Abstract
Lung cancer is a type of malignant tumor with highest morbidity and mortality. This study tested three tumor marker levels including CEA, SCCA, and bFGF to explore their value in lung cancer diagnosis and pathological type judgment. Venous blood was extracted from lung cancer patients, lung benign lesion patients and healthy control. Electrochemiluminescence immunoassay was applied to detect serum CEA and SCCA content. ELISA was used to test serum bFGF level. Serum CEA, SCCA, and bFGF levels and positive rates were significantly higher in lung cancer group than that of lung benign disease group and health control (P < 0.05). bFGF showed higher detection sensitivity than CEA in lung cancer (P < 0.05). Three joint detection sensitivity was higher than single test (P < 0.05), while its specificity was lower (P < 0.05), and the accuracy presented no significant difference. Serum CEA and SCCA levels and positive rates were obviously higher in non-small cell lung cancer patients when compared with small cell lung cancer patients (P < 0.05), while bFGF level was similar between small cell lung cancer and non-small cell lung cancer. bFGF showed higher detection rate than SCCA in small cell lung cancer (P < 0.05). Three joint detection exhibited higher positive rate in small cell lung cancer and non-small lung cancer than single test. Serum CEA, SCCA and bFGF joint detection improved detection sensitivity in lung cancer and had important reference value for pathological type deduction.
Keywords: CEA, SCCA, bFGF, lung cancer
Introduction
With the progress of industrialization and changes in lifestyle, the incidence of lung cancer has been continuing to rise. Its morbidity and mortality has reached the highest among all malignant tumors. The early diagnosis and treatment techniques are closely related to the survival rate of lung cancer. Although the treatment technologies for lung cancer have greatly improved, its five-year survival rate has not significantly increased. Therefore, the search for novel techniques for early diagnosis of lung cancer has become a research hotspot. Currently, pathological and cytological examinations are commonly used for the diagnosis of lung cancer. However, their applications are largely restricted due to the invasive procedure of tissue sampling. Occasionally, the procedure has to be repeated in order for accurate diagnosis, which is time-consuming and inconvenient. Moreover, diagnosis based on signs, symptoms and imaging may not be accurate. It is therefore necessary to develop a diagnosis method with high specificity, sensitivity, and simple operation.
The occurrence and development of tumor is a multi-stage slow process, involving a wide range of molecular biological changes. With the development of molecular technologies, increasingly more tumor markers have been applied in clinic. Diagnosis methods based on tumor markers are easily accepted by patients due to their convenience and high sensitivity. The application of tumor markers for the screening and clinical diagnosis of cancer has been increasingly acknowledged.
Carcinoembryonic antigen (CEA) is a type of acidoglycoprotein with human embryo antigen specificity, which is mainly expressed in fetal gastrointestinal epithelial tissue, pancreas and liver. While its content in normal tissue is low, its concentration is substantially increased in many types of cancer such as gastric carcinoma [1] and colorectal cancer [2,3]. It has been found that the serum CEA also increases in patients with lung cancer [4] and its concentration is correlated with the prognosis of patients [5]. For instance, Takahashi et al. [6] have shown that CEA level is an important predictive factor for the postoperative survival rate in patients with non-small cell lung cancer. CEA is a broad-spectrum tumor marker identified in a variety of tumors, and therefore its application in the diagnosis has been largely limited by its poor specificity. Numerous researches have focused on the predictive value of joint detection of CEA and other tumor markers in the diagnosis of lung cancer [7].
Squamous cell carcinoma antigen (SCCA) is a squamous epithelial related antigen TA-4 isolated from the uterus squamous epithelium by Kato et al. in 1977 [8] that belongs to the serine protease inhibitor family [9]. SCCA is closely associated with the occurrence and development of squamous cell carcinoma. It has been found that its serum concentration is elevated in a variety of tumors including lung cancer. Moreover, SCCA has been confirmed to be closely related to the prognosis of lung cancer [10]. In addition, some studies also suggest that SCCA in the breath condensate might be served as a marker for early diagnosis of lung cancer [11].
Basic fibroblast growth factor (bFGF) is a protein that can promote the proliferation and division of fibroblasts. It can also stimulate and regulate the proliferation of vascular endothelial cells and epithelial cells. Moreover, it plays an important role in the process of tumor metastasis and angiogenesis [12]. It has been shown that bFGF is overexpressed in a variety of tumors and closely associated with their development, metastasis and prognosis, such as laryngeal squamous carcinoma [13] and breast cancer [14]. Currently, serum bFGF was found to be up-regulated in lung cancer patients [15]. However, its value in the diagnosis of lung cancer has not yet been clarified. Moreover, the sensitivity or specificity of detection of single marker is usually poor. In this study, serum levels of CEA, SCCA, and bFGF in patients with lung cancer and lung benign disease, and healthy controls were compared to investigate their value in the diagnosis and determination of pathological types of lung cancer.
Materials and methods
Main reagents and instruments
Electrochemical luminescence automatic im-munity analyzer was obtained from Roche (Basel, Switzerland). Thermo Fisher Multiskan FC microplate reader was provided by Thermo Fisher Scientific Inc. (Waltham, MA, USA). CEA and SCCA detection kits were purchased from Roche. bFGF enzyme-linked immunosorbent assay kit was purchased from R&D Systems (Minneapolis, MN, USA).
Experimental subjects
A total of 135 patients with pathologically confirmed lung cancer who were admitted into our Hospital from January 2012 to December 2013 were enrolled in this study, including 84 males and 51 females. The mean age was 58.54±3.62 (32-76) years. And there were 104 cases of non-small cell lung cancer and 31 cases of small cell lung cancer. A total of 113 cases of patients with lung benign lesions including pneumonia, chronic bronchitis, emphysema, and pulmonary tuberculosis were selected, including 71 males and 42 females with a mean age of 57.96±3.45 (30-74) years. A total of 147 healthy controls were selected including 89 males and 58 females with a mean age of 58.14±3.56 (38-76) years. There was no significant difference in age and gender ratio among these three groups. All enrolled subjects had signed the informed consent, and the study was approved by the Institutional Review Board of the hospital.
Experimental methods
A total of 3 mL of venous blood was collected. Serum was isolated by centrifugation at 3000 rpm for 10 min for further analysis. Serum CEA and SCCA content was detected using electrochemiluminescence immunoassay kit according to the manufacture’s instruction. Serum bFGF level was detected by ELISA. The critical value of the three markers was as follows: CEA > 5.0 ng/mL, SCCA > 1.5 ng/mL and bFGF > 4.8 pg/mL.
Statistical analysis
All statistical analyses were performed using SPSS13.0 software. Differences between multiple groups were analyzed by ANOVA or Chi-square tests. Inspection level α=0.05. P < 0.05 was considered statistically significant.
Results
Comparison of serum CEA, SCCA, and bFGF levels in each group
As shown in Table 1. There were significant differences in serum CEA, SCCA and bFGF levels among the three groups (P < 0.05). Further, pairwise comparison was performed by Bonferroni method. Results showed that serum CEA, SCCA and bFGF levels in lung cancer group were significantly increased compared with healthy controls (P < 0.05). And lung benign disease group (P < 0.05). Although serum CEA and SCCA levels in lung benign lesion group was markedly higher than those in healthy controls (P < 0.05), they were in normal range (Figure 1). In addition, bFGF level of the two groups was similar (P > 0.05).
Table 1.
Comparison of serum CEA, SCCA, and bFGF levels in each group
| Group | n | CEA (ng/ml) | SCCA (ng/ml) | bFGF (pg/ml) |
|---|---|---|---|---|
| Lung cancer | 135 | 37.57±29.48a,b | 3.12±2.38a,b | 7.35±3.15a,b |
| Lung benign lesion | 113 | 3.01±1.97c | 0.56±0.23c | 3.01±2.12 |
| Healthy control | 147 | 2.43±1.48 | 0.47±0.29 | 2.85±2.03 |
P < 0.05 when lung cancer group was compared with healthy controls;
P < 0.05 when lung cancer group was compared with lung benign lesion group;
P < 0.05 when lung benign lesion group was compared with healthy controls.
Figure 1.
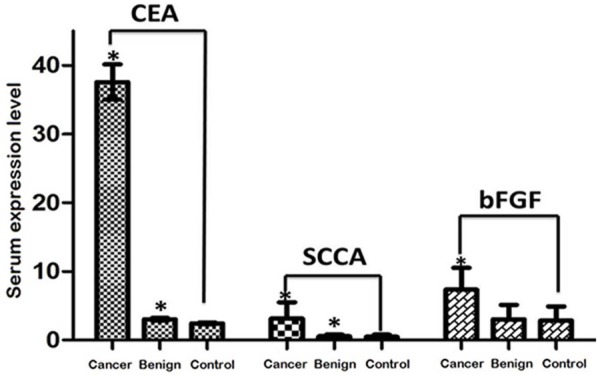
Comparison of serum CEA, SCCA, and bFGF.
Comparison of serum CEA, SCCA and bFGF positive rates in each group
Serum CEA, SCCA, and bFGF positive rates in lung cancer group were 49.6%, 51.9%, and 62.2%, respectively (Table 2). Results in chi-square tests revealed significant difference in serum CEA, SCCA, and bFGF positive rates among the three groups (P < 0.05). Pairwise comparison demonstrated that serum CEA, SCCA, and bFGF positive rates in lung cancer group were significant higher compared with lung benign disease group and healthy controls (P < 0.05). In addition, no significant difference in the positive rate of the three markers was observed between lung benign lesion group and healthy controls (P > 0.05).
Table 2.
Comparison of positive rate of serum CEA, SCCA and bFGF in each group
| Group | n | CEA | SCCA | bFGF |
|---|---|---|---|---|
| Lung cancer | 135 | 67 (49.6%) | 70 (51.9%) | 84 (62.2%) |
| Lung benign lesion | 113 | 14 (12.4%) | 16 (14.2%) | 17 (15.0%) |
| Healthy control | 147 | 13 (8.8%) | 15 (10.2%) | 16 (10.8%) |
| χ2 value | 75.91 | 74.97 | 104.64 | |
| P value | < 0.001 | < 0.001 | < 0.001 |
Sensitivity and specificity of single and joint detections of serum CEA, SCCA, bFGF in the diagnosis of lung cancer
The sensitivity, specificity and accuracy of single and joint detections of serum CEA, SCCA, bFGF levels were analyzed (Table 3). Chi-square tests revealed that the sensitivity of bFGF detection was significantly higher than that of CEA in lung cancer (χ2=4.34, P=0.037), but was similar to that of SCCA (χ2=2.96, P=0.085). The specificity and accuracy of bFGF detection was not significantly different from those of CEA and SCCA detection (P > 0.05). The sensitivity of joint detection of the three markers in lung cancer group (88.1%) was significantly higher than that of each single detection (P < 0.05), whereas the specificity of joint detection (77.7%) was significantly lower compared with single detection (P < 0.05). Additionally, the accuracy of joint detection (81.3%) was similar to that of single detection (P > 0.05).
Table 3.
Sensitivity, specificity and accuracy of single and joint detections of serum CEA, SCCA, bFGF levels
| Index | Sensitivity (%) | Specificity (%) | Accuracy (%) |
|---|---|---|---|
| CEA | 49.6 | 89.6 | 75.9 |
| SCCA | 51.9 | 88.1 | 75.7 |
| bFGF | 62.2a | 87.3 | 78.7 |
| CEA+SCCA+bFGF | 88.1a,b,c | 77.7a,b,c | 81.3 |
P < 0.05 compared with CEA;
P < 0.05 compared with SCCA;
P < 0.05 compared with bFGF.
Comparison of serum CEA, SCCA and bFGF levels in different pathological types
Serum CEA, SCCA, and bFGF levels in different pathological types were compared by t-tests. Results showed that serum CEA and SCCA levels in patients with non-small cell lung cancer were significantly higher compared with patients with small cell lung cancer (P < 0.05), whereas no significant difference in serum bFGF level was observed between in small cell lung cancer and non-small cell lung cancer groups (P > 0.05) (Table 4).
Table 4.
Comparison of serum CEA, SCCA and bFGF levels in different pathological types
| Pathological type | n | CEA (ng/ml) | SCCA (ng/ml) | bFGF (ng/ml) |
|---|---|---|---|---|
| Non-small cell lung cancer | 104 | 41.25±31.58 | 3.47±2.46 | 7.37±3.19 |
| Small cell lung cancer | 31 | 25.22±22.43 | 1.95±2.11 | 7.28±3.02 |
| T value | 3.15 | 3.11 | 0.14 | |
| P value | < 0.01 | < 0.01 | 0.44 |
Positive rates of single and joint detection of serum CEA, SCCA, and bFGF in different pathological types
The positive rates of single and joint detection of serum CEA, SCCA and bFGF in non-small cell lung cancer were significantly higher compared with small cell lung cancer (P < 0.05), whereas no significant difference in the positive rates of bFGF detection was observed between different pathological types (P > 0.05). In addition, no significant difference in the positive rates of single detection of the three markers was observed in non-small cell lung cancer (P > 0.05). The detection rate of bFGF was significantly higher than that of SCCA (χ2=4.35, P=0.037), but was similar to that of CEA (χ2=3.28, P=0.070) in small cell lung cancer. The positive rate of joint detection in both small cell lung cancer and non-small lung cancer was significantly higher compared with single detection (P < 0.05) (Table 5).
Table 5.
Positive rates of single and joint detection of serum CEA, SCCA, and bFGF in different pathological types
| Index | Non-small cell lung cancer (n=104) | Small cell lung cancer (n=31) | χ2 value | P value |
|---|---|---|---|---|
| CEA | 58 (55.8%) | 9 (29.0%) | 6.83 | < 0.01 |
| SCCA | 62 (59.6%) | 8 (25.8%) | 10.25 | < 0.01 |
| bFGF | 68 (65.4%) | 16 (51.6%) | 1.93 | 0.17 |
| CEA+SCCA+bFGF | 95 (91.3%) | 24 (77.4%) | 4.43 | 0.04 |
Discussion
Serum tumor markers have been widely used for the screening and diagnosis of primary tumor and are easily accepted by patients a convenient and noninvasive method. The search for reliable tumor markers with high sensitivity and specificity has become a research hotspot. Several tumor markers have been found to be associated with the occurrence of lung cancer, such as CEA, CA125, etc. [16]. However, the sensitivity and specificity of single detection of tumor marker is usually relatively lower compared with detection of tumor marker group composed of three or four markers with different features, sensitivity and complementation. Although it has been found that serum bFGF level was elevated in lung cancer patients, its value in the diagnosis of lung cancer is still unclarified. In this study, we investigated the value of single and joint detection of serum bFGF, CEA and SCCA levels in the diagnosis and determination of pathological type of lung cancer. It was found that the detection of serum CEA, SCCA and bFGF was quite helpful for the clinical diagnosis of lung cancer. The sensitivity of serum bFGF detection was significantly higher than that of CEA, and its positive detection rate in small cell lung cancer was higher compared with SCCA, suggesting that joint detection of bFGF could improve the sensitivity of lung cancer diagnosis, especially for small cell lung cancer. CEA and SCCA showed higher detection sensitivity for non-small cell lung cancer compared with small cell lung cancer, indicating the clinical significance of serum CEA, SCCA, and bFGF detection in the determination of pathological types of lung cancer. CEA is a broad-spectrum tumor marker and has been identified in patients with colorectal cancer. It has also been shown that serum CEA level is significantly increased in lung cancer [17]. Our results demonstrated that the sensitivity and specificity of CEA for the diagnosis of lung cancer was 49.6% and 89.6%, respectively, which is consistent with a previous report by Wang et al. [18]. Moreover, the positive rate of serum CEA detection in non-small cell lung cancer was higher than that of small cell lung cancer, which is consistent with a previous study [19]. SCCA is a type of antigen produced by squamous epithelial cells. Our results showed that serum SCCA level was increased in patients with lung cancer and was significantly higher in non-small cell lung cancer compared with small cell lung cancer, which is consistent to previous findings [19]. bFGF is also highly expressed in a variety of tumors. It has been found that [20] bFGF is highly expressed in lung cancer and is closely associated with the prognosis of lung cancer. To date, the significance of serum bFGF in the diagnosis of lung cancer has not been previously reported. In this study, we revealed a significant increase in serum bFGF level in lung cancer patients, which is consistent with previous studies [21]. Furthermore, the sensitivity of serum bFGF detection in lung cancer was significantly higher than that of CEA, indicating that joint detection of bFGF and CEA could greatly improve the diagnostic sensitivity of lung cancer. Therefore, we developed the joint detection of CEA, SCCA and bFGF, and found that its sensitivity was significantly improved in lung cancer. Nevertheless, its specificity was reduced to a certain extent.
Although the diagnostic value of CEA and SCCA in lung cancer has been frequently reported, to our knowledge, our study is the first report on the role of serum bFGF detection for the diagnosis of lung cancer. We found that its sensitivity was higher compared with CEA in lung cancer and SCCA in small cell lung cancer, suggesting the auxiliary diagnostic value of bFGF in lung cancer. Furthermore, the sensitivity of joint detection was significantly higher than that of single test. Other combinations of tumor markers may be explored in the future to improve early diagnosis and to facilitate early treatment of lung cancer in order to improve patients’ survival rate and quality of life.
Disclosure of conflict of interest
None.
References
- 1.Lai H, Jin Q, Lin Y, Mo X, Li B, He K, Chen J. Combined use of lysyl oxidase, carcino-embryonic antigen, and carbohydrate antigens improves the sensitivity of biomarkers in predicting lymph node metastasis and peritoneal metastasis in gastric cancer. Tumour Biol. 2014;35:10547–10554. doi: 10.1007/s13277-014-2355-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verberne CJ, Nijboer CH, de Bock GH, Grossmann I, Wiggers T, Havenga K. Evaluation of the use of decision-support software in carcino-embryonic antigen (CEA)-based follow-up of patients with colorectal cancer. BMC Med Inform Decis Mak. 2012;12:14. doi: 10.1186/1472-6947-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang YR, Yan JX, Wang LN. The diagnostic value of serum carcino-embryonic antigen, alpha fetoprotein and carbohydrate antigen 19-9 for colorectal cancer. J Cancer Res Ther. 2014;10(Suppl):307–309. doi: 10.4103/0973-1482.151538. [DOI] [PubMed] [Google Scholar]
- 4.Yu DH, Li JH, Wang YC, Xu JG, Pan PT, Wang L. Serum anti-p53 antibody detection in carcinomas and the predictive values of serum p53 antibodies, carcino-embryonic antigen and carbohydrate antigen 12-5 in the neoadjuvant chemotherapy treatment for III stage non-small cell lung cancer patients. Clin Chim Acta. 2011;412:930–935. doi: 10.1016/j.cca.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 5.Facchinetti F, Aldigeri R, Aloe R, Bortesi B, Ardizzoni A, Tiseo M. CEA serum level as early predictive marker of outcome during EGFR-TKI therapy in advanced NSCLC patients. Tumour Biol. 2015;36:5943–5951. doi: 10.1007/s13277-015-3269-6. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi Y, Horio H, Hato T, Harada M, Matsutani N, Kawamura M. Predictors of post-recurrence survival in patients with non-small-cell lung cancer initially completely resected. Interact Cardiovasc Thorac Surg. 2015;21:14–20. doi: 10.1093/icvts/ivv085. [DOI] [PubMed] [Google Scholar]
- 7.Doseeva V, Colpitts T, Gao G, Woodcock J, Knezevic V. Performance of a multiplexed dual analyte immunoassay for the early detection of non-small cell lung cancer. J Transl Med. 2015;13:55. doi: 10.1186/s12967-015-0419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kato H, Torigoe T. Radioimmunoassay for tumor antigen of human cervical squamous cell carcinoma. Cancer. 1977;40:1621–1628. doi: 10.1002/1097-0142(197710)40:4<1621::aid-cncr2820400435>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 9.Kato H. Expression and function of squamous cell carcinoma antigen. Anticancer Res. 1996;16:2149–2153. [PubMed] [Google Scholar]
- 10.Yu D, Du K, Liu T, Chen G. Prognostic value of tumor markers, NSE, CA125 and SCC, in operable NSCLC Patients. Int J Mol Sci. 2013;14:11145–11156. doi: 10.3390/ijms140611145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zou Y, Wang L, Zhao C, Hu Y, Xu S, Ying K, Wang P, Chen X. CEA, SCC and NSE levels in exhaled breath condensate--possible markers for early detection of lung cancer. J Breath Res. 2013;7:047101. doi: 10.1088/1752-7155/7/4/047101. [DOI] [PubMed] [Google Scholar]
- 12.Okada-Ban M, Thiery JP, Jouanneau J. Fibroblast growth factor-2. Int J Biochem Cell Biol. 2000;32:263–267. doi: 10.1016/s1357-2725(99)00133-8. [DOI] [PubMed] [Google Scholar]
- 13.Huang Z, Zeng ZY, Guan M, Xia LP. [Expression of inducible nitric oxide synthase and basic fibroblast growth factor in supraglottic squamous cell carcinoma and their clinical significance] . Ai Zheng. 2002;21:961–964. [PubMed] [Google Scholar]
- 14.Hsiung R, Zhu W, Klein G, Qin W, Rosenberg A, Park P, Rosato E, Sauter E. High basic fibroblast growth factor levels in nipple aspirate fluid are correlated with breast cancer. Cancer J. 2002;8:303–310. doi: 10.1097/00130404-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Farhat FS, Tfayli A, Fakhruddin N, Mahfouz R, Otrock ZK, Alameddine RS, Awada AH, Shamseddine A. Expression, prognostic and predictive impact of VEGF and bFGF in non-small cell lung cancer. Crit Rev Oncol Hematol. 2012;84:149–160. doi: 10.1016/j.critrevonc.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Dai H, Liu J, Liang L, Ban C, Jiang J, Liu Y, Ye Q, Wang C. Increased lung cancer risk in patients with interstitial lung disease and elevated CEA and CA125 serum tumour markers. Respirology. 2014;19:707–713. doi: 10.1111/resp.12317. [DOI] [PubMed] [Google Scholar]
- 17.Mumbarkar PP, Raste AS, Ghadge MS. Significance of tumor markers in lung cancer. Indian J Clin Biochem. 2006;21:173–176. doi: 10.1007/BF02913090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang B, He YJ, Tian YX, Yang RN, Zhu YR, Qiu H. Clinical utility of haptoglobin in combination with CEA, NSE and CYFRA21-1 for diagnosis of lung cancer. Asian Pac J Cancer Prev. 2014;15:9611–9614. doi: 10.7314/apjcp.2014.15.22.9611. [DOI] [PubMed] [Google Scholar]
- 19.Body JJ, Sculier JP, Raymakers N, Paesmans M, Ravez P, Libert P, Richez M, Dabouis G, Lacroix H, Bureau G, et al. Evaluation of squamous cell carcinoma antigen as a new marker for lung cancer. Cancer. 1990;65:1552–1556. doi: 10.1002/1097-0142(19900401)65:7<1552::aid-cncr2820650717>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 20.Joensuu H, Anttonen A, Eriksson M, Makitaro R, Alfthan H, Kinnula V, Leppa S. Soluble syndecan-1 and serum basic fibroblast growth factor are new prognostic factors in lung cancer. Cancer Res. 2002;62:5210–5217. [PubMed] [Google Scholar]
- 21.Ueno K, Inoue Y, Kawaguchi T, Hosoe S, Kawahara M. Increased serum levels of basic fibroblast growth factor in lung cancer patients: relevance to response of therapy and prognosis. Lung Cancer. 2001;31:213–219. doi: 10.1016/s0169-5002(00)00187-2. [DOI] [PubMed] [Google Scholar]


