on these functions, cancer cells are thought to be more dependent on HSP90 than normal cells. Transformed cells are exposed to oncogenic stresses induced by overexpressed abnormal oncoproteins and higher metabolic requirements; high HSP90 expression thus encourages the growth and survival of tumors [5,12,13]. Previous studies have shown that overexpression of HSP90 isoforms is involved in oncogenesis and is associated with the aggressiveness of tumors and poor prognoses in patients with various types of cancers, including gastrointestinal stromal tumor, stomach, colon, breast and lung cancer [15-21].
With regards to SCLC, however, there have been limited studies on the expression of HSP90 isoforms. Biaoxue et al. [20] suggested that overexpression of HSP90β correlates with high tumor grade, advanced stage and lymphovascular invasion of lung cancers, irrespective of histological subtype. Their study included only 11 SCLCs, 9 of which exhibited high HSP90β expression. Similarly, Wang et al. [21] showed that overexpression of GRP94 is associated with high tumor grade and advanced stage in a lung cancer cohort that included 6 SCLCs. All of these SCLCs were moderately or strongly positive for GRP94. Both studies contained very small numbers of SCLCs and used one type of HSP90 isoform, so they could not investigate the differences among the expression levels of HSP90 isoforms and the correlations between HSP90 isoforms and clinicopathological variables in SCLCs.
In our study, none of the HSP90 isoforms had any significant association with various clinicopathological factors or the survival status of patients with SCLC. However, there were considerable differences among the expression rates of HSP90 isoforms. GRP94 and TRAP1 had higher expression rates than HSP90α and HSP90β. The positive rates of HSP90α and HSP90β were 9% and 52%, respectively. In addition, almost all of these positive cases showed weak expression. On the contrary, GRP94 and TRAP1 were positive in 98% and 100% of all SCLCs, respectively, of which large proportions showed moderate or strong expression. This result indicates that GRP94 and TRAP1 might contribute more to the carcinogenesis or biology of SCLC than HSP90α and HSP90β.
HSP90 is a major inhibitor of apoptosis in SCLC, and its pharmacologic inactivation might effectively induce apoptosis in this tumor [22]. Moreover, Restall et al. [23] showed that the concentration of HSP90 inhibitor required to induce the apoptotic cell death of SCLC cells was much higher than that required to inhibit cytoplasmic HSP90s, such as HSP90α and HSP90β. They suggested that the cell death seen in SCLC cells was due to the inhibition of a target other than cytoplasmic HSP90s, and that GRP94 and TRAP1 were both candidate alternate targets for the effects of HSP90 inhibitors on SCLC cells. According to their results, GRP94 and TRAP1 are likely to play more important roles in the anti-apoptotic survival of SCLC cells than HSP90α and HSP90β, which supports the results of our study as well.
Initially, SCLC responds very well to chemotherapy and radiation, but the majority of patients relapse with resistant disease and die within two years [24]. In this study, the 2-year cumulative survival rate of SCLC patients was 8%. The development of drug resistance is the main limiting factor influencing the survival of SCLC patients. Several HSP90 inhibitors have been widely studied with the aim of overcoming resistance in different types of cancers, and although they are under evaluation in clinical trials, there have not been sufficient studies to clarify the effects of HSP90 inhibitors on SCLC [5-7]. In addition, current HSP90 inhibitors most potently interact with HSP90α and HSP90β isoforms [6]. Based on our results, HSP90 inhibitors that have a selective affinity for GRP94 or TRAP1 might be more effective against SCLC than current inhibitors. The influence of isoform selectivity on the treatment index and toxic effects of HSP90 inhibitors should be explored in future studies.
In conclusion, this study is the first to investigate the differential expression of 4 types of HSP90 isoforms and the correlation of their expression levels with various clinicopathological factors and patient survival in SCLC. There was no significant association between HSP90 isoforms and clinicopathological factors, but we found large differences among the expression rates of HSP90 isoforms in SCLC. GRP94 and TRAP1 were more highly expressed than HSP90α and HSP90β. This result implies that isoform selectivity should be seriously considered when HSP90 inhibitors are studied or adopted for the treatment of SCLC. Our study is retrospective, includes a relatively small number of cases, and lacks molecular validation. Large-scale, prospective studies with molecular validation are needed to verify our results.
Acknowledgements
This work was supported by Samsung Biomedical Research Institute grant.
Disclosure of conflict of interest
None.
References
- 1.Amini A, Byers LA, Welsh JW, Komaki RU. Progress in the management of limited-stage small cell lung cancer. Cancer. 2014;120:790–798. doi: 10.1002/cncr.28505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Argiris A, Murren JR. Staging and clinical prognostic factors for small-cell lung cancer. Cancer J. 2001;7:437–447. [PubMed] [Google Scholar]
- 3.Kalemkerian GP. Advances in pharmacotherapy of small cell lung cancer. Expert Opin Pharmacother. 2014;15:2385–2396. doi: 10.1517/14656566.2014.957180. [DOI] [PubMed] [Google Scholar]
- 4.Rossi G, Bisagni A, Cavazza A. High-grade neuroendocrine carcinoma. Curr Opin Pulm Med. 2014;20:332–339. doi: 10.1097/MCP.0000000000000061. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Carbonero R, Carnero A, Paz-Ares L. Inhibition of HSP90 molecular chaperones: moving into the clinic. Lancet Oncol. 2013;14:e358–e369. doi: 10.1016/S1470-2045(13)70169-4. [DOI] [PubMed] [Google Scholar]
- 6.Neckers L, Workman P. Hsp90 molecular chaperone inhibitors: are we there yet? Clin Cancer Res. 2012;18:64–76. doi: 10.1158/1078-0432.CCR-11-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jhaveri K, Taldone T, Modi S, Chiosis G. Advances in the clinical development of heat shock protein 90 (Hsp90) inhibitors in cancers. Biochim Biophys Acta. 2012;1823:742–755. doi: 10.1016/j.bbamcr.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schlesinger MJ. Heat shock proteins. J Biol Chem. 1990;265:12111–12114. [PubMed] [Google Scholar]
- 9.Hendrick JP, Hartl FU. Molecular chaperone functions of heat-shock proteins. Annu Rev Biochem. 1993;62:349–384. doi: 10.1146/annurev.bi.62.070193.002025. [DOI] [PubMed] [Google Scholar]
- 10.Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 11.Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 12.Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci. 2006;31:164–172. doi: 10.1016/j.tibs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Khalil AA, Kabapy NF, Deraz SF, Smith C. Heat shock proteins in oncology: Diagnostic biomarkers or therapeutic targets? Biochim Biophys Acta. 2011;1816:89–104. doi: 10.1016/j.bbcan.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Lee SD, Park SW, Hong SA, Kwon GY, Lee TJ. Expression of Survivin, HSP90, Bcl-2 and Bax Proteins in N-butyl-N-(4-hydroxybutyl) nitrosamine- induced Rat Bladder Carcinogenesis. Korean J Pathol. 2006;40:333–338. [Google Scholar]
- 15.Wang J, Cui S, Zhang X, Wu Y, Tang H. High expression of heat shock protein 90 is associated with tumor aggressiveness and poor prognosis in patients with advanced gastric cancer. PLoS One. 2013;8:e62876. doi: 10.1371/journal.pone.0062876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng HC, Takahashi H, Li XH, Hara T, Masuda S, Guan YF, Takano Y. Overexpression of GRP78 and GRP94 are markers for aggressive behavior and poor prognosis in gastric carcinomas. Hum Pathol. 2008;39:1042–1049. doi: 10.1016/j.humpath.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Milicevic Z, Bogojevic D, Mihailovic M, Petrovic M, Krivokapic Z. Molecular characterization of hsp90 isoforms in colorectal cancer cells and its association with tumour progression. Int J Oncol. 2008;32:1169–1178. [PubMed] [Google Scholar]
- 18.Diehl MC, Idowu MO, Kimmelshue K, York TP, Elmore LW, Holt SE. Elevated expression of nuclear Hsp90 in invasive breast tumors. Cancer Biol Ther. 2009;8:1952–1961. doi: 10.4161/cbt.8.20.9639. [DOI] [PubMed] [Google Scholar]
- 19.Kang GH, Lee EJ, Jang KT, Kim KM, Park CK, Lee CS, Kang DY, Lee SH, Sohn TS, Kim S. Expression of HSP90 in gastrointestinal stromal tumours and mesenchymal tumours. Histopathology. 2010;56:694–701. doi: 10.1111/j.1365-2559.2010.03550.x. [DOI] [PubMed] [Google Scholar]
- 20.Biaoxue R, Xiling J, Shuanying Y, Wei Z, Xiguang C, Jinsui W, Min Z. Upregulation of Hsp90-beta and annexin A1 correlates with poor survival and lymphatic metastasis in lung cancer patients. J Exp Clin Cancer Res. 2012;31:70. doi: 10.1186/1756-9966-31-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Q, He Z, Zhang J, Wang Y, Wang T, Tong S, Wang L, Wang S, Chen Y. Overexpression of endoplasmic reticulum molecular chaperone GRP94 and GRP78 in human lung cancer tissues and its significance. Cancer Detect Prev. 2005;29:544–551. doi: 10.1016/j.cdp.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Rodina A, Vilenchik M, Moulick K, Aguirre J, Kim J, Chiang A, Litz J, Clement CC, Kang Y, She Y, Wu N, Felts S, Wipf P, Massague J, Jiang X, Brodsky JL, Krystal GW, Chiosis G. Selective compounds define Hsp90 as a major inhibitor of apoptosis in small-cell lung cancer. Nat Chem Biol. 2007;3:498–507. doi: 10.1038/nchembio.2007.10. [DOI] [PubMed] [Google Scholar]
- 23.Restall IJ, Lorimer IA. Induction of premature senescence by hsp90 inhibition in small cell lung cancer. PLoS One. 2010;5:e11076. doi: 10.1371/journal.pone.0011076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sher T, Dy GK, Adjei AA. Small cell lung cancer. Mayo Clin Proc. 2008;83:355–367. doi: 10.4065/83.3.355. [DOI] [PubMed] [Google Scholar]


