Abstract
MircroRNA functions as a tumor suppressor or a promoter in cholangiocarcinoma (CCA). Researchers have found that miR-203 functioned as tumor suppressor in many types of cancer. However, the role of miR-203 that plays in CCA remains to be clarified. We aimed to detect the expression level and the prognostic significance of miR-203 in CCA tissues. qRT-RCR was performed to examine the miR-203 expression levels in CCA tissue specimens and corresponding normal tissues. Our findings suggest that miR-203 expression was an independent poor prognostic factor for CCA patient overall survival. Therefore, miR-203 may serve as a valuable prognostic marker and promising treatment target for CCA.
Keywords: mircroRNA, miR-203, cholangiocarcinoma, prognosis
Introduction
cholangiocarcinoma (CCA) is a highly malignant cancer of the biliary tract with a poor prognosis [1-4]. According to the statistics, the incidence and mortality of CCA is rising worldwide [5]. CCA is difficult to diagnose in early-stage and characterized by high frequency of recurrence and metastasis. What’s more, there is no effective chemoprevention or treatment, resulting in poor survival [6].
MicroRNAs (miRNAs) are non-coding RNAs of 20-22 nucleotides and regulate the translational inhibition of target mRNAs by base-pairing with their 3’-untranslated region (3’-UTR) [7]. Over the past few decades, a growing evidence suggests that an important role of miRNAs in various types of cancers [8]. In previous studies, researchers found that the expression of miR-203 was abnormal down-regulated has been observed in different types of cancers [9-11]. However, correlation between the expression of miR-203 and clinicopathological parameters in CCA patients have not yet been previously reported.
In this study, we firstly demonstrated that miR-203 expression was lower in CCA tissues compared with that in adjacent normal tissues. Next, we analyzed miR-203 expression level and its clinicopathological significance. Finally, in order to further investigate the predictor role of miR-203 in survival of CCA patients multivariate Cox regression analysis was used. Our findings will help to elucidate the roles of miRNAs in CCA.
Materials and methods
Patients
All patients had signed informed consent forms and the study was approved by the Ethics Committee of the Second Xiangya Hospital of Central South University. Clinical staging was performed according to the 6th edition 2002 American Joint Committee on Cancer (AJCC) TNM staging system. The selection criteria for patients with CCA were as follows: (1) the patients haven’t received preoperative anti-tumor therapy. (2) Two pathologists confirmed patients with CCA respectively. (3) The patients had no other cancer history. All patients were diagnosed and treated between January 2006 and April 2014. All the clinical data of the patients were also collected respectively. The mean follow-up period was 26 months. Survival time was calculated from the date of the initial surgery to death.
Tissue specimens
Human CCA and corresponding normal tissues (located >2 cm away from the tumor) were obtained from 10 patients who underwent CCA resection at the Second Xiangya Hospital Central South University. The other 138 samples of CCA tissues were collected from the Pathology Department.
Quantitative real-time RT-PCR
miRNA from tissues and cells was extracted using TRIzol (Invitrogen) reagent according to the manufacture’s instruction. Real-time qRT-PCR was carried out as described previously [12]. The qRT-PCR primers for miR-203 (HmiRQP4643) and U6 (HmiRQP1433) were purchased from Guangzhou Funeng Gene Co., Ltd (Guangzhou, China). The relative expression level of miR-203 was normalized to that of U6 by using the 2-ΔΔCt cycle threshold method.
Statistical analysis
Statistical analyses were carried out using SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 5 (GraphPad Software Inc., CA, USA). Difference of measurement data was assessed by Student’s t-test. Count data were analyzed by the χ2 or Fishers exact tests. Univariate survival analysis was performed using Kaplan-Meier method and the Log-rank test. Multivariate survival analysis was performed using the Cox multivariate analysis model. P<0.05 was considered statistically significant.
Results
Expression levels of miR-203 in CCA
We used qRT-PCR to examine miR-203 expression in 10 pairs of CCA tissues and the corresponding noncancerous tissues. As shown in Figure 1A, the expression of miR-203 was significantly down-regulated in CCA tissues when compared with adjacent normal tissues. Since the miR-203 was in lower level compared with that in the matched normal tissues, we postulated that miR-203 may play an important role in CCA. To test this hypothesis we collected other 138 CCA tissue specimens to further analyze the relationship between miR-203 expression level and clinical characters of CCA patients.
Figure 1.
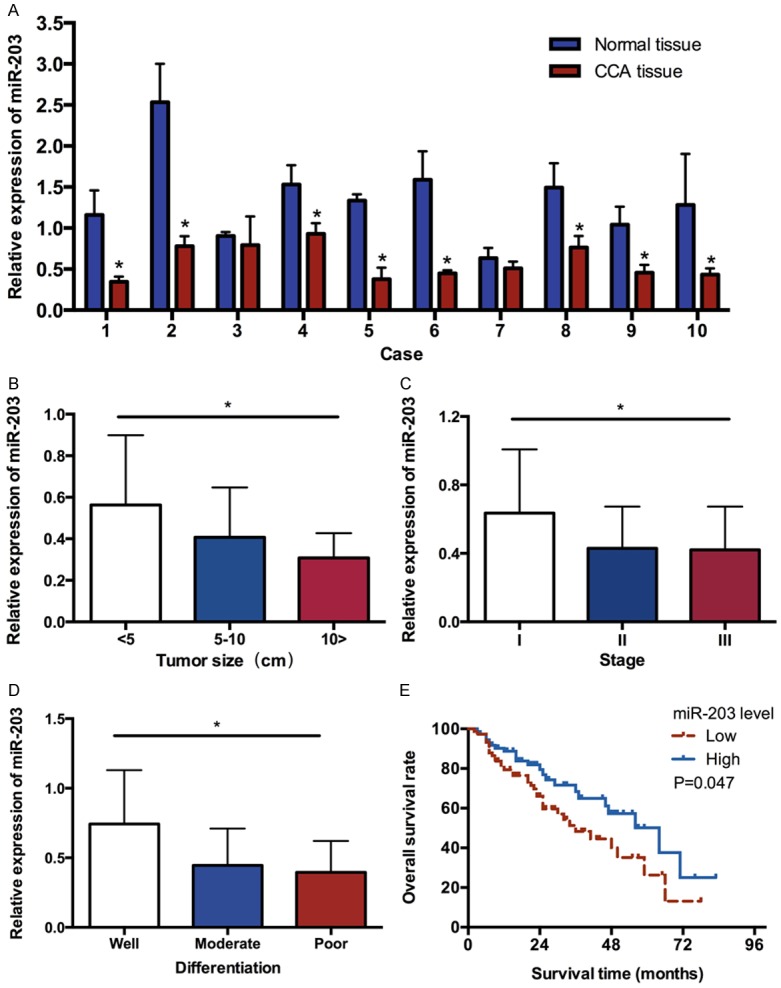
A. Comparison of miR-203 expression levels between CCA tissues and adjacent normal tissues. B. The expression level of miR-203 in CCA patients at different tumor size. C. The expression level of miR-203 in CCA patients at different clinical stages. D. The expression level of miR-203 at different differentiation of CCA tissues. E. Overall survival curve of CCA patients with different miR-203 expression. The patients with a lower expression of miR-203 had a lower survival time compared with those with a higher expression of miR-203. (*P<0.05).
Expression levels of miR-203 and clinicopathological characteristics in CCA
The miR-203 expression levels were classified as high or low in relation to the median value. Low expression of miR-203 was found to significantly correlate with tumor size (Figure 1B), clinical stage (Figure 1C) and tumor differentiation (Figure 1D), and it suggested that miR-203 might be closely related with the development of cholangiocarcinoma. However, there were no significant difference in miR-203 expression was observed with age, sex distribution, tumor number, cirrhosis, serum AFP value and cholelithiasis (shown in Table 1).
Table 1.
Correlation between the expression of miR-203 and clinicopathological parameters in CCA patients
| Characteristics | miR-203 expression | P value | |
|---|---|---|---|
|
| |||
| High | Low | ||
| Age | |||
| <55 | 41 (51.9%) | 38 (48.1%) | 0.621 |
| ≥55 | 33 (47.8%) | 36 (52.2%) | |
| Gender | |||
| Male | 39 (49.4%) | 40 (50.6%) | 0.869 |
| Female | 35 (50.7%) | 34 (49.3%) | |
| Tumor size (cm) | |||
| <5 | 45 (60.8%) | 29 (39.2%) | 0.026* |
| 5~10 | 26 (40.6%) | 38 (59.4%) | |
| ≥10 | 3 (30.0%) | 7 (70.0%) | |
| Tumor number | |||
| Solitary | 64 (52.9%) | 57 (47.1%) | 0.136 |
| Multiple | 10 (37.0%) | 17 (63.0%) | |
| Clining stage | |||
| I | 26 (68.4%) | 12 (31.6%) | 0.030* |
| II | 20 (45.5%) | 24 (54.5%) | |
| III | 28 (42.4%) | 38 (57.6%) | |
| Differentiation | |||
| Well | 18 (78.3%) | 5 (21.7%) | 0.003* |
| Moderate | 42 (50.0%) | 42 (50.0%) | |
| Poor | 14 (34.1%) | 27 (65.9%) | |
| Cholelithiasis | |||
| Yes | 34 (57.6%) | 25 (42.4%) | 0.131 |
| No | 40 (44.9%) | 49 (55.1%) | |
| Cirrhosis | |||
| Yes | 13 (39.4%) | 20 (60.6%) | 0.167 |
| No | 61 (53.0%) | 54 (47.0%) | |
| AFP | |||
| Normal | 52 (48.1%) | 56 (51.9%) | 0.459 |
| Abnormal | 22 (55.0%) | 18 (45.0%) | |
P<0.05.
Low-expression level of miR-203 predicts poor prognosis in CCA patients
Considering that the level of miR-203 expression was remarkably correlated with tumor size, clinical stage and tumor differentiation, we hypothesized that miR-203 might affect the prognosis of CCA patients. Kaplan-Meier analysis with the log-rank test indicated that low miR-203 expression had a significant impact on OS (1 year, 3 year, and 5 year survival rate were 79.42%, 48.29%, and 26.33% vs. 88.71%, 60.27%, and 37.62%, respectively; P<0.05; Figure 1E). Univariate and multivariate analyses were utilized to evaluate whether the miR-203 expression level and various clinicopathological features were independent prognostic parameters. Multivariate analysis revealed that miR-203 expression, clinical stage, tumor differentiation and cirrhosis were independently associated with the overall survival (shown in Table 2).
Table 2.
Multivariate analyses for overall survival by Cox regression model
| P value | Exp (B) | RR 95% CI | ||
|---|---|---|---|---|
|
| ||||
| Lower | Upper | |||
| Age | 0.48 | 1.23 | 0.692 | 2.188 |
| Gender | 0.094 | 1.645 | 0.918 | 2.945 |
| Tumor size | 0.311 | 1.264 | 0.803 | 1.988 |
| Tumor number | 0.201 | 0.581 | 0.253 | 1.334 |
| Differentiation | 0.020* | 1.743 | 1.092 | 2.783 |
| Clining stage | 0.001* | 2.188 | 1.383 | 3.462 |
| Cirrhosis | 0.077 | 1.835 | 0.935 | 3.602 |
| Cholelithiasis | 0.91 | 0.966 | 0.53 | 1.761 |
| AFP | 0.122 | 0.623 | 0.342 | 1.134 |
| miR-203 | 0.017* | 3.444 | 1.244 | 9.536 |
P<0.05.
Discussion
A large number of studies suggest that miRNAs play a key role in tumor development. Increasing evidence suggests that the dysregulation of miRNAs participates in CCA progression where they act as either suppressors or promoters at the post-transcriptional regulation stage. For example, Zhang J [13] et al. demonstrated that expression of miR-26a was higher in CCA tissues. miR-26a promotes CCA cell growth by inhibition of GSK-3β and subsequent activation of β-catenin. MicroRNA-144 suppresses CCA cell proliferation and invasion through targeting platelet activating factor acetylhydrolase isoform 1b (LIS1) [14]. Wang LJ [15] et al. showed that high expression levels of miR-21 were closely related to adverse clinical features, diminished survival, and poor prognosis in CCA patients.
miR-203 has been shown to down-regulate expression and inversely correlate with BMI1 expression in melanoma and inhibit invasive and proliferative abilities in part by targeting BMI1 [16]. miR-203 enhances chemosensitivity to 5-fluorouracil by targeting thymidylate synthase in colorectal cancer [17]. Wang C [18] et al. demonstrated that miR-203 played a critical role in regulating cell proliferation, apoptosis and migration by targeting of PKCα in lung cancer cells. All these findings emphasize a fundamental role of miR-203 in suppressing malignant tumor.
In this study, our results showed that miR-203 expression was significantly lower in CCA tissues compared with normal adjacent tissues. The relationship of the miR-203 with various clinical features of CCA patients was analyzed. The results showed that a low level of miR-203 expression was significantly correlated with tumor size, clinical stage and tumor differentiation, suggesting that miR-203 might be closely related with tumor development and prognosis of CCA. Furthermore, the overall survival rate of low miR-203 expression group was significantly shorter than that of high miR-203 expression group. Finally, in a multivariate Cox model, we found that miR-203 expression was an independent poor prognostic factor for overall survival rate, indicating that low miR-203 level was significantly associated with poor OS in CCA patients.
In conclusion, the expression of miR-203 was dramatically decreased in CCA tissues. Low expression of miR-203 was significantly associated with tumor progression and predicted poor prognosis in the CCA patients. These findings indicate that miR-203 serves as a novel prognostic marker and potential treatment target in CCA.
Acknowledgements
This work was supported by Project of Natural Science Foundation of Hunan Province of China (No. 13JJ3036); Technology Project of Hunan Province (2012SK3225) and the Fundamental Research Funds for the Central Universities of Central South University (2015zzts277 and 2015zzts324).
Disclosure of conflict of interest
None.
References
- 1.Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology. 2008;48:308–21. doi: 10.1002/hep.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sirica AE. Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy. Hepatology. 2005;41:5–15. doi: 10.1002/hep.20537. [DOI] [PubMed] [Google Scholar]
- 3.Chu X, Zhao P, Lv Y, Liu L. Decreased expression of TFPI-2 correlated with increased expression of CD133 in cholangiocarcinoma. Int J Clin Exp Pathol. 2015;8:328–36. [PMC free article] [PubMed] [Google Scholar]
- 4.Wu T. Cyclooxygenase-2 and prostaglandin signaling in cholangiocarcinoma. Biochim Biophys Acta. 2005;1755:135–50. doi: 10.1016/j.bbcan.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Nitta T, Sato Y, Ren XS, Harada K, Sasaki M, Hirano S, Nakanuma Y. Autophagy may promote carcinoma cell invasion and correlate with poor prognosis in cholangiocarcinoma. Int J Clin Exp Pathol. 2014;7:4913–21. [PMC free article] [PubMed] [Google Scholar]
- 6.Patel T. Cholangiocarcinoma controversies and challenges. Nat Rev Gastroenterol Hepatol. 2011;8:189–200. doi: 10.1038/nrgastro.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–73. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, Huang Z, Ye Q, Ming Y, Zhang S, Zhao Y, Liu L, Wang Q, Cheng K. Prognostic significance and anti-proliferation effect of microRNA-365 in hepatocellular carcinoma. Int J Clin Exp Pathol. 2015;8:1705–11. [PMC free article] [PubMed] [Google Scholar]
- 9.Jin J, Deng J, Wang F, Xia X, Qiu T, Lu W, Li X, Zhang H, Gu X, Liu Y, Cao W, Shao W. The expression and function of microRNA-203 in lung cancer. Tumour Biol. 2013;34:349–57. doi: 10.1007/s13277-012-0556-3. [DOI] [PubMed] [Google Scholar]
- 10.Chen HY, Han ZB, Fan JW, Xia J, Wu JY, Qiu GQ, Tang HM, Peng ZH. miR-203 expression predicts outcome after liver transplantation for hepatocellular carcinoma in cirrhotic liver. Med Oncol. 2012;29:1859–65. doi: 10.1007/s12032-011-0031-9. [DOI] [PubMed] [Google Scholar]
- 11.Viticchiè G, Lena AM, Latina A, Formosa A, Gregersen LH, Lund AH, Bernardini S, Mauriello A, Miano R, Spagnoli LG, Knight RA, Candi E, Melino G. MiR-203 controls proliferation, migration and invasive potential of prostate cancer cell lines. Cell Cycle. 2011;10:1121–31. doi: 10.4161/cc.10.7.15180. [DOI] [PubMed] [Google Scholar]
- 12.Zheng C, Li J, Wang Q, Liu W, Zhou J, Liu R, Zeng Q, Peng X, Huang C, Cao P, Cao K. MicroRNA-195 functions as a tumor suppressor by inhibiting CBX4 in hepatocellular carcinoma. Oncol Rep. 2015;33:1115–22. doi: 10.3892/or.2015.3734. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Han C, Wu T. MicroRNA-26a promotes cholangiocarcinoma growth by activating β-catenin. Gastroenterology. 2012;143:246–56.e8. doi: 10.1053/j.gastro.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mi Yang R, Chen Y, Tang C, Li H, Wang B, Yan Q, Hu J, Zou S. McroRNA-144 suppresses cholangiocarcinoma cell proliferation and invasion through targeting platelet activating factor acetylhydrolase isoform 1b. BMC Cancer. 2014;14:917. doi: 10.1186/1471-2407-14-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang LJ, He CC, Sui X, Cai MJ, Zhou CY, Ma JL, Wu L, Wang H, Han SX, Zhu Q. MiR-21 promotes intrahepatic cholangiocarcinoma proliferation and growth in vitro and in vivo by targeting PTPN14 and PTEN. Oncotarget. 2015;6:5932–46. doi: 10.18632/oncotarget.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang X, Sun Y, Han S, Zhu W, Zhang H, Lian S. MiR-203 inhibits melanoma invasive and proliferative abilities by targeting the polycomb group gene BMI1. Biochem Biophys Res Commun. 2015;456:361–6. doi: 10.1016/j.bbrc.2014.11.087. [DOI] [PubMed] [Google Scholar]
- 17.Li T, Gao F, Zhang XP. miR-203 enhances chemosensitivity to 5-fluorouracil by targeting thymidylate synthase in colorectal cancer. Oncol Rep. 2015;33:607–14. doi: 10.3892/or.2014.3646. [DOI] [PubMed] [Google Scholar]
- 18.Wang C, Wang X, Liang H, Wang T, Yan X, Cao M, Wang N, Zhang S, Zen K, Zhang C, Chen X. miR-203 inhibits cell proliferation and migration of lung cancer cells by targeting PKCα. PLoS One. 2013;8:e73985. doi: 10.1371/journal.pone.0073985. [DOI] [PMC free article] [PubMed] [Google Scholar]


