Abstract
Metastases to the breast from extramammary malignancies are extremely rare. Ruling out the diagnosis of primary breast tumor is important in order to decide on clinical management and predict prognosis. We report a case of metastasis to the breast from a pulmonary adenocarcinoma, with extensive micropapillary component, diagnosed concomitantly with the primary tumor. A 52 year-old female patient presented with mammary gland tingling and dyspnea accompanied with fatigued of 4 months duration and a nodular shadows in the front of the upper lobe was found on a chest computed tomography (CT) scan. The original clinical diagnosis was right breast cancer with lung and bone metastasis, or breast and lung double primary cancers. In addition,on physical examination a poorly defined mass was noted in the upper outer quadrant of the right breast. The patient underwent thoracocentesis and breast biopsy. By imageology, cytology, histology and immunohistochemistry, we diagnosed primary lung cancer with metastases to the right breast and bone. The metastatic anatomic sites demonstrated histologically extensive micropapillary component, which is recently recognized as an important prognostic factor. The patient was administered 4 cycles of cisplatin and docetaxel, although no clinical response was seen, the patient is still alive 9 months after diagnosis. The result of immunohistochemistry is a useful supplement in differential diagnosis.
Keywords: Lung neoplasms, breast metastasis, breast neoplasm
Introduction
Breast cancer is one of the most common malignant tumors in women, there is an upward trend in the incidence of this disease. However, metastases to the breast from extramammary malignancies are extremely rare, the prevalence of such lesions ranges from 0.4-1.3% [1-4]. Despite the rarity of metastases to the breast, the diagnosis of it from extramammary malignancies and distinction from primary mammary malignancy, is important for patient appropriate clinical management and outcome.
Sitzentfrey firstly published a case of ovarian carcinoma metastatic to the breast in 1907 [5]. So far, varies of malignancies have been reported to metastasize to the breast and according to the literature the most common primary tumors metastasizing to the breast are melanoma (29.8%), lung carcinoma (16.4%), gynecological carcinoma (12.7%), intestinal carcinoma (9.9%), leukemia and lymphoma (8.4%), rhabdomyosarcoma (7.3%), and renal cellcarcinoma (1.5%) [6].
Lung cancer is the most common malignant disease, has widespread metastasis at initial presentation or during treatment course. The most commonly affected metastatic organs are lymph node, bone, lung, brain, liver, and adrenal gland, nevertheless, there have only been a few published cases of pulmonary carcinomas metastasizing to the breast [7-10]. Recently, micropapillary carcinoma has been recognized as a rare but distinctive variant of carcinoma at several anatomical sites, including breast, urinary bladder, lung, and major salivary glands [11].
Invasive micropapillary components are being increasingly recognized as prognostic predictors in lung adenocarcinomas, many studies repeatedly find that it may be a manifestation of aggressive behavior [12,13]. Here we report a case of a female patient who presented a mass in the upper outer quadrant of right breast that had been detected through self-examination. After further systematic examinations, the patient was diagnosed with primary lung cancer that had metastasized to the right mammary gland with extensive micropapillary carcinoma in pathology. Written informed consent was obtained from the patient for publication of this case report and the corresponding images.
Case presentation
A 52-year-old, non-somking peasant woman presented to the emergency department with mammary gland tingling and dyspnea accompanied with fatigued of 4 months duration. She had not identified chronic medical conditions history and remarkable family medical history. Physical examination revealed a pain, poorly defined, firm, and non-tender mass located in the upper outer quadrant of her right breast. It was measured 5 centimeter in diameter approximately, and had some adhesion with adjacent tissue. In addition, the lesions caused the skin of right breast to become puffy and pitted, resembling orange peel. Retraction of the nipple has been observed (Figure 1). Palpable bilateral axillary lymph nodes were also noted. Examination of the chest revealed reduced breath sounds and percussion dullness at the lower left hemithorax.
Figure 1.
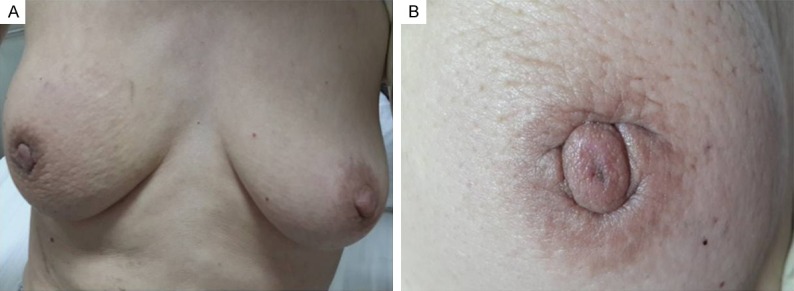
Physical findings of the chest. A. Bilateral breast were symmetrical; B. The lesions caused the skin of right breast to become puffy and pitted, resembling orange peel. Retraction of the nipple has been observed.
Imaging tests
A chest radiograph showed a little pleural effusion occupying the bilateral hemithorax (Figure 2A). A chest computed tomography (CT) scan showed nodular shadows in the front of the upper lobe. The nodular was in contact with the splanchnic pleura and approached the parietal pleura and adjacent pleural was thickened (Figure 2C, 2D). Additionly, the right breast skin was thickened, a irregular mass with lobular boundary connected to thickening of the skin (Figure 2B). A thoracolumbar magnetic resonance imaging (MRI) showed metastasis to the 10th thoracic vertebrae (Figure 2E). The original clinical diagnosis was right breast cancer with lung and bone metastasis, or breast and lung double primary cancers.
Figure 2.
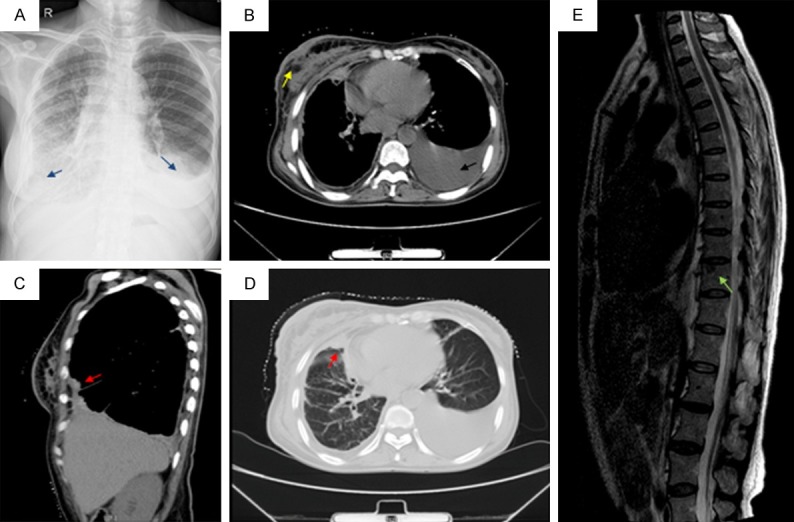
Imaging techniques. A. Chest x-ray: a little pleural effusion occupying the bilateral hemithorax (blue arrow); B. Chest computed tomography: The left lung is atelectatic and compressed by a little pleural effusion (black arrow). Additionally, right breast skin was thickened, a irregular mass with lobular boundary connected to thickening of the skin (yellow arrow); C. Chest computed tomography: Sagittal reformatted CT image demonstrates the presence of a neoplasm in the right parietal pleura (red arrow); D. Chest computed tomography: a nodular shadows in the front of the upper lobe that contact with the splanchnic pleura and approached the parietal pleura, in addition, adjacent pleural was thickened (red arrow); E. Magnetic resonance imaging: a metastasis to the 10th thoracic vertebrae (green arrow).
Cytologic and immunocytochemical findings
Moreover, the patient underwent ultrasound-guided thoracocentesis, by examination of the pleural effusion and a diagnosis of adenocarcinoma was made (Figure 3). Immunocytochemistry performed on the smears prepared from the pleural effusion sample presented the tumor cells to be strongly immunoreactive for thyroid transcription factor-1 (TTF-1), cytokeratin (CK)7, and Napsin A. Tumor cells were negative for CK20, CK 5/6, gross cystic disease fluid protein (GCDFP)15.
Figure 3.
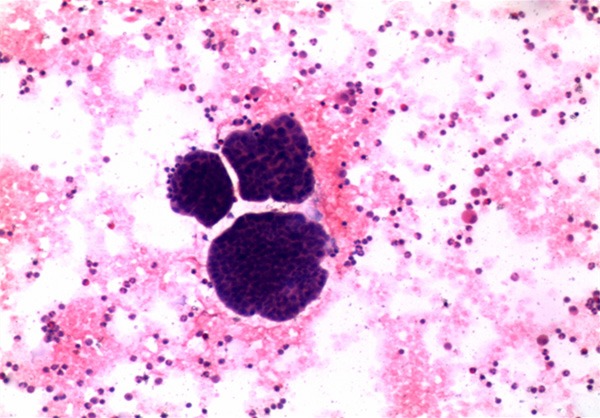
Pleural effusion aspiration smears. Hematoxylin and eosin (H&E)-stained smears prepared from the pleural effusion demonstrating low differentiated adenocarcinoma (H&E; 10×).
Histopathological and immunohistochemical findings
Finally, a breast biopsy guided by ultrasound was performed. Hematoxylin-Eosin (H&E) stained paraffin sections of the breast biopsy demonstrated poorly differentiated adenocarcinoma was infiltrated (Figure 4A). Compared with pleural effusion smear, the cells morphology are similar. In addition, within the stroma, sharply demarcated nodules of a high-grade adenocarcinoma with solid and micropapillary pattern was demonstrated (Figure 4B), and no evidence of in situ carcinoma or elastosis was observed. Immunohistochemical analysis showed tumor cells diffusely positive for cytokeratin (CK)7, NapsinA and thyroid transcription factor-1 (TTF-1) (Figure 4C-F). Moreover, the tumor cells lacked expression of Estrogen receptors (ER), progesterone receptors (PgR), Mammaglobin and gross cystic disease fluid protein 15 (GCDFP-15). Proliferation index using Ki-67 was 30%. Thus, the final diagnosis was metastatic pulmonary adenocarcinoma.
Figure 4.
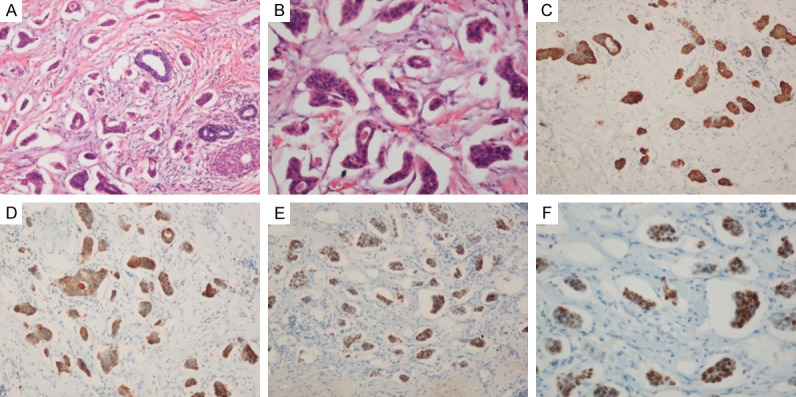
Histological findings. A. Infiltration by poorly differentiated adenocarcinoma with micropapillary pattern (Hematoxylin-eosin, ×100); B. An extensive micropapillary component was identified. (Hematoxylin-eosin, ×200); C. The tumor cells were CK-7 positive. (×100); D. The tumor cells were NapsinA positive . (×100); E. Immunohistochemical nuclear TTF-1 positivity of adenocarcinoma with solid and micropapillary component. (×100); F. Immunohistochemical nuclear TTF-1 positivity of micropapillary component (×200).
Discussion
Frequency and types of secondary breast tumours
According to Virchow’s concept, the mammary gland is a rare site of involvement by metastatic disease from extramammary malignancies [14]. The reason may be that it contained large areas of fibrous tissue with a relatively poor blood supply [15]. From clinical and autopsy cases, malignant tumors metastasizing to the breast comprise only 0.2-1.3% and 2-7% respectively [10]. Geogiannos group in a retrospectively review contain more than 14000 cases with breast malignancies diagnosis from 1907 to 1999 (with the exception of carcinoma in situ) found that only 60 cases originated from non-breast malignancies, the majority of metastasis to the breast are caused by contralateral breast cancer [3]. Williams et al reported that among extramammary solid tumors metastatic to the breast, the most common histological type was disseminated malignant melanoma in the largest published series of 169 cases [16]. Other tumor types include small cell carcinoma of the lung (SCLC), sarcoma, neuroendocrine tumors, squamous cell carcinoma.
Breast metastasis from lung adenocarcinomas
In terms of non-small cell type lung cancer (NSCLC), metastasis spread to unusual sites is less frequent and the incidence of metastasis to the breast is even rare. According to the literature, the most common metastatic organs are liver (33-40%), adrenal glands (18-38%), brain (15-43%), bone (19-33%), kidney (16-23%) and abdominal lymph nodes (29%) [17]. To the best of our knowledge, by reviewing the literatures of similar case reports between 1992 and 2012, which gave detailed clinical data regarding the primary lung cancer and breast metastasis. Only 12 cases of breast metastasis from adenocarcinomas in the PubMed database (Table 1) [4,7-9,14,18-22]. It included 8 female, their average age was 58.5 years. Moreover, 4 of them also showed ipsilateral breast metastases as ours.
Table 1.
Clinical characteristics of patients with lung adenocarcinoma and breast metastasis in previous reports
| Author, year | Age/sex | Breast tumor size/location | Primary tumor location | IHC of breast | Skin change | Follow-up (months) |
|---|---|---|---|---|---|---|
| Verger E et al., 1992 [18] | 63/male | 4 cm×3.5 cm/left breast | Right lung | Not available | Not available | Not available |
| Ramar K et al., 2003 [8] | 56/male | Not available right breast | Right lung | CK7(-) ER(-) PR(-) | Not available | Not available |
| Masmoudi A et al., 2003 [7] | 54/female | 8 cm in diameter/left breast upper quadrant | Right lung | Not available | Not available | Not available |
| Yeh CN, et al., 2004 [14] | 44/female | 4 cm×3 cm/medial lower quadrant of right breast | Not available | Not available | Not available | Not available |
| Gomez-Caro et al. 2006 [9] | 65/male | Not available/left breast | Left lung | CK4 (-) CK7(-) | Negative | 6 AWD |
| TTF-1(-) | ||||||
| Ucar et al., 2007 [19] | 63/male | 4 cm×2 cm/left breast | left lung | TTF-1(+) | Not available | Not available |
| CK7(+) | ||||||
| Fulciniti F et al., 2008 [20] | 59/female | Not available/ upper inner quadrant of right breast | Right lung | TTF-1(+) | Positive | 14 AWD |
| Klingen TA et al., 2009 [4] (2 cases) | 79/female | 8 cm indiameter/subareolar tumor in left breast | Not available | TTF1(+) | Not available | 1 DFD |
| CK7(+) | ||||||
| 70/female | 0.9 cm in diameter/subareolar tumor in right breast | Not available | TTF1(+) | Positive | 4 DFD | |
| CK7(+) | ||||||
| Maounis N et al., 2010 [21] | 73/female | 4.5 cm×3.5 cm/upper outer quadrant of left breast | Left lung | TTF-1(+) SP-A(-) | Positive | 6 DFD |
| GCDFP-15(-) | ||||||
| ER(-) | ||||||
| Mammaglobin(-) | ||||||
| Fang-Fang Ji et al., 2012 [22] (2 cases) | 49/female | 3.2 cm×3.1 cm/lower inner quadrant of the left breast | Right lung | TTF-1(+) ER(-) | Negative | 5 DFD |
| GCDFP-15(-) | ||||||
| Mammaglobin(-) | ||||||
| 40/female | 1.0 cm in diameter/upper inner quadrant of right breast | Left lung | TTF-1 (+) ER(-) | Negative | 8 DFD | |
| GCDFP-15(-) | ||||||
| Mammaglobin(-) |
AWD: alive with disease; DFD: dead from disease.
Clinical manifestation of secondary breast cancer
Previous studies have shown that the most common features of breast metastases present as palpable, rapidly growing, well-circumscribed and painless breast masses with predilection to the upper outer quadrant [1,10,14,16,23]. Unlike primary breast cancer, the retraction of skin or nipple do not demonstrate in the majority of metastases, despite their superficial location [2,4]. However, in our patient, the lesion was poorly defined. The retraction of the skin and nipple was also observed.
Imageological and pathological differential diagnosis
In the distinctiveness of a breast metastasis from a primary mammary adenocarcinoma, the clinical history, the cytopathological picture and the immunocytochemical profile of the neoplasm may be the most meaningful factors. Because the wide range of imaging manifestastions of the metastatic lesions, only based on mammographic findings, it may be extremely difficult to distinguish them, and metastasis can even mimic a benign breast tumor [3,4,24]. On the ultrasonographic manifestations, the metastatic breast lesion has also been reported as a large hypoechoic without ascoutic shadows [25,26]. Occasionally, the ultrasound images showed variable internal echoes, ranging from homogeneous hypoechogenicity to heterogeneous hypoechogenicity to homogeneous hyperechogenicity [25]. Instead, histological indicators may aid in the recognition of secondary tumors. As sited in the literature, the absence of in situ carcinoma strongly supports a metastatic tumor, although this may not be present in all primary invasive carcinomas [21]. Other clues to a metastatic malignancies rather than primary origin are often sharply circumscribed from the surrounding breast tissue [3]. Furthermore, elastosis is common in primary tumors but rare in secondary tumors [1]. Most researchers agree that calcifications are rarely seen in metastases to the breast but common in mammary carcinomas [2,10,21]. Invasive micropapillary components have been described at several anatomical sites including the breast, urinary bladder, ovary and salivary glands. With micropapillary components are being increasingly recognized as prognostic predictors in lung adenocarcinomas and it may be a manifestation of aggressive behavior [11-13]. Histologically, the micropapillary component is characterized by small papillary tufts lying freely within alveolar spaces or encased within the thin walls of connective tissue. These small, cohesive nests lack fibrovascular connective tissue cores [12]. In the present case, breast biopsies examined demonstrated tumor lesions are composed of irregular and solid malignant glands infiltrating the dense, fibrohylinized stroma. In addition, an extensive micropapillary component has been found. No evidence of in situ carcinoma or elastosis was observed. The current case also showed metastasis to the bone, may suggesting that primary lung cancer had an aggressive behavior consistent with previous researchs. It is a significant diagnostic dilemma to distinguish between metastasis from lung adenocarcinoma, particularly with an extensive micropapillary pattern, and primary mammary adenocarcinoma. The result of immunohistochemistry is a useful supplement in differential diagnosis. TTF-1 has been reported expressed in 93% of primary pulmonary small cell carcinomas, and in 63% of adenocarcinomas [27], it has not been reported positively in breast adenocarcinoma [28-30]. ERs are expressed in 80% and GCDFP-15 in 45-53% of breast carcinomas [29,31]. Occasionally, tumors from extramammary malignancies also express ER, but usually it is weak and local [32]. GCDFP-15 is expressed in 45-53% and mammaglobin in 48-72% of breast carcinoma, but mammaglobin stains negatively in pulmonary adenocarcinomas [29,31,33]. In addition, CK7 is frequently immunoreactive in lung cancer, and also in metastatic breast cancer and thyroid cancer [34]. The TTF-1, Napsin-A and CK7 is considered to be a useful marker for primary adenocarcinoma of the lung, a study involving various of immune indexes showed that the simultaneous determination of CK7 and TTF-1 contributes to distinguish between metastasis from lung adenocarcinoma and primary mammary adenocarcinoma [29]. In our case, immunohistochemical analysis showed all the tumor cells (pleura and breast) positive for TTF-1, CK7, Napsin-A and lack expression of GCDFP-15.
Treatment and outcome
The prognosis of metastasis to the breast is generally poor as most patients have widely disseminated disease [2,23]. Even though longer survival is well recognised if there is effective systemic treatment, the overwhelming majority patients die within a year of diagnosis [1,35-37]. Accurate diagnosis can prevent unnecessary mutilating surgery. In many patients, systemic treatment or palliative care is more appropriate than extensive surgery. Surgical treatment of metastases should be as conservative as possible, and excision with or without radiotherapy is considered adequate, additionally, mastectomy is only advised for very painful or deeply seated tumours. In our patient, she was administered 4 cycles of cisplatin and docetaxel, although no clinical response was seen, the patient is still alive 9 months after diagnosis.
Conclusions
Our patient illustrates that the possibility of metastasis to the breast from lung adenocarcinoma. Despite its rarity, it should be considered in the differential diagnosis of a primary mammary carcinoma because the treatment and prognosis differ significantly. It can mimic primary breast cancer in biological behavior. The result of immunohistochemistry is a useful supplement in differential diagnosis.
Disclosure of conflict of interest
None.
References
- 1.Hajdu SI, Urban JA. Cancers metastatic to the breast. Cancer. 1972;29:1691–1696. doi: 10.1002/1097-0142(197206)29:6<1691::aid-cncr2820290637>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 2.Vizcaino I, Torregrosa A, Higueras V, Morote V, Cremades A, Torres V, Olmos S, Molins C. Metastasis to the breast from extramammary malignancies: a report of four cases and a review of literature. Eur Radiol. 2001;11:1659–1665. doi: 10.1007/s003300000807. [DOI] [PubMed] [Google Scholar]
- 3.Georgiannos SN, Chin J, Goode AW, Sheaff M. Secondary neoplasms of the breast: a survey of the 20th Century. Cancer. 2001;92:2259–2266. doi: 10.1002/1097-0142(20011101)92:9<2259::aid-cncr1571>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 4.Klingen TA, Klaasen H, Aas H, Chen Y, Akslen LA. Secondary breast cancer: a 5-year population-based study with review of the literature. Apmis. 2009;117:762–767. doi: 10.1111/j.1600-0463.2009.02529.x. [DOI] [PubMed] [Google Scholar]
- 5.Sanguinetti A, Puma F, Lucchini R, Santoprete S, Cirocchi R, Corsi A, Triola R, Avenia N. Breast metastasis from a pulmonary adenocarcinoma: Case report and review of the literature. Oncol Lett. 2013;5:328–332. doi: 10.3892/ol.2012.995. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Koch A, Richter-Marot A, Wissler MP, Baratte A, Mathelin C. [Mammary metastasis of extramammary cancers: current knowledge and diagnostic difficulties] . Gynecol Obstet Fertil. 2013;41:653–659. doi: 10.1016/j.gyobfe.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Masmoudi A, Mathieu MC, Soria JC. Breast metastasis from lung adenocarcinoma: a case report. Anticancer Res. 2003;23:1825–1826. [PubMed] [Google Scholar]
- 8.Ramar K, Pervez H, Potti A, Mehdi S. Breast metastasis from non-small-cell lung carcinoma. Med Oncol. 2003;20:181–184. doi: 10.1385/MO:20:2:181. [DOI] [PubMed] [Google Scholar]
- 9.Gomez-Caro A, Pinero A, Roca MJ, Torres J, Ferri B, Galindo PJ, Parrilla P. Surgical treatment of solitary metastasis in the male breast from non-small cell lung cancer. Breast J. 2006;12:366–367. doi: 10.1111/j.1075-122X.2006.00278.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee AH. The histological diagnosis of metastases to the breast from extramammary malignancies. J Clin Pathol. 2007;60:1333–1341. doi: 10.1136/jcp.2006.046078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nassar H. Carcinomas with micropapillary morphology: clinical significance and current concepts. Adv Anat Pathol. 2004;11:297–303. doi: 10.1097/01.pap.0000138142.26882.fe. [DOI] [PubMed] [Google Scholar]
- 12.Amin MB, Tamboli P, Merchant SH, Ordonez NG, Ro J, Ayala AG, Ro JY. Micropapillary component in lung adenocarcinoma: a distinctive histologic feature with possible prognostic significance. Am J Surg Pathol. 2002;26:358–364. doi: 10.1097/00000478-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Maeda R, Isowa N, Onuma H, Miura H, Harada T, Touge H, Tokuyasu H, Kawasaki Y. Lung adenocarcinomas with micropapillary components. Gen Thorac Cardiovasc Surg. 2009;57:534–539. doi: 10.1007/s11748-009-0436-y. [DOI] [PubMed] [Google Scholar]
- 14.Yeh CN, Lin CH, Chen MF. Clinical and ultrasonographic characteristics of breast metastases from extramammary malignancies. Am Surg. 2004;70:287–290. [PubMed] [Google Scholar]
- 15.Jochimsen PR, Brown RC. Metastatic melanoma in the breast masquerading as fibroadenoma. JAMA. 1976;236:2779–2780. [PubMed] [Google Scholar]
- 16.Williams SA, Ehlers RA 2nd, Hunt KK, Yi M, Kuerer HM, Singletary SE, Ross MI, Feig BW, Symmans WF, Meric-Bernstam F. Metastases to the breast from nonbreast solid neoplasms: presentation and determinants of survival. Cancer. 2007;110:731–737. doi: 10.1002/cncr.22835. [DOI] [PubMed] [Google Scholar]
- 17.Quint LE, Tummala S, Brisson LJ, Francis IR, Krupnick AS, Kazerooni EA, Iannettoni MD, Whyte RI, Orringer MB. Distribution of distant metastases from newly diagnosed non-small cell lung cancer. Ann Thorac Surg. 1996;62:246–250. doi: 10.1016/0003-4975(96)00220-2. [DOI] [PubMed] [Google Scholar]
- 18.Verger E, Conill C, Velasco M, Sole M. Metastasis in the male breast from a lung adenocarcinoma. Acta Oncol. 1992;31:479. doi: 10.3109/02841869209088294. [DOI] [PubMed] [Google Scholar]
- 19.Ucar N, Kurt OK, Alpar S, Orsel O, Demirag F, Kurt B. Breast metastasis in a male patient with nonsmall cell lung carcinoma. South Med J. 2007;100:850–851. doi: 10.1097/SMJ.0b013e3180f62fdc. [DOI] [PubMed] [Google Scholar]
- 20.Fulciniti F, Losito S, Botti G, Di Mattia D, La Mura A, Pisano C, Pignata S. Metastases to the breast: role of fine needle cytology samples. Our experience with nine cases in 2 years. Ann Oncol. 2008;19:682–687. doi: 10.1093/annonc/mdm546. [DOI] [PubMed] [Google Scholar]
- 21.Maounis N, Chorti M, Legaki S, Ellina E, Emmanouilidou A, Demonakou M, Tsiafaki X. Metastasis to the breast from an adenocarcinoma of the lung with extensive micropapillary component: a case report and review of the literature. Diagn Pathol. 2010;5:82. doi: 10.1186/1746-1596-5-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ji FF, Gao P, Wang JG, Zhao J, Zhao P. Contralateral breast metastasis from pulmonary adenocarcinoma: two cases report and literature review. J Thorac Dis. 2012;4:384–389. doi: 10.3978/j.issn.2072-1439.2012.02.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toombs BD, Kalisher L. Metastatic disease to the breast: clinical, pathologic, and radiographic features. AJR Am J Roentgenol. 1977;129:673–676. doi: 10.2214/ajr.129.4.673. [DOI] [PubMed] [Google Scholar]
- 24.Noguera J, Martinez-Miravete P, Idoate F, Diaz L, Pina L, Zornoza G, Martinez-Regueira F. Metastases to the breast: a review of 33 cases. Australas Radiol. 2007;51:133–138. doi: 10.1111/j.1440-1673.2007.01681.x. [DOI] [PubMed] [Google Scholar]
- 25.Lee SH, Park JM, Kook SH, Han BK, Moon WK. Metastatic tumors to the breast: mammographic and ultrasonographic findings. J Ultrasound Med. 2000;19:257–262. doi: 10.7863/jum.2000.19.4.257. [DOI] [PubMed] [Google Scholar]
- 26.Derchi LE, Rizzatto G, Giuseppetti GM, Dini G, Garaventa A. Metastatic tumors in the breast: sonographic findings. J Ultrasound Med. 1985;4:69–74. doi: 10.7863/jum.1985.4.2.69. [DOI] [PubMed] [Google Scholar]
- 27.Di Loreto C, Di Lauro V, Puglisi F, Damante G, Fabbro D, Beltrami CA. Immunocytochemical expression of tissue specific transcription factor-1 in lung carcinoma. J Clin Pathol. 1997;50:30–32. doi: 10.1136/jcp.50.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klingen TA, Chen Y, Gundersen MD, Aas H, Westre B, Sauer T. Thyroid transcription factor-1 positive primary breast cancer: a case report with review of the literature. Diagn Pathol. 2010;5:37. doi: 10.1186/1746-1596-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang M, Nonaka D. A study of immunohistochemical differential expression in pulmonary and mammary carcinomas. Mod Pathol. 2010;23:654–661. doi: 10.1038/modpathol.2010.38. [DOI] [PubMed] [Google Scholar]
- 30.Zamecnik J, Kodet R. Value of thyroid transcription factor-1 and surfactant apoprotein A in the differential diagnosis of pulmonary carcinomas: a study of 109 cases. Virchows Arch. 2002;440:353–361. doi: 10.1007/s00428-001-0552-2. [DOI] [PubMed] [Google Scholar]
- 31.Takeda Y, Tsuta K, Shibuki Y, Hoshino T, Tochigi N, Maeshima AM, Asamura H, Sasajima Y, Ito T, Matsuno Y. Analysis of expression patterns of breast cancer-specific markers (mammaglobin and gross cystic disease fluid protein 15) in lung and pleural tumors. Arch Pathol Lab Med. 2008;132:239–243. doi: 10.5858/2008-132-239-AOEPOB. [DOI] [PubMed] [Google Scholar]
- 32.Dennis JL, Hvidsten TR, Wit EC, Komorowski J, Bell AK, Downie I, Mooney J, Verbeke C, Bellamy C, Keith WN, Oien KA. Markers of adenocarcinoma characteristic of the site of origin: development of a diagnostic algorithm. Clin Cancer Res. 2005;11:3766–3772. doi: 10.1158/1078-0432.CCR-04-2236. [DOI] [PubMed] [Google Scholar]
- 33.Bhargava R, Beriwal S, Dabbs DJ. Mammaglobin vs GCDFP-15: an immunohistologic validation survey for sensitivity and specificity. Am J Clin Pathol. 2007;127:103–113. doi: 10.1309/TDP92PQLDE2HLEET. [DOI] [PubMed] [Google Scholar]
- 34.Al-Zahrani IH. The value of immunohistochemical expression of TTF-1, CK7 and CK20 in the diagnosis of primary and secondary lung carcinomas. Saudi Med J. 2008;29:957–961. [PubMed] [Google Scholar]
- 35.Chaignaud B, Hall TJ, Powers C, Subramony C, Scott-Conner CE. Diagnosis and natural history of extramammary tumors metastatic to the breast. J Am Coll Surg. 1994;179:49–53. [PubMed] [Google Scholar]
- 36.McIntosh IH, Hooper AA, Millis RR, Greening WP. Metastatic carcinoma within the breast. Clin Oncol. 1976;2:393–401. [PubMed] [Google Scholar]
- 37.Alvarado Cabrero I, Carrera Alvarez M, Perez Montiel D, Tavassoli FA. Metastases to the breast. Eur J Surg Oncol. 2003;29:854–855. doi: 10.1016/s0748-7983(03)00123-9. [DOI] [PubMed] [Google Scholar]


