Abstract
Aims: The purpose of this study was to investigate the correlation between single necleotide polymorphisms (SNPs) of human epidermal growth factor receptor-2 (HER2) gene with osteosarcoma susceptibility in Chinese Han population. Methods: 90 patients with osteosarcoma and 100 healthy controls who were frequency-matched with the former by age and gender were enrolled for a case-control study. 5 SNPs of HER2, namely rs2952155, rs1810132, rs2952156, rs1136201 and rs1058808, were tested by Sequenom time of flight mass spectrometry technique. The linkage disequilibrium and haplotype were analyzed using haploview software. The risk intensity of osteosarcoma was expressed by odds ratio (OR) with 95% confidence interval (CI) which was calculated by chi-squared text. Hardy-Weinberg equilibrium (HWE) was also evaluated by chi-squared text. Results: HER2 gene rs1136201 and rs1058808 polymorphisms were associated with the increased risk of osteosarcoma (P=0.04 and 0.02, respectively). Allele G in rs1136201 was 1.67 higher risk for osteosarcoma in cases than the control group (OR=1.67, 95% CI=1.11-2.51) and G allele of rs1058808 polymorphism also significantly increased osteosarcoma susceptibility (OR=2.06, 95% CI=1.27-3.22). The haplotype analysis showed that haplotype C-T-G-G might be a susceptible haplotype to osteosarcoma (OR=1.74, 95% CI=1.01-3.00). HWE test was eligible in controls (P>0.05). Conclusion: HER2 gene rs1136201 and rs1058808 polymorphisms and haplotype C-T-G-G may be related to osteosarcoma susceptibility in Chinese Han population, indicating that the interaction of gene polrmorphism plays an role in osteosarcoma risk.
Keywords: Osteosarcoma, HER2, single nucleotide polymorphism
Introduction
Osteosarcoma is one of the common osteogenic malignant tumors, accounting for about 20% of total bone tumors, and is featured by high malignancy, rapid development, prevalence in juveniles and poor prognosis with an initial mortality of 80% [1-3]. With the improvement of chemotherapy, surgical techniques and tumor classification, most of patients with osteosarcoma can be treated by limb-salvage surgery and even healed. But there are still many patients dying of neoplasm metastasis and the 5-year tumor-free survival rate is only about 65% [4-8]. So the hot area of researches both at home and abroad currently lies in how to detect high-risk group of osteosarcoma early and how to early diagnose and treat timely. There are abundant studies exploring the pathogenesis of osteosarcoma, especially genetic variant has attracted more attention. Genetic variants in microRNA, DNA repair, GST and VEGF polymorphisms and so on have been verified that have influences on the occurrence of osteosarcoma risk [9-11].
Epidermal growth factor receptor-2 (HER2) is a member of human epidermal growth factor receptor family. It is encoded by HER2 proto-oncogene, also called ERBB2, located on chromosome 17q21 and related to the development and progression of multiple tumors [12,13]. Ile655Val polymorphism caused by the mutation of A→G in transmembrane transduction domain 655 site of HER2 gene represents one of the differences between HER2 proto-oncogene and oncogene [14,15]. The majority of clinical observations show that the metastasis and invasion of gastric cancer are strong when HER2 is expressed [16]. Some reports have demonstrated the close relationship of Ile655Val polymorphism with breast cancer risk [17].
To ascertain the correlation of HER2 single necleotide polymorphisms (SNPs) with the pathogenesis of osteosarcoma in Chinese Han population, 5 SNPs of HER2 gene were tested using time of flight mass spectrometry technique in patients with osteosarcoma. 90 patients with osteosarcoma and 100 healthy controls were enrolled to explore the effect of SNPs on osteosarcoma in present study. We hope that our study can provide experimental basis for searching drug targets related to the pathogenesis of osteosarcoma.
Materials and methods
Clinical data
Ninty patients with osteosarcoma including 53 males and 37 females were collected in the case group. They were aged 16-53 with a median age of 19.6, and were histopathologically confirmed with osteosarcoma through needle or open biopsy. All patients without the history of genetic cancer syndromes did not experience radiothe rapy or chemotherapy before operation. 100 healthy people were frequency-matched by gender and age with cases as the controls. They were enrolled in regular physical examination center included 59 males and 41 females aged 12-51 with a median age of 20.3. People were excluded from controls if they suffered from diabetes, coronary heart disease or tumors. Samples were collected in accordance with the national ethics criteria for human genome research. All subjects were unrelated by blood.
Primary reagents and instruments
DNA extraction kit and Taq enzyme were purchased from Beijing Aidelai Biological Science and Technology Co. Ltd., meanwhile alkaline phosphatase, iPLEX enzyme and cation exchange resin were from Shanghai North Connaught Biotechnology Co. Ltd. SpectroCHIP and MassARRAYTyper software system were provided by Shanghai Pan Ke Industrial Co. Ltd.
Primer design and synthesis
Primers were designed by Primer 5.0 software and synthesized by Shanghai Genecore Biotechnologies Co. Ltd. and primer sequences are listed in Table 1.
Table 1.
Primer sequences of HER2 polymorphisms
| SNP | Primer Sequences | PCR product length |
|---|---|---|
| rs2952155 | Forward 5’-CTGGCAGCAGGGCGTTATTT-3’ | 201 bp |
| Reverse 5’-CAAAATAACGCCCTGCTGCC-3’ | ||
| rs1810132 | Forward 5’-TGCCTCTTCATCTCTGGGGT-3’ | 352 bp |
| Reverse 5’-GAACCCCAGAGATGAAGAGG-3’ | ||
| rs2952156 | Forward 5’-ACTTTGGGGAGAAAAACAGA-3’ | 289 bp |
| Reverse 5’-GTTTTTCTCCCCAAAGTCCT-3’ | ||
| rs1136201 | Forward 5’-AGGCAGGTTTTAGAGTAGGA-3’ | 355 bp |
| Reverse 5’-TACTCTAAAACCTGCCTTGG-3’ | ||
| rs1058808 | Forward 5’-TAATGGGTCACCTTCTCTTG-3’ | 300 bp |
| Reverse 5’-CAAGAGAAGGTGACCCATTA-3’ |
DNA extraction
2~3 mL peripheral venous blood was collected in morning from every subject with an empty stomach, and was conducted anticoagulation using 20 g/L EDTA 200 µL. Genome DNA was extracted using Qiagen genome DNA extraction kit following the operating manual, standardized the concentration to 50 µg/L, and preserved in freezer at -20°C for later.
PCR system
PCR reaction system is a volume of 20 µL solution, including 1 µL of DNA sample diluted to 5 µg/L previously, 0.5 µL forward and reverse primers, respectively, 2.0 µL PCR buffer (containing 15 mmol/L MgCl2), 0.2 L of 2.5 mmol/L dNTP, 0.1 µL HotStarTaq enzyme and 15.7 µL ddH2O. PCR conditions were as follows: 94°C for 15 min; 45 cycles of 94°C for 20 s, 56°C for 30 s and 72°C for 1 min; 72°C for 3 min. Residual dNTP was digested through dephosphorylation after PCR amplification, containing 1.53 µL water, 0.17 µL SAP buffer and 0.3 U alkaline phosphatase. The reaction was proceeded at 37°C for 40 min and then at 85°C for 5 min to make enzyme inactive. The primer extension reaction was conducted according to Sequenom program.
Genotyping analysis
SNP genotyping was operated by Shanghai Pan Ke Industrial Co. Ltd. utilizing MassARRAY system of American Sequenom company. The final reactant was added with 6 mg cation exchange resin for desalination and mixed with 25 µL water for suspension. The final typing products were operated spotting to a spectroCHIP with 384 holes using MassARRAY Nanodispenser system and were analyzed by matrix assisted laser desorption ionizing time of flight mass spectrometry. Final results were read in real time by MassARRAYRT software system and conducted genotyping analysis through MassARRAYTyper software system.
Statistical analysis
Hardy-Weinberg equilibrium (HWE) was tested by chi-squared test in the control group. Statistical analysis was operated using SPSS 18.0 software. The distributions of allele and genotype in HER2 SNPs both in cases and controls were calculated by χ2 test. The linkage disequilibrium and haplotype were analyzed using haploview software. Odds ratio (OR) and 95% confidence interval (CI) were calculated to evaluate the effects of genotypes, alleles and haplotypes in HER2 SNPs on the risk of osteosarcoma. P<0.05 represents statistical significance.
Results
Test of Hardy-Weinberg equilibrium
Genotype distributions of HER2 rs2952155, rs1810132, rs2952156, rs1136201 and rs1058808 polymorphisms in the control group were all consistent with Hardy-Weinberg equilibrium (P>0.05), indicating that the study objects came from the same community and had the representativeness of regional population.
Correlation analysis between genotypes and alleles of HER2 SNPs with the pathogenesis of osteosarcoma
Table 2 lists the distribution of genotypes and alleles of rs2952155, rs1810132, rs2952156, rs1136201 and rs1058808 polymorphisms in HER2 gene. The genotypes frequencies of HER2 rs1136201 and rs1058808 polymorphisms were significantly different between in cases and the control group (P=0.04 and 0.02, respectively). They might be associated with the increased risk of osteosarcoma. Allele G in rs1136201 was 1.67 higher risk for osteosarcoma in cases compared with the control group (OR=1.67, 95% CI=1.11-2.51). Similarly, G allele of rs1058808 polymorphism was also a risk factor for osteosarcoma (OR=2.06, 95% CI=1.27-3.22).
Table 2.
Genotype and allele frequencies of HER2 polymorphisms
| SNP | Genotype (n) | P | Allele (2n) | P | |||
|---|---|---|---|---|---|---|---|
| rs2952155 | CC | CT | TT | 0.28 | C | T | 0.15 |
| Case | 33 | 31 | 26 | 97 | 83 | ||
| Control | 26 | 41 | 33 | 93 | 107 | ||
| rs1810132 | CC | CT | TT | 0.26 | C | T | 0.09 |
| Case | 19 | 39 | 32 | 77 | 103 | ||
| Control | 31 | 41 | 28 | 103 | 97 | ||
| rs2952156 | GG | GA | AA | 0.18 | G | A | 0.97 |
| Case | 47 | 26 | 17 | 120 | 60 | ||
| Control | 46 | 41 | 13 | 133 | 67 | ||
| rs1136201 | AA | AG | GG | 0.04 | A | G | 0.01a |
| Case | 20 | 47 | 23 | 87 | 93 | ||
| Control | 38 | 46 | 16 | 122 | 78 | ||
| rs1058808 | CC | CG | GG | 0.02 | C | G | 3.00×10-3 b |
| Case | 48 | 28 | 14 | 124 | 56 | ||
| Control | 69 | 26 | 5 | 164 | 36 | ||
represents OR=1.67 and 95% CI=1.11-2.51;
means OR=2.06 and 95% CI=1.27-3.22.
Haplotype analysis
Linkage disequilibrium and haplotype analysis of rs2952155, rs1810132, rs2952156 and rs1058808 polymorphisms (Table 3 and Figure 1) were conducted with haploview software. The correlation analysis of these four haplotypes with osteosarcoma susceptibility demonstrated that haplotype C-T-G-G had significant differences between cases and controls (P<0.05) and might be a susceptible factor to osteosarcoma (OR=1.74, 95% CI=1.01-3.00).
Table 3.
Linkage disequilibrium and haplotype analysis of HER2 rs2952155, rs1810132, rs2952156 and rs1508808
| Haplotype | Cases, 2n | Controls, 2n | OR (95% CI) | P value |
|---|---|---|---|---|
| T-C-A-C | 60 (34.48) | 67 (34.18) | 1.00 (Ref.) | - |
| C-T-G-C | 41 (22.99) | 57 (29.08) | 0.80 (0.47-1.37) | 0.42 |
| C-T-G-G | 56 (32.76) | 36 (18.37) | 1.74 (1.01-3.00) | 4.60×10-2 |
| T-C-G-C | 17 (9.77) | 36 (18.37) | 0.53 (0.23-1.03) | 0.06 |
Figure 1.
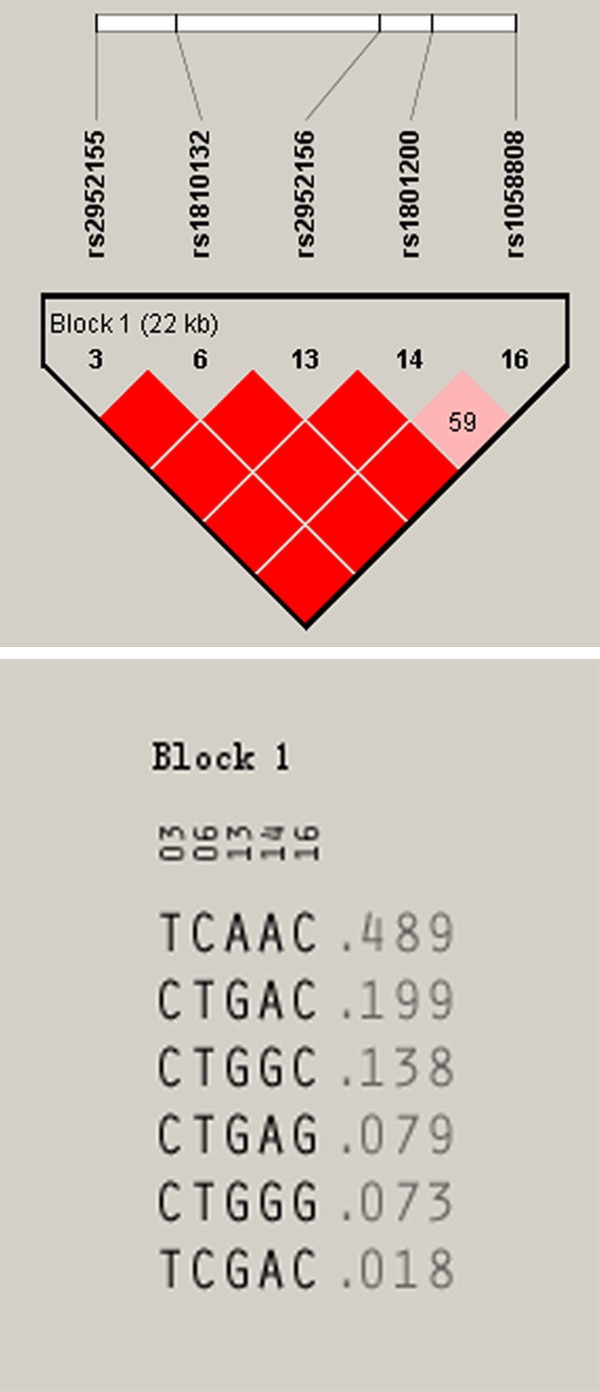
Haplotype analysis diagram. Darker block represent a stronger linkage disequilibrium, while the lighter one stands for a weaker linkage disequilibrium.
Discussion
Osteosarcoma is a connective tissue malignant tumor which tumor cells can directly produce tumor bones and osteoid tissues and its morbidity ranks the first in all primary malignancies [18,19]. It has taken place mostly on distal femur and proximal tibia, accounting for 75% of all osteosarcoma patients [20]. Distant metastasis is the main cause of treatment failure and death in the majority of patients with osteosarcoma [21]. In recent years, with the development of tumor molecular biology, it has been realized that cell carcinogenesis is mainly caused by the changes of genetic information [22-24]. According to the study of Chang et al. in a meta-analysis, genetic variants of CD 152 can significantly increased the development of osteosarcoma risk in Chinese population [25]. As far as the study of Wang et al., MDM2 plays a vital role in the carcinogenesis of osteosarcoma, the polymorphism rs2279744 of it is associated with the increased risk of osteosarcoma [26].
HER2 participates in the regulation of cell proliferation, apoptosis, metastasis and invasion through various signal transduction pathways, including Ras/Raf/MAPK, PI3K/Akt and STAT [27]. Studies have shown that Ile655Val polymorphism caused by the conversion of A to G in transmembrane transduction domain of HER2 protein can significant activate similar ligand mediated receptor and tyrosine kinase and improve the autophosphorylation, tyrosine kinase activity and cell transformation [28]. What is more, multiple studies have reported the relationship of HER2 gene polymorphism Ile655Val with the risk of breast cancer [29]. Recently, with the increasing number of studies on the association of HER2 with gastric cancer risk, monoclonal antibody Herceptin targeting at HER2 is applied to treating advanced gastric cancer patients, which demonstrates promising effects. At the moment, observed results mainly show that HER2/neu is related to tumor size, serosal invasion degree and lymph node metastasis [30]. Lots of researches suggest that HER2 is closely related to multiple cancers. The high expression of HER2 protein affects the malignant biological behavior of osteosarcoma and always predict a poor prognosis. Scotlandi et al. researched 84 cases of osteosarcoma using immunohistochemical staining and found that HER2 gene was over-expressed in 32% of the cases [31].
In present study, 5 SNPs from HER2 were analyzed and their functions were different. Two of them rs1136201 and rs1508808 have demonstrated that either genotypes or alleles are associated with the increased risk of osteosarcoma. But the other three SNPs rs1810132, rs2952155 and rs2952156 had no significant relevance with osteosarcoma susceptibility. Further haplotype analysis manifested that haplotype C-T-G-G constituted by rs2952155, rs1810132, rs2952156 and rs1508808 had significant differences between cases and controls, being the potential susceptible haplotype to osteosarcoma. Additionally, as haplotype C-T-G-G contains susceptible allele G, further evidence has been offered to prove that G allele at rs1508808 may be the susceptible factor for osteosarcoma.
There are more than 20 SNPs in human HER2 gene, and in this study we only primarily analyzed the association between the incidence of osteosarcoma with the SNPs of minor allele frequency (MAF) larger than 0.05. Based on the present and previous studies, we speculate genetic variant of HER2 affect the development of osteosarcoma through regulating the expression level of HER in patients with osteosarcoma. Rs2952155 and rs2952156 are the mutations in intron domain, which may be a reason that both of two don’t make woke in the pathogenesis of osteosarcoma. Additionally, the sample size of the study was relatively small. To fully understand the functions of HER2 SNPs on the onset of osteosarcoma in Chinese Han population, more efforts should be made to seek more SNPs and expand the scale of samples in studies so as to provide experimental evidence for the pathogenesis, procession and early diagnosis of osteosarcoma.
Disclosure of conflict of interest
None.
References
- 1.Picci P. Osteosarcoma (osteogenic sarcoma) Orphanet J Rare Dis. 2007;2:6. doi: 10.1186/1750-1172-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caudill JS, Arndt CA. Diagnosis and management of bone malignancy in adolescence. Adolesc Med State Art Rev. 2007;18:62–78. [PubMed] [Google Scholar]
- 3.Rosen G, Tan C, Sanmaneechai A, Beattie EJ Jr, Marcove R, Murphy ML. The rationale for multiple drug chemotherapy in the treatment of osteogenic sarcoma. Cancer. 1975;35:936–945. doi: 10.1002/1097-0142(197503)35:3+<936::aid-cncr2820350714>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 4.Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M, Winkelmann W, Zoubek A, Jurgens H, Winkler K. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J. Clin. Oncol. 2002;20:776–790. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 5.Liang W, Gao B, Xu G, Weng D, Xie M, Qian Y. Possible contribution of aminopeptidase N (APN/CD13) to migration and invasion of human osteosarcoma cell lines. Int J Oncol. 2014;45:2475–2485. doi: 10.3892/ijo.2014.2664. [DOI] [PubMed] [Google Scholar]
- 6.Huang H, Zheng HY, Liu ZL, Zhang L. Prognostic significance of relaxin-2 and S100A4 expression in osteosarcoma. Eur Rev Med Pharmacol Sci. 2014;18:2828–2834. [PubMed] [Google Scholar]
- 7.Jaffe N. Osteosarcoma: review of the past, impact on the future. The American experience. Cancer Treat Res. 2009;152:239–262. doi: 10.1007/978-1-4419-0284-9_12. [DOI] [PubMed] [Google Scholar]
- 8.Bacci G, Briccoli A, Longhi A, Ferrari S, Mercuri M, Faggioli F, Versari M, Picci P. Treatment and outcome of recurrent osteosarcoma: experience at Rizzoli in 235 patients initially treated with neoadjuvant chemotherapy. Acta Oncol. 2005;44:748–755. doi: 10.1080/02841860500327503. [DOI] [PubMed] [Google Scholar]
- 9.Weng Y, Chen Y, Chen J, Liu Y, Bao T. Common genetic variants in microRNA processing machinery genes are associated with risk and survival in patients with osteosarcoma. Mol Genet Genomics. 2015 [Epub ahead of print] [Google Scholar]
- 10.Goricar K, Kovac V, Jazbec J, Zakotnik B, Lamovec J, Dolzan V. Genetic variability of DNA repair mechanisms and glutathione-S-transferase genes influences treatment outcome in osteosarcoma. Cancer Epidemiol. 2015;39:182–188. doi: 10.1016/j.canep.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Tie Z, Bai R, Zhai Z, Zhang G, Zhang H, Zhao Z, Zhou D, Liu W. Single nucleotide polymorphisms in VEGF gene are associated with an increased risk of osteosarcoma. Int J Clin Exp Pathol. 2014;7:8143–8149. [PMC free article] [PubMed] [Google Scholar]
- 12.Cho HS, Leahy DJ. Structure of the extracellular region of HER3 reveals an interdomain tether. Science. 2002;297:1330–1333. doi: 10.1126/science.1074611. [DOI] [PubMed] [Google Scholar]
- 13.Krawczyk P, Nicooe M, Powrozek T, Mlak R, Sawicki M, Jarosz B, Pajak B, Kucharczyk K, Stencel D, Trojanowski T, Milanowski J. Sensitive methods for the detection of an insertion in exon 20 of the gene in the metastasis of non-small cell lung cancer to the central nervous system. Oncol Lett. 2013;6:1063–1067. doi: 10.3892/ol.2013.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson SE, Gould MN, Hampton JM, Trentham-Dietz A. A case-control study of the HER2 Ile655Val polymorphism in relation to risk of invasive breast cancer. Breast Cancer Res. 2005;7:R357–364. doi: 10.1186/bcr1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frank B, Hemminki K, Wirtenberger M, Bermejo JL, Bugert P, Klaes R, Schmutzler RK, Wappenschmidt B, Bartram CR, Burwinkel B. The rare ERBB2 variant Ile654Val is associated with an increased familial breast cancer risk. Carcinogenesis. 2005;26:643–647. doi: 10.1093/carcin/bgh342. [DOI] [PubMed] [Google Scholar]
- 16.Ameyaw MM, Tayeb M, Thornton N, Folayan G, Tariq M, Mobarek A, Evans DA, Ofori-Adjei D, McLead HL. Ethnic variation in the HER-2 codon 655 genetic polymorphism previously associated with breast cancer. J Hum Genet. 2002;47:172–175. doi: 10.1007/s100380200019. [DOI] [PubMed] [Google Scholar]
- 17.Benusiglio PR, Lesueur F, Luccarini C, Conroy DM, Shah M, Easton DF, Day NE, Dunning AM, Pharoah PD, Ponder BA. Common ERBB2 polymorphisms and risk of breast cancer in a white British population: a case-control study. Breast Cancer Res. 2005;7:R204–209. doi: 10.1186/bcr982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao W, You CC, Zhuang JP, Zu JN, Chi ZY, Xu GP, Yan JL. Viability inhibition effect of gambogic acid combined with cisplatin on osteosarcoma cells via mitochondria-independent apoptotic pathway. Mol Cell Biochem. 2013;382:243–252. doi: 10.1007/s11010-013-1740-5. [DOI] [PubMed] [Google Scholar]
- 19.Yang JS, Lin CW, Hsieh YS, Cheng HL, Lue KH, Yang SF, Lu KH. Selaginella tamariscina (Beauv. ) possesses antimetastatic effects on human osteosarcoma cells by decreasing MMP-2 and MMP-9 secretions via p38 and Akt signaling pathways. Food Chem Toxicol. 2013;59:801–807. doi: 10.1016/j.fct.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 20.Li H, Min D, Zhao H, Wang Z, Qi W, Zheng S, Tang L, He A, Sun Y, Yao Y, Shen Z. The Prognostic Role of Ezrin Immunoexpression in Osteosarcoma: A Meta-Analysis of Published Data. PLoS One. 2013;8:e64513. doi: 10.1371/journal.pone.0064513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, He ML, Zhao JM, Qing HH, Wu Y. Meta-analysis of associations of the ezrin gene with human osteosarcoma response to chemotherapy and prognosis. Asian Pac J Cancer Prev. 2013;14:2753–2758. doi: 10.7314/apjcp.2013.14.5.2753. [DOI] [PubMed] [Google Scholar]
- 22.Nakase M, Inui M, Okumura K, Kamei T, Nakamura S, Tagawa T. p53 gene therapy of human osteosarcoma using a transferrin-modified cationic liposome. Mol Cancer Ther. 2005;4:625–631. doi: 10.1158/1535-7163.MCT-04-0196. [DOI] [PubMed] [Google Scholar]
- 23.Hellwinkel OJ, Muller J, Pollmann A, Kabisch H. Osteosarcoma cell lines display variable individual reactions on wildtype p53 and Rb tumour-suppressor transgenes. J Gene Med. 2005;7:407–419. doi: 10.1002/jgm.684. [DOI] [PubMed] [Google Scholar]
- 24.Xie XK, Yang DS, Ye ZM, Tao HM. Enhancement effect of adenovirus-mediated antisense c-myc and caffeine on the cytotoxicity of cisplatin in osteosarcoma cell lines. Chemotherapy. 2009;55:433–440. doi: 10.1159/000265527. [DOI] [PubMed] [Google Scholar]
- 25.Chang Z, Song R, Xu S, Xu M, Yu X. CD 152 gene polymorphisms and risk of osteosarcoma in Chinese population. Tumour Biol. 2014;35:6809–6814. doi: 10.1007/s13277-014-1891-3. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Liu Z, Jing P, Shao L, Chen L, He X, Gong W. Effects of murine double minute 2 polymorphisms on the risk and survival of osteosarcoma: a systemic review and meta-analysis. Tumour Biol. 2014;35:1649–1652. doi: 10.1007/s13277-013-1227-8. [DOI] [PubMed] [Google Scholar]
- 27.Liu SQ, Yu JP, Yu HG, Lv P, Chen HL. Activation of Akt and ERK signalling pathways induced by etoposide confer chemoresistance in gastric cancer cells. Dig Liver Dis. 2006;38:310–318. doi: 10.1016/j.dld.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Kuraoka K, Matsumura S, Hamai Y, Nakachi K, Imai K, Matsusaki K, Oue N, Ito R, Nakayama H, Yasui W. A single nucleotide polymorphism in the transmembrane domain coding region of HER-2 is associated with development and malignant phenotype of gastric cancer. Int J Cancer. 2003;107:593–596. doi: 10.1002/ijc.11450. [DOI] [PubMed] [Google Scholar]
- 29.Xie D, Shu XO, Deng Z, Wen WQ, Creek KE, Dai Q, Gao YT, Jin F, Zheng W. Population-based, case-control study of HER2 genetic polymorphism and breast cancer risk. J Natl Cancer Inst. 2000;92:412–417. doi: 10.1093/jnci/92.5.412. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Ma YH, Sun ZZ, Rui YJ, Yin QD, Song S, Wei XM, Liu J, Liu XG, Hu KJ. Effect of c-erbB2 overexpression on prognosis in osteosarcoma: evidence from eight studies. Tumour Biol. 2014;35:8939–8943. doi: 10.1007/s13277-014-2165-9. [DOI] [PubMed] [Google Scholar]
- 31.Scotlandi K, Manara MC, Hattinger CM, Benini S, Perdichizzi S, Pasello M, Bacci G, Zanella L, Bertoni F, Picci P, Serra M. Prognostic and therapeutic relevance of HER2 expression in osteosarcoma and Ewing’s sarcoma. Eur J Cancer. 2005;41:1349–1361. doi: 10.1016/j.ejca.2005.03.015. [DOI] [PubMed] [Google Scholar]


