Abstract
Synchronous colorectal carcinoma defines as multiple malignant lesions presented in a single patient at initial diagnosis. We report a case of triple synchronous colorectal carcinoma without related familial history. Preoperative computed tomography (CT) scan and endoscopic examination suggested multiple malignant lesions occurred in separate segments of colon. Then we performed laparoscopic total colectomy and ileorectal anastomosis with a J-type pouch. Post operative pathological examination confirmed the malignant characteristics of the triple lesions. The mini review summarizes the clinicopathological and molecular features of synchronous colorectal carcinoma based on current literatures. It appears to probably have significant distinctions with solitary tumors in terms of pathological type, primary locations and microsatellite instability.
Keywords: Synchronous colorectal carcinoma, case report
Introduction
Synchronous colorectal carcinoma refers to more than one primary cancers discovered in a single patient at initial presentation, which is a special and rarer type of colorectal malignancy compared to solitary tumors [1]. Here we report a case of synchronous triple colorectal carcinoma of a middle-aged male patient and present an associated mini review on current research of synchronous colorectal carcinoma.
Case description
A 51-year-old man sought medical consultation in Gastrointestinal Surgery of Union Hospital presented with a three-month history of hematochezia and intermittent abdominal pain. The patient had no family history of cancer or hereditary intestinal disorders. Physical examination revealed a soft tenderness in the lower left quadrant of the abdomen without any mass palpated. Hemorrhoids or anal fissures were not discovered via digital rectal examination. Regular laboratory examinations demonstrated a hemoglobin level of 83 g/L and the occult blood test was positive as well. Tumor markers were in the normal range (CEA 1.5 μg/L). The abdominal plain film revealed flatulence in splenic flexure and descending colon, while sporadic air fluid levels were presented as well (Figure 1A). Further abdominal contrast CT scan identified a mass lesion in ascending and descending colon with associated circumferential thickening and local infiltration (Figure 1B). The electronic colonoscopy (Figure 1C) and endoscopic ultrasonography (EUS) examination (Figure 1D) were partially blocked in descending colon due to the canal stenosis, showing an iso-echoic polypoid lesion in sigmoid colon (28 cm from anal verge) and suspected malignant lesion in descending colon (ulcerative infiltration type, 50 cm from anal verge). Biopsy confirmed both lesions as adenocarcinoma preoperatively (Figure 1E, 1F).
Figure 1.
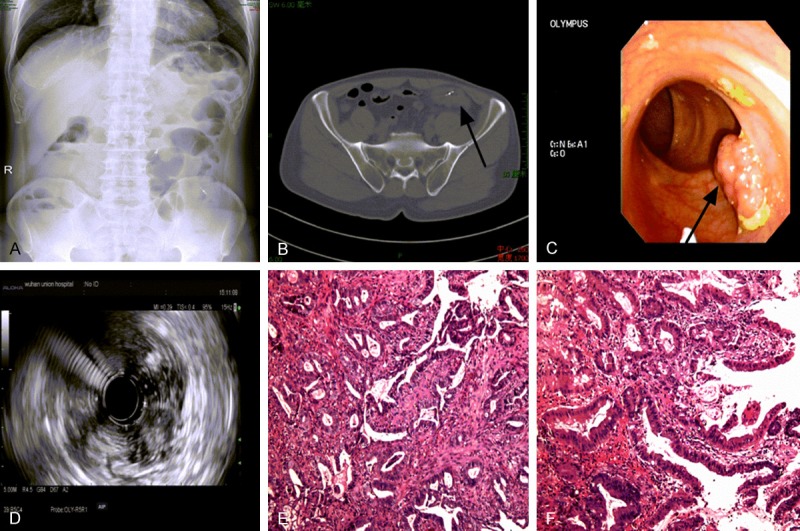
Preoperative examination results of this triple synchronous colorectal carcinoma patient. A. Abdominal plain film presented with severe flatulence and sporadic air fluid levels; B. Abdominal CT scan with a infiltrative mass and circumferential thickening in descending colon (→); C. Colonoscopy showing a polypoid lesion within sigmoid colon; D. Iso-echoic feature of the sigmoid polypoid lesion by EUS examination; E. Biopsy examination revealing adenocarcinoma of the lesion in descending colon; F. Biopsy examination revealing well-differentiated adenocarcinoma of the polypoid lesion in sigmoid colon; Tissue slides: HE×100.
Based on results of preoperative evaluation, the patient underwent total colectomy in a laparoscopic operation. 10 cm of distal ileum, total colon and partial rectum were removed completely with regional lymph nodes dissection. We constructed a J-type pouch with the residual ileum before ileorectal anastomosis to partially replace the stool storage function of colon and rectal ampulla. Postoperative pathological examination confirmed triple synchronous malignant lesions in total colon. Tumor A was a protruding 4.5 cm×4 cm mass in proximal ascending colon (1.5 cm away from ileocecal valve) with moderate differentiation and full thickness infiltration (pT4N0M0). Tumor B was an ulcerative 6 cm×3 cm mass in descending colon (13 cm from distal incisal margin) with moderate differentiation and full thickness infiltration (pT4N0M0). Tumor C was confirmed as a polypoid 1.5 cm×1 cm lesion in sigmoid colon (8 cm from distal incisal margin) with well differentiation and submucosal infiltration (pT1N0M0) (Figure 2). There were no residual tumors and metastasis in incisal margins and regional lymph nodes respectively. Three days later, the patient began liquid diet and the ileal pouch successfully controlled the frequency of defecation to 2-3 times per day after surgery. No severe complications such as anastomotic leakage occurred during hospital stay post-operatively. The TNM clinical grading of this patient was stage II, thus we performed 6 cycles of XELOX regimen as adjuvant therapy and the patient revealed good tolerance and compliance without tumor relapse.
Figure 2.
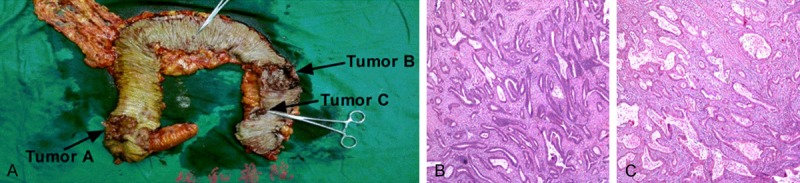
Post operative examination results of this triple synchronous colorectal carcinoma patient. A. Gross morphology of total colon and triple malignant lesions (→); B. A representative image of post operative pathological examination results of tumor A and tumor B (both were moderate differentiated adenocarcinoma); C. A representative image of post operative pathological examination results of tumor C (well differentiated adenocarcinoma); Tissue slides: HE×40.
Discussion
Synchronous colorectal carcinoma: epidemiological, clinical, pathological features and molecular mechanisms.
Synchronous colorectal carcinoma indicates more than one primary malignant lesions discovered in a single patient simultaneously. Once multiple colorectal cancers are detected in different time points, it is identified as metachronous colorectal carcinoma. The initial presence of multiple tumors is the major point of identification as a synchronous cancer [2].
Synchronous colorectal carcinoma accounts for a wide-range 1%-8% of all colorectal cancers by review on different studies [3-5]. Four convincing large sample-size (>800 cases) studies reported a prevalence of 3.1%, 3.7%, 3.9% and 3.5% respectively [3,6-8]. A systemic review reported an overall incidence of 3.5% of synchronous colorectal carcinoma versus all colorectal cancers by pooling data from 39 studies (3667/105686) [2]. These results suggest synchronous cancers as a relative rare entity with overall incidence rate probably below 4%.
Compared to solitary cancers, synchronous colorectal carcinoma presents a higher male to female ratio indeed [6]. An 884-case study reported a ratio of 1.6 in synchronous colorectal carcinoma in contrast to 1.2 of solitary cancer [3]. By pooling data from the majority of related studies, the male to female ratio was 1.7 in total (2797/1588) [2,3]. The origination of sex difference is currently unknown, but sex hormones most likely contribute [9]. Therefore further investigations are needed to explain the even more remarkable male predominance in synchronous colorectal carcinoma.
With respect to age, there is no consensus on its correlation with synchronous colorectal carcinoma occurrence. An earlier literature review reported an average age of 63 years when patients presented with synchronous colorectal carcinoma based on large quantity of pooling data [2]. Recent studies presented a discrepant mean-age 47, 72 and 79 years of synchronous cancer patients respectively [3,10,11]. Mainstream viewpoint supports a higher presentation age of synchronous cancer patients compared to solitary patients. However, there are still reports of synchronous cancer patients diagnosed at a similar mean-age as solitary patients [6]. Hence, more evidence is needed to elucidate the existence and impact of age disparity.
Location preference of synchronous carcinoma is still in controversy. As is reported that primary locations of synchronous colorectal carcinoma differed from solitary cancers with an ascending colon predominance (43% in synchronous carcinoma to 37% in solitary carcinoma) and less frequency of lesions located in sigmoid colon and rectum compares to that of solitary cancers (48% in synchronous carcinoma to 57% in solitary carcinoma) [3]. Nevertheless, some literature reported a sigmoid advantage of synchronous carcinoma against solitary malignancy [4]. In addition, most synchronous carcinoma is believed to occur separately in different portions of large intestine, with exception of small amount of synchronous carcinoma located closely in the same segment of the colon [8,12]. These findings are consistent with the right colon predominance of some susceptible factors.
Patients with possible predisposing factors (inflammatory bowel diseases, familial adenomatous polyposis) are reported to have higher risk of synchronous colorectal carcinoma [13-15]. A case series study of inflammatory bowel disease related colorectal cancer presented a surprising ratio of 20% cases diagnosed with synchronous colorectal carcinoma (22/108), which was much higher compared to sporadic incidence rate 3.5% of synchronous carcinoma [14]. Ulcerative colitis is known to overcome Crohn’s disease significantly in triggering synchronous colorectal carcinoma as well [15]. A study performed by Greenstein and colleagues unveiled that synchronous colorectal carcinoma accounted for 21% of familial adenomatous polyposis related cancer while it merely accounted for 2.5% of de novo colorectal cancer [16]. The reason behind this has been linked to inflammation-induced dysplasia, and adenomas are believed to be more closely involved in development of synchronous tumors especially the serrated sessile adenomas or polyps [4,17]. In spite of the higher risk of patients with predisposing factors to develop into synchronous colorectal carcinoma, a large-scale study revealed that patients with predisposing factors accounted for only 10% among all synchronous colorectal carcinoma cases [4], suggesting further unknown mechanisms or factors besides predisposing conditions may involve in the development of synchronous colorectal carcinoma.
Whether synchronous colorectal carcinoma possesses specific pathological features has not reached a consensus yet. A retrospective study concluded mucinous adenocarcinoma was slightly more common observed in synchronous colorectal carcinoma than solitary cancers by cases analysis [18]. This result seems rational since mucinous adenocarcinoma is a pathological trait of hereditary nonpolyposis colorectal cancer, which is a common predisposing factor of synchronous cancers. Nevertheless, a convincing series (158 cases) summarized to an opposite result that metachronous cancer but not synchronous carcinoma appeared to have higher ratio of mucinous adenocarcinoma in post-operative pathology examination [19].
More than one primary lesions diagnosed simultaneously is defined as synchronous colorectal carcinoma. Most of the published cases reported only two carcinomas in the large intestine. Patients with three or more primary lesions accounted for 1.8% to 16.7% among all synchronous colorectal carcinoma cases [6,20]. Up to now, the maximum of tumor count discovered and reported in a single patient is six lesions [21]. Moreover, synchronous colorectal carcinoma is found to display smaller size and lower pathological grading and T staging than standard colorectal cancers [15].
The majority of mechanism studies of synchronous colorectal carcinoma blame it on microsatellite instability (MSI) of the genome [22,23]. Synchronous colorectal carcinoma has a higher proportion of MSI-positive cancers than solitary cancers. There are two responsible mechanisms to generate microsatellite instability in synchronous cancers: 1. With respect to most inherited cases, the hereditary mutation on mismatch repair genes disables the correction process against errors on microsatellite repetition region during DNA replication, resulting in microsatellite instability; 2. In sporadic synchronous cancer patients, methylation of mismatch repair genes mainly results in MSI instead of hereditary factors. Particularly, BRAF related methylation on MLH1 promoters is a strongly indicator for sporadic cases. That is the reason why BRAF is a recommended gene to examine on synchronous colorectal carcinoma patient. The methylation of certain genes may be due to the regional carcinogenic environment of colon and rectum [24-26]. Apart from microsatellite instability, mutations on KRAS and GSRM1 are linked to synchronous cancer as well, revealing a complex mechanism network of carcinogenesis on synchronous colorectal carcinoma [27].
Surgical decision is quite difficult to make since the complex clinical features of synchronous carcinoma and individual differences must be taken into account. Pre-operative examination is necessary for accurate surgical decision. Colonoscopy (including EUS) is an essential approach for the appraisal of tumors count as well as locations and taking a biopsy meanwhile. CT scan is commonly used to make assisted assessment in case of the malignant stenosis and has an advantage on local infiltration evaluation over endoscopy. Even with these measures, there are still some lesions failed to identify preoperatively due to their tiny sizes.
In terms of surgical procedures, a retrospective large scale cohort study represented by van Leersum and colleagues reported that patients with synchronous colorectal carcinoma were more likely to receive deviating or permanent stoma (37% versus 33%) and less likely to undergo a laparoscopic operation compared to solitary cancer patients [3]. The reason may be due to the difficulty in dealing with extensive resection range and more concerns on anastomotic leakage than managing solitary regional cancers. Early-stage lesions can be removed under colonoscopy. Patients with tumors located in the adjacent segment are recommended for a hemicolectomy, otherwise a sub-total or total colectomy is preferred.
In summary, synchronous colorectal carcinoma is a unique subtype of colorectal cancer and shows great disparity against solitary tumors with all probable clinical and molecular implications confirmed by current studies. However, it is premature to make conclusions with those controversies unsolved and further researches are needed to finally judge.
Acknowledgements
We sincerely thank all pretty nurses in our department to provide considerate nursing service during hospital stay, which is the basis of rapid recovery on this special patient. This article has not been published yet and is not considered for publication by other journals at this time. Written informed consent has been given by this patient.
Disclosure of conflict of interest
None.
References
- 1.Yeh CC, Hsi SC, Chuu CP, Kao YH. Synchronous triple carcinoma of the colon and rectum. World J Surg Oncol. 2013;11:66. doi: 10.1186/1477-7819-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lam AK, Chan SS, Leung M. Synchronous colorectal cancer: clinical, pathological and molecular implications. World J Gastroenterol. 2014;20:6815–6820. doi: 10.3748/wjg.v20.i22.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Leersum NJ, Aalbers AG, Snijders HS, Henneman D, Wouters MW, Tollenaar RA, Eddes EH. Synchronous colorectal carcinoma: a risk factor in colorectal cancer surgery. Dis Colon Rectum. 2014;57:460–466. doi: 10.1097/DCR.0000000000000068. [DOI] [PubMed] [Google Scholar]
- 4.Lam AK, Carmichael R, Gertraud BP, Gopalan V, Ho YH, Siu S. Clinicopathological significance of synchronous carcinoma in colorectal cancer. Am J Surg. 2011;202:39–44. doi: 10.1016/j.amjsurg.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Box JC, Rodriguez-Bigas MA, Weber TK, Petrelli NJ. Clinical implications of multiple colorectal carcinomas in hereditary nonpolyposis colorectal carcinoma. Dis Colon Rectum. 1999;42:717–721. doi: 10.1007/BF02236924. [DOI] [PubMed] [Google Scholar]
- 6.Latournerie M, Jooste V, Cottet V, Lepage C, Faivre J, Bouvier AM. Epidemiology and prognosis of synchronous colorectal cancers. Br J Surg. 2008;95:1528–1533. doi: 10.1002/bjs.6382. [DOI] [PubMed] [Google Scholar]
- 7.Mulder SA, Kranse R, Damhuis RA, de Wilt JH, Ouwendijk RJ, Kuipers EJ, van Leerdam ME. Prevalence and prognosis of synchronous colorectal cancer: a Dutch population-based study. Cancer Epidemiol. 2011;35:442–447. doi: 10.1016/j.canep.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Kaibara N, Koga S, Jinnai D. Synchronous and metachronous malignancies of the colon and rectum in Japan with special reference to a coexisting early cancer. Cancer. 1984;54:1870–1874. doi: 10.1002/1097-0142(19841101)54:9<1870::aid-cncr2820540917>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 9.Van Leersum NJ, Snijders HS, Henneman D, Kolfschoten NE, Gooiker GA, ten Berge MG, Eddes EH, Wouters MW, Tollenaar RA Dutch Surgical Colorectal Cancer Audit Group. Bemelman WA, van Dam RM, Elferink MA, Karsten TM, van Krieken JH, Lemmens VE, Rutten HJ, Manusama ER, van de Velde CJ, Meijerink WJ, Wiggers T, van der Harst E, Dekker JW, Boerma D. The Dutch surgical colorectal audit. Eur J Surg Oncol. 2013;39:1063–1070. doi: 10.1016/j.ejso.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Wang HZ, Huang XF, Wang Y, Ji JF, Gu J. Clinical features, diagnosis, treatment and prognosis of multiple primary colorectal carcinoma. World J Gastroenterol. 2004;10:2136–2139. doi: 10.3748/wjg.v10.i14.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norrie MW, Hawkins NJ, Todd AV, Meagher AP, O’Connor TW, Ward RL. The role of hMLH1 methylation in the development of synchronous sporadic colorectal carcinomas. Dis Colon Rectum. 2002;45:674–680. doi: 10.1007/s10350-004-6266-1. [DOI] [PubMed] [Google Scholar]
- 12.Eu KW, Seow-Choen F, Goh HS. Synchronous colorectal cancer in an Oriental population. Int J Colorectal Dis. 1993;8:193–196. doi: 10.1007/BF00290304. [DOI] [PubMed] [Google Scholar]
- 13.Greenstein AJ, Slater G, Heimann TM, Sachar DB, Aufses AJ. A comparison of multiple synchronous colorectal cancer in ulcerative colitis, familial polyposis coli, and de novo cancer. Ann Surg. 1986;203:123–128. doi: 10.1097/00000658-198602000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu X, Goldblum JR, Zhao Z, Landau M, Heald B, Pai R, Lin J. Distinct clinicohistologic features of inflammatory bowel disease-associated colorectal adenocarcinoma: in comparison with sporadic microsatellite-stable and Lynch syndrome-related colorectal adenocarcinoma. Am J Surg Pathol. 2012;36:1228–1233. doi: 10.1097/PAS.0b013e318253645a. [DOI] [PubMed] [Google Scholar]
- 15.Kiran RP, Khoury W, Church JM, Lavery IC, Fazio VW, Remzi FH. Colorectal cancer complicating inflammatory bowel disease: similarities and differences between Crohn’s and ulcerative colitis based on three decades of experience. Ann Surg. 2010;252:330–335. doi: 10.1097/SLA.0b013e3181e61e69. [DOI] [PubMed] [Google Scholar]
- 16.Fante R, Roncucci L, Di Gregorio C, Tamassia MG, Losi L, Benatti P, Pedroni M, Percesepe A, De Pietri S, Ponz de Leon M. Frequency and clinical features of multiple tumors of the large bowel in the general population and in patients with hereditary colorectal carcinoma. Cancer. 1996;77:2013–2021. doi: 10.1002/(SICI)1097-0142(19960515)77:10<2013::AID-CNCR8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 17.Mohammadi M, Kristensen MH, Nielsen HJ, Bonde JH, Holck S. Qualities of sessile serrated adenoma/polyp/lesion and its borderline variant in the context of synchronous colorectal carcinoma. J Clin Pathol. 2012;65:924–927. doi: 10.1136/jclinpath-2012-200803. [DOI] [PubMed] [Google Scholar]
- 18.Adloff M, Arnaud JP, Bergamaschi R, Schloegel M. Synchronous carcinoma of the colon and rectum: prognostic and therapeutic implications. Am J Surg. 1989;157:299–302. doi: 10.1016/0002-9610(89)90555-2. [DOI] [PubMed] [Google Scholar]
- 19.King-Yin LA, Ong K, Ho YH. Colorectal mucinous adenocarcinoma: the clinicopathologic features and significance of p16 and p53 expression. Dis Colon Rectum. 2006;49:1275–1283. doi: 10.1007/s10350-006-0650-y. [DOI] [PubMed] [Google Scholar]
- 20.Kimura T, Iwagaki H, Fuchimoto S, Hizuta A, Orita K. Synchronous colorectal carcinomas. Hepatogastroenterology. 1994;41:409–412. [PubMed] [Google Scholar]
- 21.Fegiz G, Ramacciato G, Indinnimeo M, Gozzo P, Valabrega S, De Angelis R, Barillari P. Synchronous large bowel cancer: a series of 47 cases. Ital J Surg Sci. 1989;19:23–28. [PubMed] [Google Scholar]
- 22.Aslanian HR, Burgart LJ, Harrington JJ, Mahoney DW, Zinsmeister AR, Thibodeau SN, Ahlquist DA. Altered DNA mismatch repair expression in synchronous and metachronous colorectal cancers. Clin Gastroenterol Hepatol. 2008;6:1385–1388. doi: 10.1016/j.cgh.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 23.Nosho K, Kure S, Irahara N, Shima K, Baba Y, Spiegelman D, Meyerhardt JA, Giovannucci EL, Fuchs CS, Ogino S. A prospective cohort study shows unique epigenetic, genetic, and prognostic features of synchronous colorectal cancers. Gastroenterology. 2009;137:1609–1620. doi: 10.1053/j.gastro.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pakneshan S, Salajegheh A, Smith RA, Lam AK. Clinicopathological relevance of BRAF mutations in human cancer. Pathology. 2013;45:346–356. doi: 10.1097/PAT.0b013e328360b61d. [DOI] [PubMed] [Google Scholar]
- 25.Pritchard CC, Grady WM. Colorectal cancer molecular biology moves into clinical practice. Gut. 2011;60:116–129. doi: 10.1136/gut.2009.206250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogino S, Goel A. Molecular classification and correlates in colorectal cancer. J Mol Diagn. 2008;10:13–27. doi: 10.2353/jmoldx.2008.070082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ueda E, Watanabe T, Umetani N, Ishigami H, Sasaki S, Nagawa H. Microsatellite instability of cancers and concomitant adenomas in synchronous multiple colorectal cancer patients. J Exp Clin Cancer Res. 2002;21:149–154. [PubMed] [Google Scholar]


