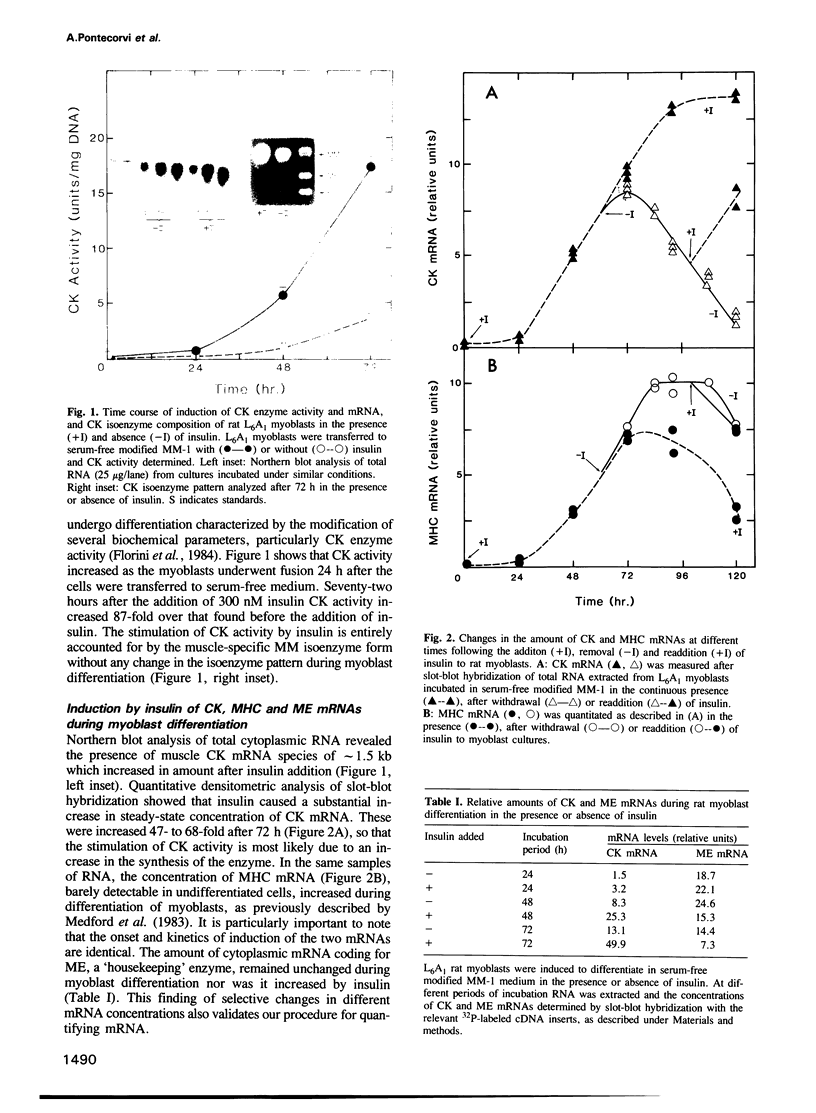
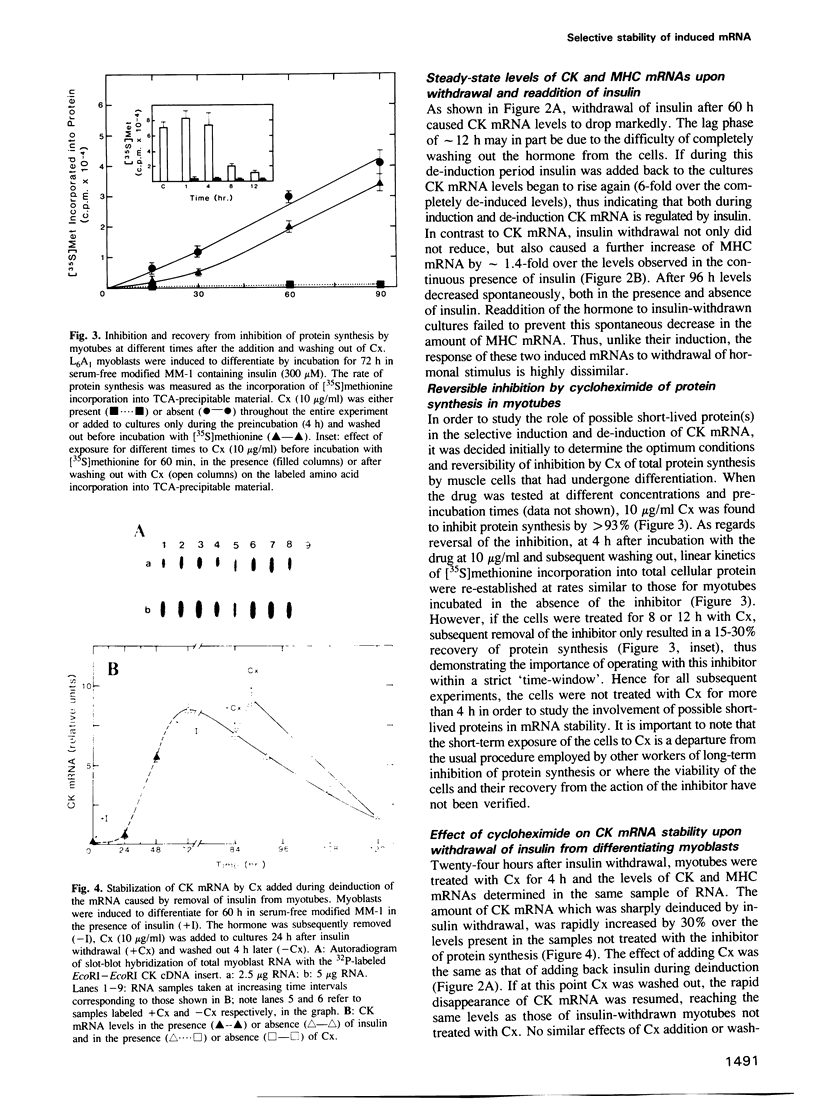
Abstract
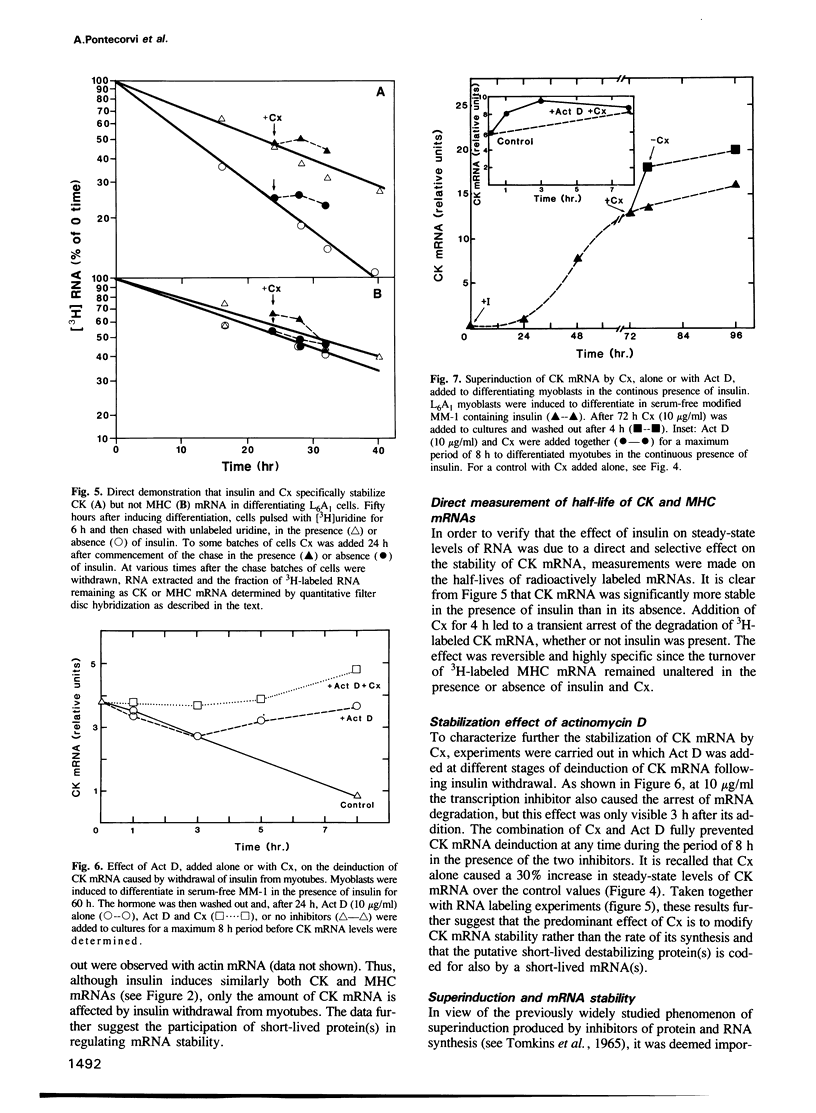
This investigation concerns the combined effects of removal and readdition of insulin and inhibition of protein and RNA synthesis on the stability of insulin-induced mRNAs during and after differentiation of rat L6A1 myoblast cells in culture. Addition of insulin accompanying the withdrawal of the mitogenic stimulus of serum to myoblasts caused an 80-fold increase in creatine phosphokinase (CK) activity which was largely accounted for by a similar increase in the amount of CK mRNA. The latter was co-ordinately induced with myosin heavy chain (MHC) mRNA but not malic enzyme (ME) mRNA. Measurements of steady-state levels of mRNA showed that removal of insulin caused CK mRNA, but not MHC mRNA, to be rapidly degraded, the effect being reversed upon readdition of the hormone. Direct measurement of 3H-labeled CK, MHC and beta-actin mRNAs confirmed the selective stabilization and destabilization of CK mRNA by the hormone. Conditions were established for a time-window during which cycloheximide (Cx) produced a virtually total arrest of protein synthesis in myotubes that was reversible upon removal of the inhibitor. Under these conditions, Cx selectively prevented the degradation of CK mRNA in a reversible manner. Actinomycin D (Act D) also arrested the loss of this mRNA. Under the same conditions of mRNA stabilization during de-induction, a superinduction of CK mRNA, but not MHC mRNA, was observed if the two inhibitors were added during induction in the continuous presence of insulin. We conclude that a short-lived protein(s), encoded by a short-lived mRNA(s), selectively regulates the stability of reversibly inducible mRNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker E. J., Keller L. R., Schloss J. A., Rosenbaum J. L. Protein synthesis is required for rapid degradation of tubulin mRNA and other deflagellation-induced RNAs in Chlamydomonas reinhardi. Mol Cell Biol. 1986 Jan;6(1):54–61. doi: 10.1128/mcb.6.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayard B., Bisbal C., Lebleu B. Activation of ribonuclease L by (2'-5')(A)4-poly(L-lysine) conjugates in intact cells. Biochemistry. 1986 Jun 17;25(12):3730–3736. doi: 10.1021/bi00360a038. [DOI] [PubMed] [Google Scholar]
- Benfield P. A., Zivin R. A., Miller L. S., Sowder R., Smythers G. W., Henderson L., Oroszlan S., Pearson M. L. Isolation and sequence analysis of cDNA clones coding for rat skeletal muscle creatine kinase. J Biol Chem. 1984 Dec 10;259(23):14979–14984. [PubMed] [Google Scholar]
- Brawerman G. Determinants of messenger RNA stability. Cell. 1987 Jan 16;48(1):5–6. doi: 10.1016/0092-8674(87)90346-1. [DOI] [PubMed] [Google Scholar]
- Caput D., Beutler B., Hartog K., Thayer R., Brown-Shimer S., Cerami A. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caravatti M., Minty A., Robert B., Montarras D., Weydert A., Cohen A., Daubas P., Buckingham M. Regulation of muscle gene expression. The accumulation of messenger RNAs coding for muscle-specific proteins during myogenesis in a mouse cell line. J Mol Biol. 1982 Sep;160(1):59–76. doi: 10.1016/0022-2836(82)90131-0. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. S., Jaynes J. B., Hauschka S. D. Regulation of creatine kinase induction in differentiating mouse myoblasts. Mol Cell Biol. 1985 Mar;5(3):484–492. doi: 10.1128/mcb.5.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clemens M. J. A potential role for RNA transcribed from B2 repeats in the regulation of mRNA stability. Cell. 1987 Apr 24;49(2):157–158. doi: 10.1016/0092-8674(87)90553-8. [DOI] [PubMed] [Google Scholar]
- Colletta G., Cirafici A. M., Vecchio G. Induction of the c-fos oncogene by thyrotropic hormone in rat thyroid cells in culture. Science. 1986 Jul 25;233(4762):458–460. doi: 10.1126/science.3726540. [DOI] [PubMed] [Google Scholar]
- Csako G., Papadopoulos N. M., Jett G. K., McIntosh C. L. Five creatine kinase isoenzymes in serum of a patient with severe heart disease. Clin Chem. 1982 Oct;28(10):2170–2172. [PubMed] [Google Scholar]
- Devlin R. B., Emerson C. P., Jr Coordinate accumulation of contractile protein mRNAs during myoblast differentiation. Dev Biol. 1979 Mar;69(1):202–216. doi: 10.1016/0012-1606(79)90286-0. [DOI] [PubMed] [Google Scholar]
- Dym H., Turner D. C., Eppenberger H. M., Yaffe D. Creatine kinase isoenzyme transition in actinomycin D-treated differentiating muscle cultures. Exp Cell Res. 1978 Apr;113(1):15–21. doi: 10.1016/0014-4827(78)90082-4. [DOI] [PubMed] [Google Scholar]
- Ewton D. Z., Florini J. R. Effects of the somatomedins and insulin on myoblast differentiation in vitro. Dev Biol. 1981 Aug;86(1):31–39. doi: 10.1016/0012-1606(81)90312-2. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Florini J. R., Ewton D. Z., Evinger-Hodges M. J., Falen S. L., Lau R. L., Regan J. F., Vertel B. M. Stimulation and inhibition of myoblast differentiation by hormones. In Vitro. 1984 Dec;20(12):942–958. doi: 10.1007/BF02619668. [DOI] [PubMed] [Google Scholar]
- Florini J. R., Roberts S. B. A serum-free medium for the growth of muscle cells in culture. In Vitro. 1979 Dec;15(12):983–992. doi: 10.1007/BF02619157. [DOI] [PubMed] [Google Scholar]
- Guyette W. A., Matusik R. J., Rosen J. M. Prolactin-mediated transcriptional and post-transcriptional control of casein gene expression. Cell. 1979 Aug;17(4):1013–1023. doi: 10.1016/0092-8674(79)90340-4. [DOI] [PubMed] [Google Scholar]
- Hashimoto C., Steitz J. A. A small nuclear ribonucleoprotein associates with the AAUAAA polyadenylation signal in vitro. Cell. 1986 May 23;45(4):581–591. doi: 10.1016/0092-8674(86)90290-4. [DOI] [PubMed] [Google Scholar]
- Kerr I. M., Cayley P. J., Silverman R. H., Knight M. The antiviral action of interferon. Philos Trans R Soc Lond B Biol Sci. 1982 Sep 24;299(1094):59–67. doi: 10.1098/rstb.1982.0106. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Magnuson M. A., Nikodem V. M. Molecular cloning of a cDNA sequence for rat malic enzyme. Direct evidence for induction in vivo of rat liver malic enzyme mRNA by thyroid hormone. J Biol Chem. 1983 Oct 25;258(20):12712–12717. [PubMed] [Google Scholar]
- Medford R. M., Nguyen H. T., Nadal-Ginard B. Transcriptional and cell cycle-mediated regulation of myosin heavy chain gene expression during muscle cell differentiation. J Biol Chem. 1983 Sep 25;258(18):11063–11073. [PubMed] [Google Scholar]
- Medford R. M., Wydro R. M., Nguyen H. T., Nadal-Ginard B. Cytoplasmic processing of myosin heavy chain messenger RNA: evidence provided by using a recombinant DNA plasmid. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5749–5753. doi: 10.1073/pnas.77.10.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell R. L., Henning-Chubb C., Huberman E., Verma I. M. c-fos expression is neither sufficient nor obligatory for differentiation of monomyelocytes to macrophages. Cell. 1986 May 23;45(4):497–504. doi: 10.1016/0092-8674(86)90281-3. [DOI] [PubMed] [Google Scholar]
- Nadal-Ginard B. Commitment, fusion and biochemical differentiation of a myogenic cell line in the absence of DNA synthesis. Cell. 1978 Nov;15(3):855–864. doi: 10.1016/0092-8674(78)90270-2. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Quantitation of parameters that determine the rate of ovalbumin synthesis. Cell. 1975 Mar;4(3):189–189. doi: 10.1016/0092-8674(75)90167-1. [DOI] [PubMed] [Google Scholar]
- Ralhan R., Johnson G. S. Destabilization of cytoplasmic mouse mammary tumor RNA by heat shock: prevention by cycloheximide pretreatment. Biochem Biophys Res Commun. 1986 Jun 30;137(3):1028–1033. doi: 10.1016/0006-291x(86)90328-1. [DOI] [PubMed] [Google Scholar]
- Reeves R., Elton T. S., Nissen M. S., Lehn D., Johnson K. R. Posttranscriptional gene regulation and specific binding of the nonhistone protein HMG-I by the 3' untranslated region of bovine interleukin 2 cDNA. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6531–6535. doi: 10.1073/pnas.84.18.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Shainberg A., Yagil G., Yaffe D. Alterations of enzymatic activities during muscle differentiation in vitro. Dev Biol. 1971 May;25(1):1–29. doi: 10.1016/0012-1606(71)90017-0. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steinberg R. A., Levinson B. B., Tomkins G. M. "Superinduction" of tyrosine aminotransferase by actinomycin D: a reevaluation. Cell. 1975 May;5(1):29–35. doi: 10.1016/0092-8674(75)90088-4. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widelitz R. B., Magun B. E., Gerner E. W. Effects of cycloheximide on thermotolerance expression, heat shock protein synthesis, and heat shock protein mRNA accumulation in rat fibroblasts. Mol Cell Biol. 1986 Apr;6(4):1088–1094. doi: 10.1128/mcb.6.4.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffe A. P., Glover J. F., Martin S. C., Tenniswood M. P., Williams J. L., Tata J. R. Deinduction of transcription of Xenopus 74-kDa albumin genes and destabilization of mRNA by estrogen in vivo and in hepatocyte cultures. Eur J Biochem. 1985 Feb 1;146(3):489–496. doi: 10.1111/j.1432-1033.1985.tb08678.x. [DOI] [PubMed] [Google Scholar]