Abstract
Background:
Urinary tract infection (UTI) is deemed the most prevalent infectious disease in that it has now touched the overall incidence of 18/1000 persons per year in the general population.
Objectives:
This study sought to determine the characteristics of isolates from patients with UTI and their susceptibility to commonly used antibiotics in Punjab, Pakistan.
Patients and Methods:
Totally, 1429 urine samples were analyzed from UTI patients for the isolation of uropathogens at Chughtai’s Lahore Lab, Lahore, Pakistan, during a period of 14 months. The antimicrobial susceptibility test was performed via the disc diffusion method for the isolates obtained from 392 (26%) positive cultures.
Results:
The highest percentage (67%) of isolates was from females in comparison to males (33%). The frequency of Escherichia coli was the highest (62%) in culture-positive urine samples, followed by E. faecalis (15%), Candida (14%), Pseudomonas (6%), Klebsiella spp. (1%), Proteus (1%), and Staphylococcus aureus (1%). E. coli was highly resistant to antimicrobial drugs, viz. cephalexin (95%), cephradine (95%), pipemidic acid (92%), amikacin (91%), and nalidixic acid (91%). Most of the routine β-lactam antibiotics like amoxicillin/clavulanic acid, ampicillin, and aztreonam were also ineffective against E. coli, with resistance rates of 84%, 84%, and 72%, correspondingly. This pathogen showed maximum susceptibility (97%) against three drugs, namely imipenem, meropenem, and cefoperazone. Piperacillin and fosfomycin also provided significant results against E. coli with respective susceptibility rates of 96% and 90%.
Conclusions:
Our results showed that broad-spectrum antibiotics such as imipenem, meropenem, fosfomycin, cefoperazone/sulbactam, and vancomycin would be the first line and the most effective drugs for the empirical treatment of urinary tract pathogens due to their higher resistance rates against other drugs like cephalexin, cephradine, ciprofloxacin, levofloxacin, and norfloxacin.
Keywords: Urinary Tract Infections, Escherichia coli, Antimicrobial Drugs
1. Background
Urinary tract infection (UTI) is considered the most prevalent infectious disease insofar as it has reached the overall incidence of 18/1000 persons per year in the general population (1, 2). UTI affects both sexes, irrespective of their age, although it is more common in females (3, 4) due to anatomical predisposition or large bacterial load in urothelial mucosa or other host factors (5) including obstruction in the urinary tract, sexual activity, and pregnancy (6). One out of every two females contracts UTI at least once in her life (7). Diverse groups of uropathogens (gram-positive and negative) are involved in the etiology of UTI, but the Gram-negative facultative anaerobe and uropathogenic Escherichia coli is responsible for the majority of the infections in the general population (8, 9). It is estimated that 80% of UTI cases in healthy women aged 18-39 years are caused by E. coli, followed by Staphylococcus saprophyticus (15 - 20%) (10). Other probably less common uropathogens include Klebsiella, Enterobacter, Serratia, Proteus, Pseudomonas, and Enterococcus (11).
In the treatment of UTI, mostly empirical antibiotic therapy is used by clinicians; thus, frequent misuse of antibiotics may increase resistance in uropathogens (12, 13). The gradual increasing antimicrobial resistance among uropathogens has become a challenge worldwide (14). The antibiotic susceptibility of bacterial strains isolated from urine cultures decides the best choice of antimicrobial drugs for the treatment and prophylaxis of UTI. Prompt diagnosis and timely antimicrobial treatment help to minimize renal scarring and progressive kidney damage (15, 16).
2. Objectives
The present study was designed to investigate antibiotic susceptibility and resistant pattern in isolates obtained from UTI patients. This would be helpful to clinicians to make new choices of antibiotic therapy for the management of UTI.
3. Patients and Methods
3.1. Isolation
During the period of December 2012 to January 2014, a total of 1429 urine samples were collected from UTI patients in Chughtais’ Lahore Lab, Lahore, Pakistan. Sterile plastic containers were used to collect mid-stream urine samples, and the samples were immediately processed for different procedures such as Gram staining, culture, and antibiotic susceptibility testing.
3.2. Identification of Microorganisms
The urine samples were streaked on cysteine lactose electrolyte deficient (CLED) media (Oxoid, England) for the isolation of uropathogens and incubated at 37°C for 24 hours. Urine volume (0.01 mL) (loopful) was inoculated with a sterile calibrated wire loop and was used for the colony count of the isolates. Bacterial growth was reflected significant as per Kass count (single species count > 105 organisms/mL of urine) (17). The colonies were biochemically characterized according to the guidelines (18). The isolates were subcultured on the MacConkey agar (Oxoid, England) and incubated at 37°C for 24 hours in order to obtain pure growth. Standardized identification (API 20 E System) (BioMérieux, France) was used to identify and confirm the groups of different isolates.
3.3. Antibiotic Susceptibility
The antibiotic susceptibility pattern of the isolates was determined by the Kirby-Bauer disk diffusion method. Plates of Müller-Hinton agar were used to find the sensitivity pattern and incubated at 37°C for 24 hours. The zone of the inhibition of the bacterial growth was measured after incubation and compared with the clinical and laboratory standards institute (CLSI, 2013) guidelines. Isolates with intermediate susceptibility were considered as resistant. E. coli (ATCC® 25922), Pseudomonas aeruginosa (ATCC® 27853), E. faecalis (ATCC2® 9212), and S. aureus (ATCC® 29213) were used as reference strains according to the CLSI protocol. A total of 392 different gram-negative and gram-positive isolates were subjected to sensitivity testing for 38 different antibiotics, viz. ampicillin (30 µg), amoxicillin (25 µg), amoxicillin/clavulanic acid (30 µg), ampicillin/sulbactam (30 µg), cefepime (30 µg), cefotaxime (30 µg), cefoxitin (30 µg), cefuroxime (30 µg), ceftazidime (30 µg), ceftriaxone (30 µg), cephalexin (30 µg), cefradine (30 µg), cefaclor (30 µg), cefixime (30 µg), imipenem (10 µg), meropenem (10 µg), vancomycin (30 µg), amikacin (30 µg), gentamycin (30 µg), tobramycin (30 µg), azithromycin (30 µg), erythromycin (30 µg), doxycycline (30 µg), ciprofloxacin (10 µg), levofloxacin (10 µg), norfloxacin (10 µg), nitrofurantoin (300 µg), fosfomycin (50 µg), clindamycin (10 µg), fusidic acid (50 µg), linezolid (30 µg), cefoperazone (30 µg), aztreonam (30 µg), nalidixic acid (30 µg), ofloxacin (5 µg), moxifloxacin (5 µg), trimethoprim/sulfamethoxazole (25 µg), pipemidic acid (20 µg), cefoperazone/sulbactam (30 µg), and piperacillin/tazobactam (110 µg). These antibiotics are routinely prescribed for UTI patients by the clinicians in the Punjab region.
The detection of extended-spectrum beta-lactamases (ESBL) production was carried out using the cephalosporin/clavulanic acid combination discs method. This test was performed on Müller-Hinton Agar via the disc diffusion method as suggested by the CLSI. The organism was considered as ESBL producing if the zone diameter was ≥ 5 mm for ceftazidime (30 μg) tested in combination with clavulanic acid versus its zone diameter when tested alone.
4. Results
In this study a total of 1429 urine samples were analyzed. Significant bacteriuria was observed in 392 (27.4%) samples, while the remaining 1037 samples demonstrated either nonsignificant bacteriuria, very low bacterial count, or were sterile. Among all the patients, 62.6% were female and the remaining 37.4% were male. Out of the 392 UTI patients with significant bacteriuria, 263 (67%) were female and 129 (33%) male. Overall, 244/392 (64%) samples were positive for the presence of E. coli: 163 (67%) samples in the females and 81 (33%) in the males. The presence of the Gram-negative and Gram-positive uropathogens among the UTI patients is depicted in Table 1. Isolates other than E. coli were Klebsiella spp., which was isolated from 3 (60%) females and 2 (40%) males; Proteus spp. in 3 (100%) females only; Pseudomonas in 15 (65%) females and 8 (35%) males; S. aureus in 2 (67%) females and 1 (33%) male; E. faecalis in 42 (72%) females and 16 (28%) males; and Candida in 35 (63%) females and 21 (38%) males.
Table 1. Gender-Wise Frequency of Microorganisms Isolated from the Urine Specimens a.
| Bacteria | Female | Male | Total |
|---|---|---|---|
| Gram-negative Bacilli | |||
| Escherichia coli | 163 (67) | 81 (33) | 244 (62) |
| Klebsiella spp. | 3 (60) | 2 (40) | 5 (1) |
| Proteus | 3 (100) | 0 (0) | 3 (1) |
| Pseudomonas | 15 (65) | 8 (35) | 23 (6) |
| Gram-positive Cocci | |||
| Staphylococcus aureus | 2 (67) | 1 (33) | 3 (1) |
| Enterococcus faecalis | 42 (72) | 16 (28) | 58 (15) |
| Candida | 35 (63) | 21 (38) | 56 (14) |
| Total | 263 (67) | 129 (33) | 392 (100) |
a Data are presented as No. (%).
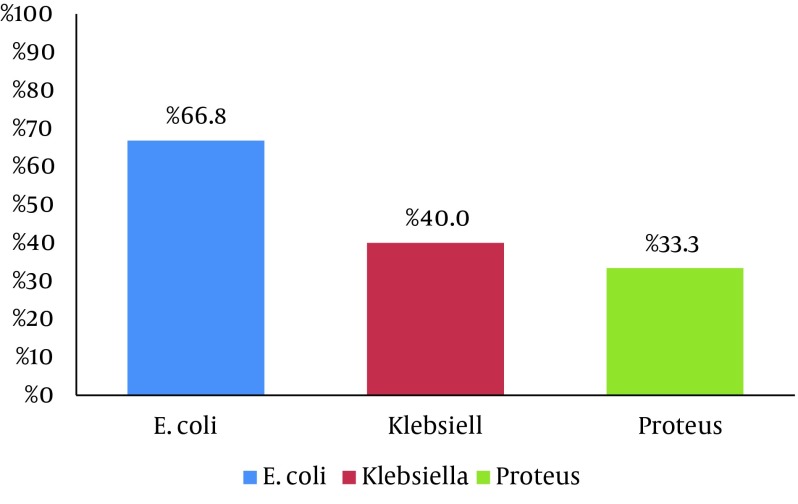
The overall number of the ESBL-producing organisms was 166/252 (65.9%). The frequency of ESBL positivity within the individual organism group was the highest amongst E coli (66.8%), followed by Klebsiella spp. (40%) and Proteus mirabilis (33.3%), as is shown in Figure 1. The isolates exhibited variation in their resistant patterns. The E. coli isolates were resistant to cephalexin (95%), cephradine (95%), pipemidic acid (92%), amikacin (91%), and nalidixic acid (91%) and had the highest sensitivities for imipenem (97%), meropenem (97%), cefoperazone (97%), and tazobactam (94%). Klebsiella spp. had a pattern of microbial resistance, viz. cephalexin (100%) and cephradine (100%), with high sensitivity (100%) for nitrofurantoin. Proteus spp. showed maximum resistance (100%) against 12/31 different antibiotics. The most sensitive antibiotics were imipenem, meropenem, cefoperazone, and tazobactam, as is illustrated in Table 2. The Pseudomonas isolates exhibited the least susceptibility (43%) to ciprofloxacin, levofloxacin, norfloxacin, ofloxacin, and moxifloxacin and the highest susceptibility to tazobactam, imipenem, meropenem, Amikacin and aztreonam (87%, 78%, 78%, 78%, and 70%, respectively).
Figure 1. Percentage Frequency of Extended-Spectrum Beta-Lactamases (ESBL) Among the Gram-Negative Urinary Isolates.
Table 2. Prevalence and Percentage of Antimicrobial Resistance among the Gram-Negative Isolates a.
| No. | AMC | SAM | FEP | CFP | CTX | CXM | CAZ | CRO | CL | CE | CEC | CFM | IPM | MEM | ATM | AK | CN | TOB | DO | NA | CIP | LEV | NOR | OFX | MOX | SXT | F | PIP | SCF | TZP | FOS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli | 244 | 84 | 84 | 71 | 72 | 72 | 80 | 71 | 71 | 95 | 95 | 79 | 71 | 3 | 3 | 72 | 91 | 47 | 59 | 81 | 91 | 82 | 82 | 81 | 82 | 82 | 78 | 20 | 92 | 3 | 4 | 10 |
| Klebsiella spp. | 5 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 100 | 100 | 40 | 40 | 20 | 20 | 40 | 20 | 40 | 40 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 40 | 0 | 20 | 20 | 20 | 40 |
| Proteus spp. | 3 | 67 | 67 | 67 | 67 | 67 | 67 | 67 | 67 | 100 | 100 | 67 | 67 | 0 | 0 | 67 | 33 | 67 | 67 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 0 | 0 | 67 |
| Pseudomonas spp. | 23 | NT | NT | 39 | NT | NT | NT | 48 | NT | NT | NT | NT | NT | 22 | 22 | 30 | 22 | 43 | NT | NT | NT | 57 | 57 | 57 | 57 | 57 | NT | NT | NT | NT | 13 | NT |
a Abbreviations: AMC, Amoxicillin/clavulanic acid; AK, Amikacin; ATM, Aztreonam; CAZ, Ceftazidime; CE, Cephradine; CEC, Cefaclor; CFP, Cefoperazone; CFM, Cefixime; CL, Cephalexin; CN, Gentamicin; CIP, Ciprofloxacin; CRO, Ceftriaxone; CTX, Cefotaxime; CXM, Cefuroxime; DO, Doxycycline; FEP, Cefepime; FOS, Fosfomycin; IPM, Imipenem; LEV, Levofloxacin; MEM, Meropenem; MOX, Moxifloxacin; NA, Nalidixic acid; NOR, Norfloxacin; NT, Not Tested; OFX, Ofloxacin; PIP, Pipemidic acid; SAM, Ampicillin/sulbactam; SCF, Cefoperazone/sulbactam; SXT, Trimethoprim/sulfamethoxazole F Nitrofurantoin; TOB, Tobramycin; TZP, Piperacillin/tazobactam.
Among the Gram-positive isolates, S. aureus exhibited utmost resistance (67%) against ampicillin, amoxicillin, tobramycin, ciprofloxacin, levofloxacin, norfloxacin, and nitrofurantoin and had the highest (100%) susceptibility to vancomycin, amikacin, fosfomycin, clindamycin, fusidic acid, and linezolid. S. faecalis presented maximum sensitivity rates (97%, 93%, and 86%) against vancomycin, linezolid, and fosfomycin, respectively, and maximum antimicrobial resistance rates (88%, 83%, 83%, and 83%) against gentamicin, ciprofloxacin, levofloxacin, and norfloxacin, correspondingly. The corresponding resistance patterns of the 31 antimicrobial agents on the Gram-negative and Gram-positive uropathogens are illustrated in Tables 2 and 3.
Table 3. Prevalence and Percentage of Antimicrobial Resistance among the Gram-Positive Isolates a.
| No. | AMP | AML | AMC | SAM | FEP | CTX | FOX | CXM | CAZ | CRO | CL | CE | CEC | CFM | IPM | MEM | VA | AK | CN | TOB | AZM | E | DO | CIP | LEV | NOR | F | FOS | DA | FD | LZD | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus | 3 | 67 | 67 | 33 | 33 | 33 | 33 | 33 | 33 | 33 | 33 | 33 | 33 | 33 | 33 | 33 | 33 | 0 | 0 | 33 | 67 | 33 | 33 | 33 | 67 | 67 | 67 | 67 | 0 | 0 | 0 | 0 |
| S. fecalis | 58 | 22 | 22 | 22 | 22 | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | 3 | NT | 88 | NT | NT | NT | 60 | 83 | 83 | 83 | 21 | 14 | NT | NT | 7 |
a Abbreviations: AK, Amikacin; AMP, Ampicillin; AML, Amoxicillin; AMC, Amoxicillin/clavulanic acid; AZM, Azithromycin; CAZ, Ceftazidime; CE, Cephradine; CEC, Cefaclor; CFP, Cefoperazone; CFM, Cefixime; CL, Cephalexin; CN, Gentamicin; CIP, Ciprofloxacin; CRO, Ceftriaxone; CTX, Cefotaxime; CXM, Cefuroxime; DA, Clindamycin; DO, Doxycycline; E, Erythromycin; F, Nitrofurantoin; FD, Fusidic acid; FEP, Cefepime; FOS, Fosfomycin; IPM, Imipenem; MEM, Meropenem; LEV, Levofloxacin; NOR, Norfloxacin; LZD, Linezolid; NT, Not Tested; SAM, Ampicillin/sulbactam; TOB, Tobramycin; VA, Vancomycin.
5. Discussion
The present study focused on the local status of antimicrobial susceptibility pattern in uropathogens with a view to offering assistance in monitoring the continuous changing environment of bacterial resistance and further improvements in UTI treatment. This is a retrospective study where routine diagnostic results and susceptibility analysis are used. The present data belong to patients bearing the cost of medical check-up in a private laboratory; therefore, they may not imitate the true prevalence of UTI as most of the patients were treated empirically for this infection.
It has been reported previously that in 80% of acute and recurrent UTI cases in women, E. coli is involved as the primary organism, followed by S. saprophyticus (10% - 15%). Other less common uropathogens with the potential to cause UTI include Klebsiella, Enterobacter, Serratia, Proteus, Pseudomonas, and Enterococcus (11). Our findings indicate that the frequency of E. coli was 62% in UTI from a total of 392 culture-positive urine samples. In some previous reports, the E. coli prevalence was 68% (19, 20), followed by Pseudomonas (6%), Klebsiella spp. (1%), and Proteus (1%). Akram et al. reported that in the majority of their UTI patients, E. coli was the predominant organism (21): E. coli was found in 67% of the females and 33% of the males from a total of 62% of the UTI cases, in comparison with Pseudomonas, which was detected in 65% of the females and 35% of the males from only 6% of the UTI cases. Our results are relatively similar to a previous study from Lahore, Pakistan, which reported that the prevalence rate of E. coli was 73% as opposed to only 27% in the other UTI-causing organisms (22).
Among the Gram-positive pathogens, E. faecalis was found as the most frequent organism (15%), followed by S. aureus (1%). The prevalence rate of E. Candida was 14%, which is quite similar to the finding of a previous study from Nepal, stating that the prevalence rate of E. faecalis was 18% (23). A previous study conducted in 2008 found resistant patterns in E. coli against ampicillin (92%), co-trimoxazole (80%), ciprofloxacin (62%), gentamicin (47%), nitrofurantoin (20%), and amikacin (4%) (24). In contrast, our study revealed significant changes in the E. coli resistant patterns, especially against amikacin, where E. coli exhibited high resistance (91%). Our results also demonstrated that 23 out of the 31 different drugs showed greater than 70% antimicrobial resistance against E. coli, the most common pathogen. β-lactam drugs like amoxicillin/clavulanic acid, ampicillin, and aztreonam were also ineffective against E. coli as it had high antimicrobial resistance (84%, 84%, and 72%, respectively). A study conducted in the Lahore region indicated high resistance of E. coli to β-lactam antibiotics such as amoxicillin/ clavulanic acid, ampicillin, and aztreonam (22). E. coli showed the maximum susceptibility (97%) against drugs like imipenem, meropenem, and cefoperazone/sulbactam. Tazobactam/piperacillin and fosfomycin also provided significant activity against E. coli (96% and 90%, respectively).
In our study, Pseudomonas was found as the second most frequent Gram-negative isolate and had maximum resistance against ciprofloxacin, levofloxacin, norfloxacin, ofloxacin, and moxifloxacin, while it was highly susceptible to tazobactam/piperacillin. Klebsiella has a resistance pattern comparatively similar to E. coli with high resistance (100%) to cephalexin and cephradine and less resistance against nalidixic acid (20%). A previous study from Pakistan reported high sensitivity (80%) to cefepime versus a greater resistance rate (87%) against ciprofloxacin (25). Nitrofurantoin was the most efficient drug against this pathogen as it had peak sensitivity (100%). Proteus was the least common pathogen and had maximum resistance (100%) against 12 antibacterial drugs but was found to have 100% sensitivity against meropenem, cefoperazone/sulbactam, and piperacillin/tazobactam.
Our results indicated that only 16% of the UTI cases were caused by Gram-positive microorganisms. E. faecalis was detected as a more resistant uropathogen than S. aureus. Moreover, E. faecalis showed a high rate of resistance to gentamicin (88%), ciprofloxacin (83%), levofloxacin (83%), and norfloxacin (83%) but linezolid was the most effective antimicrobial drug. Vancomycin, amikacin, fosfomycin, clindamycin, fusidic acid, and linezolid had strong antimicrobial activity against the Gram-positive isolates. The S. aureus isolates from UTI were susceptible to amikacin, augmentin, and oxacillin in a previous study by Bano et al. while marked resistance to amoxicillin, ampicillin, tobramycin, ciprofloxacin, levofloxacin, norfloxacin, and fusidic acid was found in another study (25). Dash et al. (2013) also reported that nitrofurantoin was the most effective drug against gram-positive uropathogens (13). Our study demonstrated the highest frequency for ESBL production in E. coli (66.8%), followed by Klebsiella spp. (40%) and Proteus mirabilis (33.3%). Our results are similar to a previous study from Karachi, Pakistan, which reported that the ESBL frequencies among E. coli and Proteus mirabilis isolates were 68.55% and 28.57%, respectively. In contrast, the frequency of Klebsiella was different (84.61%) from our finding, which may be due to the lower number of Klebsiella isolates in our study (26).
The antibiotic resistance of uropathogens to trimethoprim/sulfamethoxazole, ampicillin, and cephalothin is increasing worldwide (27). In developing countries, the frequent prescription of antibiotics for the treatment of UTI and other infections, self-medication, suboptimal concentration and quality of antimicrobial agents, and community level poor hygiene are reasons for the ever-growing antimicrobial resistance in uropathogens. In conclusion, cefoperazone/sulbactam and vancomycin would be the first-line drug and most effective for the empirical treatment of UTI. In conclusion, the present study demonstrated increased antibiotic resistance in UTI isolates, which necessitates the careful selection of antimicrobials and their conservative use.
Acknowledgments
We highly acknowledge the permission and support of Dr. Omar Chughtai for this study in microbiology department of his laboratory.
Footnotes
Authors’ Contributions:Study concept and design: Muhammad Sohail and Mohsin Khurshid; Acquisition of data: Muhammad Sohail; Analysis and interpretation of data: Mohsin Khurshid; Drafting of the manuscript: Muhammad Sohail, Mohsin Khurshid, and Hafiz Ghulam Murtaza Saleem; Critical revision of the manuscript for important intellectual content: Mohsin Khurshid, Hasnain Javed, and Abdul Arif Khan; Statistical analysis: Mohsin Khurshid and Hasnain Javed; Administrative, technical, and material support: Muhammad Sohail and Hafiz Ghulam Murtaza Saleem; Study supervision: Mohsin Khurshid and Abdul Arif Khan.
References
- 1.Bader MS, Hawboldt J, Brooks A. Management of complicated urinary tract infections in the era of antimicrobial resistance. Postgrad Med. 2010;122(6):7–15. doi: 10.3810/pgm.2010.11.2217. [DOI] [PubMed] [Google Scholar]
- 2.Mittal R, Aggarwal S, Sharma S, Chhibber S, Harjai K. Urinary tract infections caused by Pseudomonas aeruginosa: a minireview. J Infect Public Health. 2009;2(3):101–11. doi: 10.1016/j.jiph.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Valiquette L. Urinary tract infections in women. Can J Urol. 2001;8 Suppl 1:6–12. [PubMed] [Google Scholar]
- 4.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002;113 Suppl 1A:5S–13S. doi: 10.1016/s0002-9343(02)01054-9. [DOI] [PubMed] [Google Scholar]
- 5.Schaeffer AJ, Rajan N, Cao Q, Anderson BE, Pruden DL, Sensibar J, et al. Host pathogenesis in urinary tract infections. Int J Antimicrob Agents. 2001;17(4):245–51. doi: 10.1016/s0924-8579(01)00302-8. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen H, Weir M. Urinary tract infection as a possible marker for teenage sex. South Med J. 2002;95(8):867–9. [PubMed] [Google Scholar]
- 7.Franz M, Horl WH. Common errors in diagnosis and management of urinary tract infection. II: clinical management. Nephrol Dial Transplant. 1999;14(11):2754–62. doi: 10.1093/ndt/14.11.2754. [DOI] [PubMed] [Google Scholar]
- 8.Tabibian JH, Gornbein J, Heidari A, Dien SL, Lau VH, Chahal P, et al. Uropathogens and host characteristics. J Clin Microbiol. 2008;46(12):3980–6. doi: 10.1128/JCM.00339-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katouli M. Population structure of gut Escherichia coli and its role in development of extra-intestinal infections. Iran J Microbiol. 2010;2(2):59–72. [PMC free article] [PubMed] [Google Scholar]
- 10.Stamm WE. Scientific and clinical challenges in the management of urinary tract infections. Am J Med. 2002;113 Suppl 1A:1S–4S. doi: 10.1016/s0002-9343(02)01053-7. [DOI] [PubMed] [Google Scholar]
- 11.Ronald A. The etiology of urinary tract infection: traditional and emerging pathogens. Dis Mon. 2003;49(2):71–82. doi: 10.1067/mda.2003.8. [DOI] [PubMed] [Google Scholar]
- 12.Falagas ME, Polemis M, Alexiou VG, Marini-Mastrogiannaki A, Kremastinou J, Vatopoulos AC. Antimicrobial resistance of Esherichia coli urinary isolates from primary care patients in Greece. Med Sci Monit. 2008;14(2):CR75–9. [PubMed] [Google Scholar]
- 13.Dash M, Padhi S, Mohanty I, Panda P, Parida B. Antimicrobial resistance in pathogens causing urinary tract infections in a rural community of Odisha, India. J Family Community Med. 2013;20(1):20–6. doi: 10.4103/2230-8229.108180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pape L, Gunzer F, Ziesing S, Pape A, Offner G, Ehrich JH. [Bacterial pathogens, resistance patterns and treatment options in community acquired pediatric urinary tract infection]. Klin Padiatr. 2004;216(2):83–6. doi: 10.1055/s-2004-823143. [DOI] [PubMed] [Google Scholar]
- 15.Conway PH, Cnaan A, Zaoutis T, Henry BV, Grundmeier RW, Keren R. Recurrent urinary tract infections in children: risk factors and association with prophylactic antimicrobials. JAMA. 2007;298(2):179–86. doi: 10.1001/jama.298.2.179. [DOI] [PubMed] [Google Scholar]
- 16.Chishti AS, Maul EC, Nazario RJ, Bennett JS, Kiessling SG. A guideline for the inpatient care of children with pyelonephritis. Ann Saudi Med. 2010;30(5):341–9. doi: 10.4103/0256-4947.68549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kass EH. Asymptomatic infections of the urinary tract. Trans Assoc Am Physicians. 1956;69:56–64. [PubMed] [Google Scholar]
- 18.Barrow GI, Feltham RKA. Cowan and Steel's Manual for the Identification of Medical Bacteria. 3th ed. New York: Cambridge University Press; 1993. [DOI] [Google Scholar]
- 19.Sharifian M, Karimi A, Tabatabaei SR, Anvaripour N. Microbial sensitivity pattern in urinary tract infections in children: a single center experience of 1,177 urine cultures. Jpn J Infect Dis. 2006;59(6):380–2. [PubMed] [Google Scholar]
- 20.Kothari A, Sagar V. Antibiotic resistance in pathogens causing community-acquired urinary tract infections in India: a multicenter study. J Infect Dev Ctries. 2008;2(5):354–8. doi: 10.3855/jidc.196. [DOI] [PubMed] [Google Scholar]
- 21.Akram M, Shahid M, Khan AU. Etiology and antibiotic resistance patterns of community-acquired urinary tract infections in J N M C Hospital Aligarh, India. Ann Clin Microbiol Antimicrob. 2007;6:4. doi: 10.1186/1476-0711-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rabia T, Hafeez R, Hasnain S. Prevalenceof Multi Drug resistant E.coli in patients of UTI registering at diagnostic laboratory. Pak. J. Zool. 2012;44(3):702–12. [Google Scholar]
- 23.Baral R, Timilsina S, Jha P, Bhattarai NR, Poudyal N, Gurung R, et al. Study of antimicrobial susceptibility pattern of Gram positive organisms causing UTI in a tertiary care hospital in eastern region of Nepal. Health Renaiss. 2013;11(2):119–124. doi: 10.3126/hren.v11i2.8218. [DOI] [Google Scholar]
- 24.Bashir MF, Qazi JI, Ahmad N, Riaz S. Diversity of Urinary Tract Pathogens and Drug Resistant Isolates of <i>Escherichia coli</i> in different age and gender Groups of Pakistanis. Trop J Pharm Res. 2008;7(3):1025–1031. doi: 10.4314/tjpr.v7i3.14687. [DOI] [Google Scholar]
- 25.Bano K, Khan J, Begum RH, Munir S, Akbar N, Ansari JA, et al. Patterns of antibiotic sensitivity of bacterial pathogens among urinary tract infections (UTI) patients in a Pakistani population. Afr J Microbiol Res. 2012;6(2):414–20. [Google Scholar]
- 26.Afridi FI, Farooqi BJ, Hussain A. Frequency of extended spectrum beta lactamase producing enterobacteriaceae among urinary pathogen isolates. J Coll Physicians Surg Pak. 2011;21(12):741–4. [PubMed] [Google Scholar]
- 27.Gupta K, Hooton TM, Stamm WE. Increasing antimicrobial resistance and the management of uncomplicated community-acquired urinary tract infections. Ann Intern Med. 2001;135(1):41–50. doi: 10.7326/0003-4819-135-1-200107030-00012. [DOI] [PubMed] [Google Scholar]