Abstract
Introduction: Tracheobronchopathia osteochondroplastica (TO) is a rare disorder involving the lumen of the trachea-bronchial tree and characterized by multiple sub-mucosal osseous and cartilaginous nodules in the trachea and bronchus, sparing the posterior wall. We here report two cases of patients with tracheobronchopathia osteochondroplastica and review the relevant literature briefly. Case presentation: Case 1 was a 64-year-old woman with a history of Chronic Obstructive Pulmonary Disease (COPD) who presented with frequent non-productive cough for 2 years. Chest computed tomography (CT) showed signs consistent with COPD and evident irregular narrowing of the tracheal and both main bronchial lumen caused by calcific foci. Fibre optic bronchoscope (FOB) was performed and showed dozens of sub-mucosal nodules protruding into the lumen of lower half of the trachea and both main bronchi. Histopathological exam demonstrated sub-mucosal ossification and cartilage in the sample. Her follow-up has been uneventful for 3 years. Case 2 was a 37-year-old man presented with hoarseness, exertional dyspnea, and intermittent dry cough for about 3 years. Chest CT scans showed irregular nodules around the entire circumference of the trachea extending from sub-glottic region to lower trachea. FOB showed glottic stenosis and diffused sub-mucosal calcified nodules protruding from the antero-lateral portion of the trachea in the subglottic region. Over the following 12 months, his disease is stable. Conclusions: TO is a rare, benign disease with slow progression, clinicians should be aware of TO and should consider it in patients with chronic cough, recurrent respiratory infection and evolving exertional dyspnea.
Keywords: Tracheobronchopathia osteochondroplastica
Introduction
Tracheobronchopathia osteochondroplastica (TO) is a rare, slow-growing, benign disorder involving the lumen of the trachea-bronchial tree and characterized by multiple sub-mucosal osseous and cartilaginous nodules in the trachea and bronchus, sparing the posterior wall. It was first described by Wilks in the 19th century in a 38 year old man with tuberculosis. Since then hundreds cases have been reported worldwide, however, many TO patients were unable to be diagnosed for the unawareness of clinicians. The etiology and pathology are still unclear. TO shows a wide variety of clinical manifestations, from asymptomatic to non-specific respiratory symptoms. TO can be confirmed by the specific representation through fiber optic bronchoscopy (FOB) and histopathological examination. In this report, we describe 2 cases of TO with typical radiographic and endoscopic appearance.
Case presentation
Case 1 was a 64-year-old female presented with frequent non-productive cough since 2 years with a history of Chronic Obstructive Pulmonary Disease (COPD). Two months prior to admission, the cough had started to occur more frequently despite treatment with salmeterol/fluticasone (50/500 μg twice daily). She was a non-smoker. The review of other systemic symptoms yielded negative results. Following admission, physical examinations was unremarkable. Routine blood investigations were within normal range. The results of an arterial blood gas analysis were normal, with a pH of 7.35, PaO2 of 88 mmHg, PaCO2 of 40 mmHg and HCO3 - of 2 mmol/l. Also, immunological and rheumatologic tests, including antinuclear antibody, deoxyribonucleic acid antibody, perinuclear anti-neutrophil cytoplasmic antibodies, antineutrophil cytoplasmic antibodies, extractable nuclear antigens, and rheumatoid factor were normal. A lung function test indicated moderate obstructive ventilation disorder with a FEV1/FVC ratio of 60%. Chest computed tomography (CT) showed signs consistent with COPD and evident irregular narrowing of the tracheal and both main bronchial lumen caused by calcific foci (Figure 1A, 1B). No swelling of mediastinal lymph nodes were found. Because of the presence of this lesion, FOB was performed and showed dozens of sub-mucosal nodules protruding into the lumen of lower half of the trachea and both main bronchi. The nodules were distributed along the antero-lateral wall of the trachea and the main bronchi, sparing the posterior wall of the trachea. The membranous structures were intact (Figure 1C, 1D). The nodules were found to be firm and hard in consistency when grasped with the biopsy forceps. Histopathological exam demonstrated sub-mucosal ossification and cartilage in the sample (Figure 2). Hence, the evidence pointed towards a diagnosis of TO. Her symptoms was alleviated after receiving oral antitussives. Three years after the initial visit to our hospital, his chest X-ray and pulmonary function test revealed no deterioration.
Figure 1.
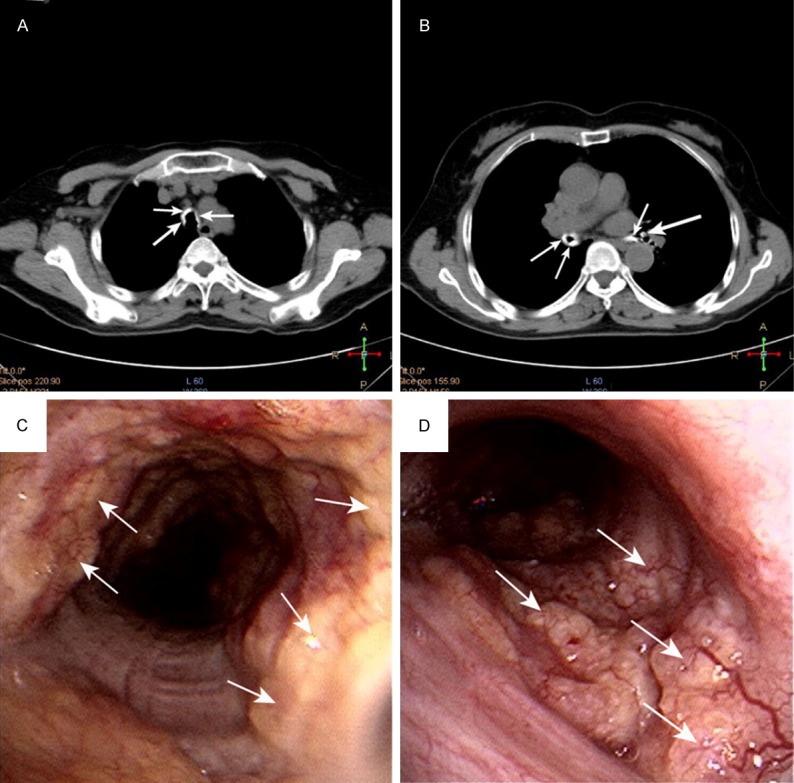
Images and appearance of TO (A, B) Chest CT scan showing a ring (arrow) of multiple calcified nodules surrounding the tracheal lumen. (C, D) FOB showing dozens of sub-mucosal nodules (arrow) protruding into the lumen of lower half of the trachea and right main bronchi.
Figure 2.
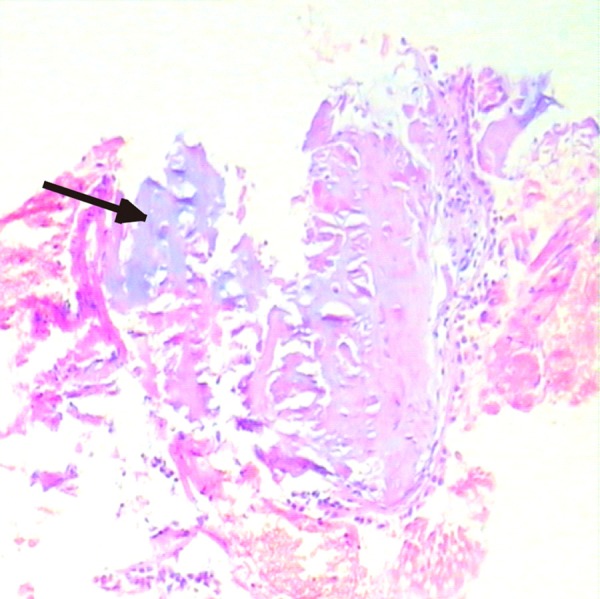
Histopathological examination demonstrating sub-mucosal ossification and cartilage in the sample.
Case 2 was a 37-year-old male presented with hoarseness, exertional dyspnea, and intermittent dry cough for about 3 years. Other than fever and productive cough during acute episodes of chest infection, he did not have haemoptysis, wheezing or weight loss. He was not known to have any chronic illness. He had no history of smoking, recent travel or usage of illicit drugs. He is a pediatrician. His family history was unremarkable. On physical examination, his was in good general condition, with no clubbing or lymphadenopathy and systemic examination was unremarkable. All routine blood tests were normal. Chest radiographs did not show any apparent pulmonary parenchymal lesion. Chest CT scans showed irregular nodules around the entire circumference of the trachea causing narrowing of the tracheal lumen from the sub-glottic region to the lower trachea, with the glottis involved (Figure 3A, 3B). FOB showed glottic stenosis and diffused sub-mucosal calcified nodules protruding from the antero-lateral portion of the trachea in the subglottic region (Figure 3C). Because of the stenosis of the airway, our fiberoptic bronchoscope failed to pass through the glottis. But with the characteristic appearance on radiography and images we obtained through FOB, the diagnosis of TO was confirmed. He was also relieved from accepting antitussives. Over the following 12 months, his disease was stable.
Figure 3.
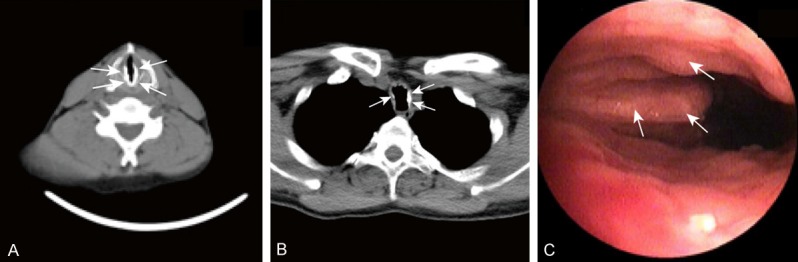
Radiographic and bronchoscopic images of TO (A, B) Chest CT scan showing ossified nodules around the entire circumference of the trachea causing stenosis of sub-glottic trachea. (C) FOB showed glottic stenosis and diffused sub-mucosal calcified nodules protruding from the antero-lateral portion of the trachea in the subglottic region.
Discussion
Tracheobronchopathia osteochondroplastica (TO) is a rare, slow-growing, benign disorder involving the lumen of the trachea-bronchial tree and characterized by multiple sub-mucosal osseous and cartilaginous nodules in the anterior and lateral wall of trachea and bronchus, except the posterior wall. This malady was first reported by Wilkes and colleagues in 1857. Since then, hundreds of cases of TO have been reported in the literature. The accurate incidence of TO in general population is hard to estimate since most patients are asymptomatic or present with nonspecific respiratory symptoms [1]. But the prevalence of TO may be higher than expected. Studies render that only half of patients with TO are accurately diagnosed during their life-time [2]. We have performed approximately 2,000 procedures in our bronchoscopy laboratory and 2 cases were noted, similar to the incidence found in the large cohort study from China (n=41,600) [3]. The onset of TO generally occurs in the 4th and the 7th decades of life without sexual predominance [1]. However, a few cases of TO have developed in children and young adults [4-6]. More interestingly, Sellon et al. [7] reported that TO can even affect dog’s trachea.
The etiologic and pathogenetic mechanisms contributing to the formation of a TO are poorly understood. Chronic infections, irritants, metabolic disorders, and genetic predisposition are possibly associated with development of TO [8]. Direct relationship with either calcium or phosphorus metabolism has not been established [9], albeit cartilaginous and osseous nodules compose most part of the lesions of TO. In our patients, no predisposing condition could be found and no causal association could be inferred. COPD is also not considered as a risk factor for TO despite our first case with concurrent COPD.
Manifestations of TO are quite variable. Patients may be asymptomatic or they may present with minor cough or more severe symptoms. Symptom severity depends on the extent of tracheal/bronchial obstruction and complicated respiratory infections leading to dyspnea [10]. In asymptomatic individuals, TO is an incidental finding at autopsy, routine bronchoscopy or CT-scan, this performed to detect an unrelated problem. In rare cases, TO was exceptionally discovered secondary to difficult intubation [11-13].
Chest physical examinations are unremarkable in the majority of patients. Chest radiographs are usually normal. However irregularity and/or stenosis of the trachea or a lobar atelectasis may be manifested in some patients [9,14]. Thoracic CT scans most likely show pathognomonic findings of TO that diffuse sub-mucosal calcified nodules protruding from the antero-lateral trachea into the lumen, sparing the posterior wall. It is also important in detecting the complications such as lobar collapse and post obstructive bronchiectasis. Nevertheless, CT still has shortcomings such as unreliability for atypical cases or insensitivity for early changes.
In addition to routine radiological examination, bronchoscopy is gaining the most attention for its utility in TO detection. In bronchoscopy, TO presents as diffuse sub-mucosal ivory nodules 1 to 10 mm in size protruding into the tracheal lumen, most frequently involve the distal 2/3 of trachea, from the anterior and lateral wall except the posterior wall, creating a “cobblestone” or “rock garden” appearance [15,16]. Occasionally the larynx could also be involved [10,11,17,18]. In our second case, ossific nodules began in the sub-glottic region and extended to carina, causing hoarseness and stenosis of lower trachea. This is a rare condition.
Histopathologic examination usually reveals metaplastic cartilage and bone in the tracheal sub-mucosa, often in conjunction with perichondrium of the tracheal cartilage. The overlying mucosa is intact and may appear to be normal or metaplastic [5]. However, histopathologic examination for confirming TO has not been a mandatory part of most clinical practices, partly because the combination of radiographic and endoscopic appearance of diffuse sub-mucosal cartilaginous and osseous nodules is characteristic and sufficient for clinical diagnosis, on the other hand the lesions are too tough enough to obtain during bronchoscopy [19].
At present, no definitive therapy can eradicate TO. Treatment of TO is nonspecific and aims at symptom control, including anti-tussives and inhaled corticosteroids for cough, antibiotics for infectious complications. Surgical resection, cryotherapy, laser excision, external beam irradiation, radiotherapy, or stent insertion is generally only indicated in cases of patients with severe trachea-bronchial stenosis to relieve airway obstruction [20]. Therapeutic guidelines for the treatment of renal artery embolism have not been established, and because of the rarity of the disease, it is unlikely that the superiority of a particular therapy will be evaluated in prospective randomized clinical trials.
The prognosis of patients with TO is usually favorable, most cases shows very little evolution over a period of years. However, reports show a few patients died of complications of TO, especially severe respiratory infections [21]. Our patients had been followed for 3 years, their conditions were unchanged.
In conclusion, TO is a rare and benign disorder with characterization of multiple sub-mucosal osseous and cartilaginous nodules in the anterior and lateral wall of trachea and bronchus. The clinical symptoms of TO vary depending upon severity of tracheal stenosis or subsequent infection. FOB is the diagnostic modality of choice for TO. The prognosis of TO is usually favorable. Nevertheless, long-term patient follow-up is imperative. Our cases highlight the fact that clinicians should consider TO as one of the differentials while dealing with patients with recurrent respiratory symptoms who are over their fourth decade of life and are not relieved on routine medical treatment.
Acknowledgements
Many thanks to our patient who consented to the publication of this case report.
Disclosure of conflict of interest
None.
References
- 1.Ulasli SS, Kupeli E. Tracheobronchopathia osteochondroplastica: a review of the literature. Clin Respir J. 2014 doi: 10.1111/crj.12166. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Thakur A, Yang T, Chen T, Rana N, Zhu B, Wei X, Yang L, Zhang G, Zhang M, Chen M. Atypical presentation of tracheobronchopathia osteochondroplastica: is chronic inflammation a perpetrator? Med Princ Pract. 2013;22:503–505. doi: 10.1159/000346662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu Y, Wu N, Huang HD, Dong YC, Sun QY, Zhang W, Wang Q, Li Q. A clinical study of tracheobronchopathia osteochondroplastica: findings from a large Chinese cohort. PLoS One. 2014;9:e102068. doi: 10.1371/journal.pone.0102068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sant’Anna CC, Pires-de-Mello P, Morgado Mde F, March Mde F. Tracheobronchopathia osteochondroplastica in a 5 year-old girl. Indian Pediatr. 2012;49:985–986. doi: 10.1007/s13312-012-0223-1. [DOI] [PubMed] [Google Scholar]
- 5.Swamy TL, Hasan A. Tracheopathia osteoplastica presenting with haemoptysis in a young male. Indian J Chest Dis Allied Sci. 2010;52:119–121. [PubMed] [Google Scholar]
- 6.Jindal S, Nath A, Neyaz Z, Jaiswal S. Tracheobronchopathia osteochondroplastica--a rare or an overlooked entity? J Radiol Case Rep. 2013;7:16–25. doi: 10.3941/jrcr.v7i3.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sellon RK, Johnson JL, Leathers CW, Sporn T, Beckley JC. Tracheobronchopathia osteochondroplastica in a dog. J Vet Intern Med. 2004;18:359–362. doi: 10.1892/0891-6640(2004)18<359:toiad>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Danckers M, Raad RA, Zamuco R, Pollack A, Rickert S, Caplan-Shaw C. A complication of tracheobronchopathia osteochondroplastica presenting as acute hypercapnic respiratory failure. Am J Case Rep. 2015;16:45–49. doi: 10.12659/AJCR.892427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hantous-Zannad S, Sebai L, Zidi A, Ben Khelil J, Mestiri I, Besbes M, Hamzaoui A, Ben Miled-M’rad K. Tracheobronchopathia osteochondroplastica presenting as a respiratory insufficiency: diagnosis by bronchoscopy and MRI. Eur J Radiol. 2003;45:113–116. doi: 10.1016/s0720-048x(02)00028-1. [DOI] [PubMed] [Google Scholar]
- 10.Meyer CN, Dossing M, Broholm H. Tracheobronchopathia osteochondroplastica. Respir Med. 1997;91:499–502. doi: 10.1016/s0954-6111(97)90117-7. [DOI] [PubMed] [Google Scholar]
- 11.Warner MA, Chestnut DH, Thompson G, Bottcher M, Tobert D, Nofftz M. Tracheobronchopathia osteochondroplastica and difficult intubation: case report and perioperative recommendations for anesthesiologists. J Clin Anesth. 2013;25:659–661. doi: 10.1016/j.jclinane.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Bachy A, Saroul N, Darcha C, Bellini R, Mom T, Gilain L. An unusual cause of tracheal stenosis: diagnosis and management? Tracheopathia osteochondroplastica. Eur Ann Otorhinolaryngol Head Neck Dis. 2012;129:211–213. doi: 10.1016/j.anorl.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Martens PR, Van den Brande FG. Failure of one-lung ventilation because of tracheobronchopathia osteoplastica during a heartport procedure with EZ-Blocker. J Cardiothorac Vasc Anesth. 2012;26:e35. doi: 10.1053/j.jvca.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 14.Hodges MK, Israel E. Tracheobronchopathia osteochondroplastica presenting as right middle lobe collapse. Diagnosis by bronchoscopy and computerized tomography. Chest. 1988;94:842–844. doi: 10.1378/chest.94.4.842. [DOI] [PubMed] [Google Scholar]
- 15.Gautam HP. Tracheopathia osteoplastica. Postgrad Med J. 1968;44:186–189. doi: 10.1136/pgmj.44.508.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ulasli SS, Kupeli E. Tracheobronchopathia osteochondroplastica: A review of the literature. Clin Respir J. 2014 doi: 10.1111/crj.12166. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Thomas D, Stonell C, Hasan K. Tracheobronchopathia osteoplastica: incidental finding at tracheal intubation. Br J Anaesth. 2001;87:515–517. doi: 10.1093/bja/87.3.515. [DOI] [PubMed] [Google Scholar]
- 18.Paaske PB, Tang E. Tracheopathia osteoplastica in the larynx. J Laryngol Otol. 1985;99:305–310. doi: 10.1017/s0022215100096742. [DOI] [PubMed] [Google Scholar]
- 19.Raess PW, Cowan SW, Haas AR, Zhang PJ, Litzky LA, Miller WT Jr, Cooper JD, Deshpande CG. Tracheobronchopathia osteochondroplastica presenting as a single dominant tracheal mass. Ann Diagn Pathol. 2011;15:431–435. doi: 10.1016/j.anndiagpath.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Willms H, Wiechmann V, Sack U, Gillissen A. Tracheobronchopathia osteochondroplastica: A rare cause of chronic cough with haemoptysis. Cough. 2008;4:4. doi: 10.1186/1745-9974-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leske V, Lazor R, Coetmeur D, Crestani B, Chatte G, Cordier JF. Tracheobronchopathia osteochondroplastica: a study of 41 patients. Medicine (Baltimore) 2001;80:378–390. doi: 10.1097/00005792-200111000-00004. [DOI] [PubMed] [Google Scholar]


