Abstract
Primary squamous cell carcinoma (SCC) of the stomach is rare. Its pathogenesis is also unclear and there are conflicting reports about it in the past. Only about 100 cases have been reported so far in the literature. The current study discusses a new case of gastric squamous cell carcinoma, from a 50-year-old Chinese male patient diagnosed via subtotal gastrectomy with Roux-en-Y reconstruction and D2 lymphadenectomy. In the stomach, an ulcerated mass in the antrum, measuring 12×8×6 cm, was observed. Further, pathological examination of the resected specimen revealed a well-differentiated SCC. Observations indicated tumor cell invasion into the serosa, and encroachment into perigastric regional lymph node. A follow-up abdominal CT scan three months later revealed tumor invasion into the ascending colon. We assume that this invaded mass was transferred from the gastric squamous cell carcinoma. Interestingly, the patient is still alive.
Keywords: Squamous cell carcinoma, stomach
Introduction
Most of the gastric cancer cases include adenocarcinoma. Squamous cell carcinoma (SCC) of the stomach is an extremely rare entity, with an annual incidence rate of 0.04 to 0.07% [1]. The first SCC case was described in 1905 [2]. Since then, less than 100 cases have been reported in the literature [3]. Despite the available diagnostic criteria, SCC pathogenesis is still unclear and different hypotheses have been proposed for its diagnosis. In addition, no effective systemic standardized treatment options are available and the prognosis usually seems less favorable. In terms of its occurrence, SCC is mostly prevalent in males with a male-to-female ratio of 5:1 and is usually diagnosed in the sixth decade of life when it has already progressed to an advanced stage [4].
Case report
A 50-year-old Chinese male with a two-month history of upper abdominal pain was admitted to the First Affiliated Hospital of Wenzhou Medical University. Initially, the patient did not show any symptoms of nausea, vomiting, dysphagia or melena. The physical examination and routine laboratory tests upon admission did not show any abnormal signs and appeared normal. However, the upper gastrointestinal endoscopy revealed a protruding mass located in the antrum. This mass was approximately 12 cm in diameter and displayed surface bleeding (as seen in Figure 1). Subsequently, the patient underwent subtotal gastrectomy with Roux-en-Y reconstruction and D2 lymphadenectomy. As a result, an ulcerated mass measuring 12×8×6 cm, was observed in the gastric antrum. The tumor had apparently infiltrated into the serosal layer, pancreas head, transverse mesocolon and partial lymph node of the stomach. Further pathological examination of the resected specimen revealed SCC characteristics, displaying a typical keratin pearl formation and intracellular bridges (as observed in Figure 2). Immunohistochemical staining was positive for cytokeratin as seen in Figure 3. Interestingly, the tumor had not invaded the mucosa of the esophagogastric junction. Also, no squamous cells were identified in the normal portion of the stomach. Based on the TNM classification of UICC 2010, the tumor was diagnosed as stage IIB (T4bN3aM0). Surgery was performed and one month post-surgery, a combination chemotherapy consisting of cisplatin (200 mg) and docetaxel (100 mg) was administered for four days after every 35 days. In total, the patient received three cycles of the chemotherapy regimen. A follow-up abdominal CT scan, three months later, revealed tumor invasion into the ascending colon as illustrated in Figure 4. We assume that this invaded mass originated from the gastric squamous cell carcinoma. The patient is still alive today.
Figure 1.
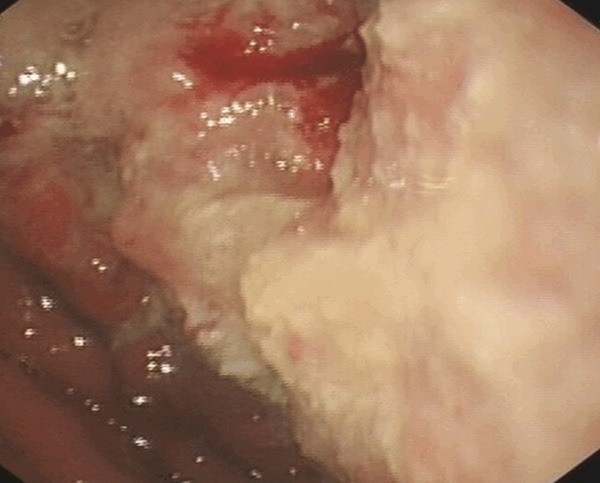
Endoscopic image of the large tumor predominantly located in the antrum without involving the gastroesophageal junction.
Figure 2.
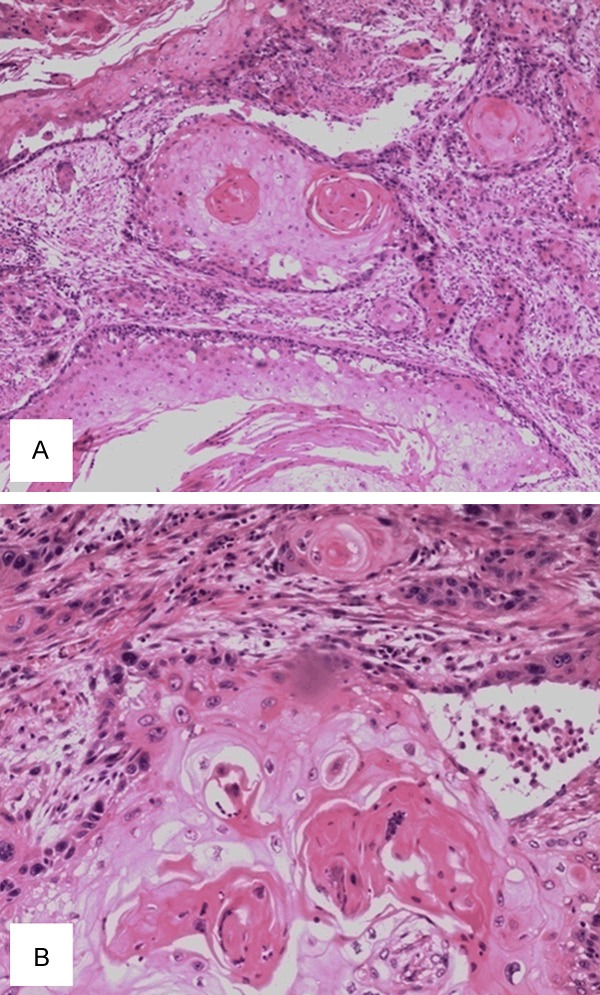
A. HE staining of the tumor showing tumor nests (40×, magnification). B. HE staining showing the typical keratin pearl formation and intracellular bridges, characteristic of SCC, at a higher (magnification of 100×).
Figure 3.
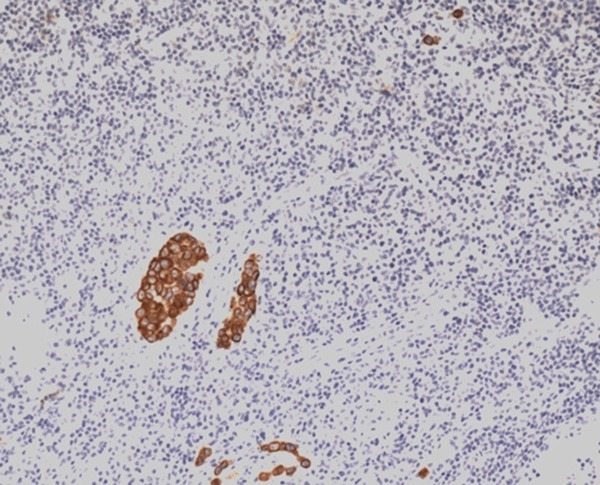
Immunohistochemical staining of cytokeratin (magnification, 40×).
Figure 4.
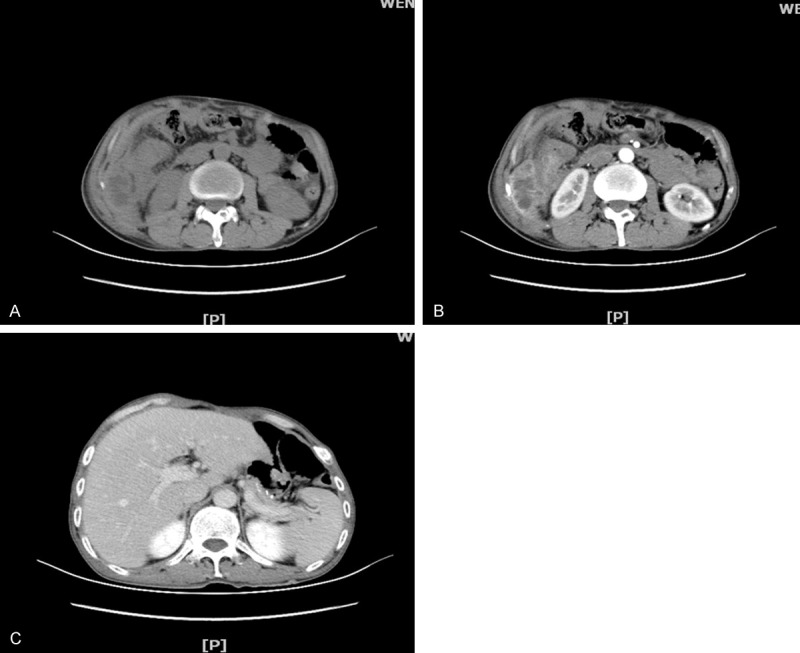
A. Abdominal CT scan depicting an irregular mass in the ascending colon, resulting in a narrow lumen. B. Enhanced CT revealing increase in heterogeneous mass. C. CT showing the stomach after postoperative changes.
Discussion
Several diagnostic criteria exist for squamous cell carcinoma. According to the Japanese classification of gastric carcinoma [5], the diagnostic criteria include: 1) all tumor cells are SCC cells, and 2) there is distinct evidence supporting the origin of SCC directly in the gastric mucosa. However, Parks RE [6] proposed another set of following criteria: 1) the tumor should not be located in the cardia, 2) the tumor should also not extend into the oesophagus, and 3) there should be no evidence of SCC elsewhere in the body.
Consistently, the patient described in our study also had the tumor located in the antrum as detected by endoscopy and laparotomy, indicating that the cardia was intact and the tumor proximity was normal. In addition, we did not find any SCC in other organs of the body upon clinical examination that included abdominal CT, endoscopic examination, pulmonary imaging and radiological studies. Therefore, the patient was diagnosed with a primary SCC of the stomach.
Boswell and Helwig [1] also defined four histopathological features to diagnose a primary gastric SCC. They include the presence of keratinizing cell masses with pearl formation, a mosaic pattern of cell arrangement, intercellular bridges and high concentrations of sulfhydryl or disulfide groups indicative of keratin production. Similarly, we also detected typical keratin pearl formation and intercellular bridges in the tumor sections, which further reinforced the SCC diagnosis.
In addition, the pathogenesis of gastric SCC is still very obscure. Several mechanisms underlying its diagnosis include: 1) differentiation of the squamous from pre-existing adenocarcinoma, 2) squamous metaplasia of the gastric mucosa preceding malignant transformation, 3) pluripotent stem cells capable of developing into any cell type, and 4) the presence of ectopic squamous epithelial nests in the gastric mucosa [7-9]. Mori et al. [10] hypothesized that neoplastic pluripotent cells first transform into adenocarcinoma followed by squamous metaplasia, which eventually results in SCC. In addition, Takita et al. [11] suggested that Epstein-Barr virus (EBV) infection may also be involved in the pathogenesis of gastric SCC. They used immunohistochemistry and liquid hybridization assays for detection of human papilloma virus (HPV) infection, and polymerase chain reaction method for Epstein-Barr virus (EBV) infection.
Wakabayashi et al. [12] reviewed 56 cases of gastric squamous cell carcinomas that have been reported in Japan. The median age of onset was between 29 and 81 years (mean, 64.7±1.7 years), and the male-to-female ratio was 44:12. The frequency of tumor location in the upper, lower and middle portions of the stomach were 57.1%, 21.4% and 19.6%, respectively and the tumor diameters ranged from 2.1 to 13 cm (mean, 6.6±0.3 cm). In this study, we also reviewed the clinical features of 11 SCC patients based on literature search (English language studies), which are summarized in Table 1. The mean age of the patients was 63.8 years and the tumor size ranged between 4 and 18 cm (mean- 8.2 cm).
Table 1.
Clinical features of 11 SCC patients
| Author | Year | Age (yr) | Sex | Chief clinical presentation | Position | Size (cm) | Survival (mo) |
|---|---|---|---|---|---|---|---|
| Raju et al [13] | 1987 | 59 | M | Abdominal pain | ps | 12 | ND |
| Dursun et al [4] | 2003 | 65 | M | Abdominal pain | lc | 4 | 3 |
| Hara et al [14] | 2004 | 85 | M | Abdominal pain | gc | 8 | 171 |
| Choi et al [15] | 2007 | 40 | M | Without any symptoms | gc | 7 | 121 |
| Callacondo et al [3] | 2009 | 83 | M | Abdominal pain, vomiting weight loss | Antrum | 18 | 241 |
| Guttmann et al [16] | 2012 | 81 | F | Abdominal pain, weight loss | lc | 4 | ND |
| Tokuhara et al [17] | 2012 | 67 | M | Difficulty swallowing | lc | 6 | 13 |
| Hwang et al [18] | 2014 | 61 | M | Weight loss | Fundus | 6.5 | 6 |
| Wakabayashi et al [12] | 2014 | 69 | M | Difficulty swallowing | lc | 9 | 36 |
| Mardi et al [19] | 2015 | 42 | M | Abdominal pain, vomiting | Antrum | 4 | ND |
| Present case | 2015 | 50 | M | Abdominal pain | Antrum | 12 | 31 |
Alive patients;
ps: posterior wall of the stomach; lc: the lesser curvature of the stomach; gc: the greater curvature of the stomach; ND: Not described.
Depending on the stage of diagnosis, the prognosis for primary gastric SCC has been reported to be either more favorable [20] or less favorable [21] than gastric adenocarcinoma. However, usually due to the late diagnosis of gastric SCC, it is already in an advanced stage, with a poor prognosis. To date, no standard chemotherapy regimen for gastric SCC has been defined. Previous reports suggested that radical surgical excision was the best option to potentially cure the localized disease. However, aggressive surgery plus adjuvant chemotherapy was appropriate for advanced-stage SCC of the stomach in some patients [2,9]. The overall survival rates of the patient range from 7 months to 8 years [2]. A high incidence of stomach SCC is observed in the sixth decade of life, although 17-year-old patients have also been reported [22]. Interestingly, Marubashi et al. [23] reported a case of gastric SCC in a 70-year-old male who responded effectively to neoadjuvant and low-dose FP chemotherapy.
To conclude, in this study, we have reported a patient who underwent subtotal gastrectomy with Roux-en-Y reconstruction and D2 lymphadenectomy. Based on the pathological characteristics, the patient was diagnosed with gastric SCC. Post-surgery, the patient received definitive chemotherapy with docetaxel and cisplatin to manage lymph node metastasis. Although the prognosis is somewhat controversial, the disease stage and surgical resectability are the key determinants of outcomes in gastric SCC.
Disclosure of conflict of interest
None.
References
- 1.Boswell JT, Helwig EB. squamous cell carcinoma and adenoacanthoma of the stomach. A clinicopathologic study. Cancer. 1965;18:181–192. doi: 10.1002/1097-0142(196502)18:2<181::aid-cncr2820180209>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 2.Bonnheim DC, Sarac OK, Fett W. Primary squamous cell carcinoma of the stomach. Am J Gastroenterol. 1985;80:91–94. [PubMed] [Google Scholar]
- 3.Callacondo D, Ganoza-Salas A, Anicama-Lima W, Quispe-Mauricio A, Longacre TA. Primary squamous cell carcinoma of the stomach with paraneoplastic leucocytosis: a case report and review of the literature. Hum Pathol. 2009;40:1494–1498. doi: 10.1016/j.humpath.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Dursun M, Yaldiz M, Isikdogan A, Yilmaz G, Canoruc F, Ormeci N, Yilmaz S. Primary squamous cell carcinoma of the stomach: a case report and review of the literature. Eur J Gastroenterol Hepatol. 2003;15:329–330. doi: 10.1097/01.meg.0000050011.68425.82. [DOI] [PubMed] [Google Scholar]
- 5.Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma. 13th edition. Tokyo: Kanchara; 1999. [DOI] [PubMed] [Google Scholar]
- 6.Parks RE. Squamous neoplasms of the stomach. Am J Roentgenol Radium Ther Nucl Med. 1967;101:447–449. doi: 10.2214/ajr.101.2.447. [DOI] [PubMed] [Google Scholar]
- 7.Straus R, Heschel S, Fortmann DJ. Primary adenosquamous carcinoma of the stomach. A case report and review. Cancer. 1969;24:985–995. doi: 10.1002/1097-0142(196911)24:5<985::aid-cncr2820240518>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 8.Amuluru K, Gupta H. Primary squamous cell carcinoma of the stomach: a case report. J Gastrointest Cancer. 2010;41:24–26. doi: 10.1007/s12029-009-9097-4. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt C, Schmid A, Lüttges JE, Kremer B, Henne-Bruns D. Primary squamous cell carcinoma of the stomach. Report of a case and review of literature. Hepatogastroenterology. 2001;48:1033–1036. [PubMed] [Google Scholar]
- 10.Mori M, Iwashita A, Enjoji M. Squamous cell carcinoma of the stomach: report of three cases. Am J Gastroenterol. 1986;81:339–342. [PubMed] [Google Scholar]
- 11.Takita J, Kato H, Miyazaki T, Nakajima M, Fukai Y, Masuda N, Manda R, Fukuchi M, Kuwano H. Primary squamous cell carcinoma of the stomach: a case report with immunohistochemical and molecular biologic studies. Hepatogastroenterology. 2005;52:969–974. [PubMed] [Google Scholar]
- 12.Wakabayashi H, Matsutani T, Fujita I, Kanazawa Y, Nomura T, Hagiwara N, Hosone M, Katayama H, Uchida E. A rare case of primary squamous cell carcinoma of the stomach and a review of the 56 cases reported in Japan. J Gastric Cancer. 2014;14:58–62. doi: 10.5230/jgc.2014.14.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raju GC, Barton EN, Marchack D, Naraynsingh V. Hypercalcaemia in primary squamous cell carcinoma of the stomach. J R Soc Med. 1987;80:587–588. doi: 10.1177/014107688708000920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hara J, Masuda H, Ishii Y, Aoki N, Nakayama H, Komura K, Karube H, Funada T, Hemmi A, Takayama T. Exophytic primary squamous cell carcinoma of the stomach. J Gastroenterol. 2004;39:299–300. doi: 10.1007/s00535-003-1294-5. [DOI] [PubMed] [Google Scholar]
- 15.Choi SB, Park SS, Oh SY, Kim JH, Kim WB, Lee JH, Choi JW, Kim SJ, Kim CS, Mok YJ. Primary squamous cell carcinoma of the stomach that developed with Menetrier’s disease. Dig Dis Sci. 2007;52:1722–1724. doi: 10.1007/s10620-006-9191-4. [DOI] [PubMed] [Google Scholar]
- 16.Guttmann S, Fromer N, Shamah S, Braha J, Vlodov J, Badalov N. A case of two primary gastric malignancies: adenocarcinoma and squamous cell carcinomaof the stomach. Gastrointest Endosc. 2012;75:1113–1114. doi: 10.1016/j.gie.2011.05.037. [DOI] [PubMed] [Google Scholar]
- 17.Tokuhara K, Nakano T, Inoue K, Nakane Y, Kwon AH. Primary squamous cell carcinoma in the gastric remnant. Surg Today. 2012;42:666–669. doi: 10.1007/s00595-012-0144-6. [DOI] [PubMed] [Google Scholar]
- 18.Hwang SH, Lee JH, Kim K, Shin DH, Kim JY, Sol MY, Choi KU. Primary squamous cell carcinoma of the stomach: A case report. Oncol Lett. 2014;8:2122–2124. doi: 10.3892/ol.2014.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mardi K, Mahajan V, Sharma S, Singh S. Primary squamous cell carcinoma of stomach: A rare case report. South Asian J Cancer. 2013;2:199. doi: 10.4103/2278-330X.119897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Altshuler JH, Shaka JA. Squamous cell carcinoma of the stomach. Review of the literature and report of a case. Cancer. 1966;19:831–838. doi: 10.1002/1097-0142(196606)19:6<831::aid-cncr2820190613>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 21.Volpe CM, Hameer HR, Masetti P, Pell M, Shaposhnikov YD, Doerr RJ. Squamous cell carcinoma of the stomach. Am Surg. 1995;61:1076–1078. [PubMed] [Google Scholar]
- 22.Schwab G, Wetscher G, Dietze O, Schmid K, Pointner R. Primary squamous cell carcinoma of the stomach in a seventeen-year-old boy. Surg Today. 1992;22:561–564. doi: 10.1007/BF00308905. [DOI] [PubMed] [Google Scholar]
- 23.Marubashi S, Yano H, Monden T, Tateishi H, Kanoh T, Iwazawa T, Matsui S, Nakano Y, Kinuta M, Takahashi H, Okamura J. Primary squamous cell carcinoma of the stomach. Gastric Cancer. 1999;2:136–141. doi: 10.1007/s101200050036. [DOI] [PubMed] [Google Scholar]


