Abstract
Background:
Acute and chronic inflammations are difficult to control. Using chemical anti-inflammatory medications along with their complications considerably limit their use. According to Traditional Iranian Medicine (TIM), there is an important relation between inflammation and Imtila (food and blood accumulation in the body); food reduction or its more modern equivalent Caloric Restriction (CR) may act against both Imtila and inflammation.
Objectives:
This experimental study aimed to investigate the effect of 30% reduction in daily calorie intake on inflammation in rats.
Materials and Methods:
A total of 18 male rats (Rattus rattus) weighing 220 to 270 g were obtained. Then, the inflammation was induced by injecting formalin in their paws. Next, the rats were randomized by generating random numbers into two equal groups (9 + 9) putting on either normal diet (controls) or a similar diet with 30% reduction of calorie (cases). Paw volume changes were recorded twice per day by one observer in both groups using a standard plethysmometer for 8 consecutive days. Serum C-reactive protein (CRP), Erythrocyte Sedimentation Rate (ESR), complete blood count (erythrocyte, platelet, and white blood cell) and hemoglobin were compared between the groups.
Results:
Decline of both body weight and paw volume was significantly more prominent in the case than in the control rats within the study period (P < 0.001 and < 0.001, respectively). Paw volume decrease was more prominent after day 3. On day 8, serum CRP-positive (1 or 2 +) rats were more frequent in ad libitum fed group comparing with those received CR (33.3% vs. 11.1%). This difference, however, was insignificant (P = 0.58). At the same time, mean ESR was significantly higher in the control rats comparing with that in the case group (29.00 ± 2.89 h vs. 14.00 ± 1.55 h; P = 0.001). Other serum parameters were not significantly different between the two groups at endpoint.
Conclusions:
Rats fed with a 30% calorie-restricted diet in comparison with to ad libitum fed controls for 8 days had significantly more prominent regression of inflammation.
Keywords: Caloric Restriction, Inflammation, Animal Models, Iran
1. Background
Caloric Restriction (CR), which is the reduction in calorie intake without inducing malnutrition (1), is a well-known method for reducing weight, diminishing morbidity and mortality, and retarding aging (2). Therefore, CR has been suggested as a robust nutritional intervention for fighting against obesity and extending lifespan (3).
Although there are ample anecdotal proofs with regard to health benefits of caloric restriction and fasting in human, such dietary changes have recently received enthusiastic attention from scientists and undergone rigorous studies in laboratory animals and human subjects (4).
In traditional Iranian medicine, inflammation has strong relationship with Imtila (food and blood accumulation in the body). Rhazes, Avicenna, and Haly Abbas described the Imtila as the accumulation of waste products in the body due to excess food, alcohol, and rest in addition to lack of exercise, which is, whether beneficial (Mahmooda) or Non-beneficial (Ghair-mahmooda) both would be toxic for the body. The accumulation of these waste products might lead to increase in blood volume, vessel wall tension, and vascular pressure (5). Food restriction can help reducing this accumulation and accordingly reducing inflammation too.
Reduced generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS), deactivation of redox-sensitive transcription factors, and preventing the expression of clusters of inflammatory and oxidative stress genes are the most recent cutting-edge findings regarding possible beneficial effects of CR on diverse organisms (6).
Modern studies have shown that energy metabolism and immune function are tightly interconnected through shared signaling mechanisms (7).
Obesity, which is characterized by increased mass of adipose tissue, is accompanied with systemic inflammation. This simple link between obesity and inflammation justifies the influence of CR on systemic inflammation, immune response, and antioxidant defense (8). Based on this connection, some investigators believe that CR acts through weight loss. In this model, CR-induced weight loss augments insulin sensitivity, reduces circulating inflammation-related products, and increases anti-inflammatory factors produced by adipocytes (9). Reduced levels of serum interleukin (IL) IL-6, C-Reactive Protein (CRP) and Tumor Necrosis Factor (TNF)-α, suppress up-regulation of NuclearF (NF)-κB, cyclooxygenase-2 (COX-2), and inducible Nitric Oxide Synthase (iNOS) after CR-induced weight-loss, which have corroborated this hypothesis (10). Many of the scientists, however, believe that the anti-inflammatory effect of CR is not limited to adipose tissue but is a systemic mechanism, affecting serum, liver, heart, hypothalamus, etc. (11).
Similarity between rodents and humans in terms of association between CR and inflammation (12) has enabled scientists to carry out studies in this regard on mice and rats instead of performing on humans.
It has been previously shown that CR protects laboratory rodents against a variety of inflammatory agents (13). However, these reports are scant in the literature, particularly in terms of the effect of short-term CR strategies on inflammation. In addition, majority of available reports have focused only on laboratory changes and mechanisms.
2. Objectives
The objective of the present study is to examine short-term effects of a 30% reduction in daily calorie intake on both laboratory (CRP and ESR) and clinical (Perceptional) aspects of inflammation in a rat model. We did not find similar study about the effect of CR on clinical aspects like changes of paw edema.
3. Materials and Methods
3.1. Animals and Diets
A total of 23 male rats (Rattus rattus) 5-6 weeks old, and weighing 100 to 150 g were obtained from animal laboratory of Urmia University in Iran. The rats were housed individually in a temperature-controlled (20 - 22°C) room under 12 h : h light/dark cycles with free access to food and tap water for 6 - 7 weeks. After this acclimation period, at the age of 12 weeks, 18 rats were randomized into two equal and weight-matched groups: a control diet group (controls, n = 9) and a 30% CR diet group (cases, n = 9) by generating random numbers using standard software. Based on prior and similar studies about edema volume changes after formalin injection (14), we decided to select 9 + 9 cases.
To determine daily requirement of food, more than enough food was placed in each case in the morning and the remaining was retrieved at night. The rats had ad libitum access to food in the interim. Accordingly, mean daily amount of required food was calculated (total food in the morning in g - remained food at night in g) as 15 g/rat. Thus, each rat in the case group had ad libitum access to 10.5 g food daily during the study period, while the control rats had free access to more than 15 g of food daily. Caloric restriction diets were adjusted to provide the same intake levels for total protein, minerals, and vitamins as in the control diet.
All conditions and handling of the animals were approved by the Ethics Committee of Shahed University (No. 41/168152) and conducted according to the Declaration of Helsinki.
3.2. Inducing Inflammation
For anti-inflammatory activity, the formalin-induced edema model was used (15). Rats were injected with 0.05 mL of 2.5 % formaldehyde solution into the sub-plantar region of the right hind paw. The paw volume was measured before and after formalin injection and the difference in these two volumes was used as an indication of inflammation. The paw volume was measured using a mercury-balance plethysmometer technique (16).
Paw volume and body weight were recorded before injection, one hour after injection and then daily at a certain hour in all case and control rats for 8 consecutive days.
We measured paw volume and body weight twice by one observer daily.
3.3. Blood Sampling and Serum Parameters
To determine baseline serum parameters, 5 rats were randomly selected and their blood samples (2 mL) were collected. In day 8, blood samples were obtained from all the study rats. Serum CRP, ESR, white blood cell count, red blood cell count, platelet count, and hemoglobin level were measured using conventional laboratory methods.
3.4. Statistical Analysis
All results were expressed as means ± standard deviation, median (interquartile range), or frequency (%). Statistical analysis was conducted using SPSS statistical software (V 18.0). Distribution of the numerical data was tested using Kolmogorov-Smirnov test and QQ-plots. Differences were determined using Independent samples t test, repeated measures analysis, Mann-Whitney U test, or Fisher exact test, where appropriate. Significance was set at P < 0.05.
4. Results
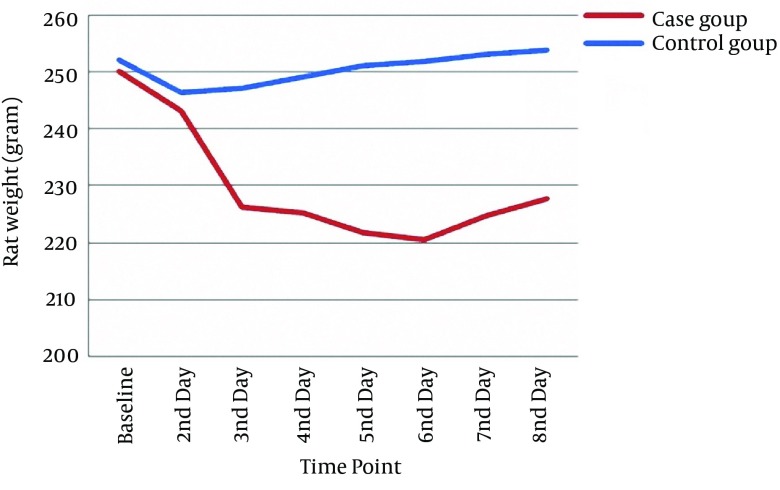
At baseline, case and control groups were comparable in terms of weight (250.11 ± 29.25 g vs. 252.22 ± 12.28 g, respectively); independent samples t test (P = 0.84) and paw volume (4.97 ± 0.67 mL vs. 4.56 ± 0.58 mL, respectively); and independent samples t test (P = 0.18) (Tables 1 and 2). Based on the result of repeated measures analysis, weight loss was significantly more prominent in cases than in controls during the study period (P < 0.001) (Figure 1). Percentage of weight change of the studied rats was significant too (Table 3).
Table 1. Mean Weight of the Studied rats in Different Occasions a,b.
| Group | Baseline | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 |
|---|---|---|---|---|---|---|---|---|
| Case | 250.11 ± 29.25 | 243.11 ± 27.14 | 226.22 ± 25.23 | 225.22 ± 26.60 | 221.78 ± 27.16 | 220.56 ± 27.58 | 224.78 ± 27.82 | 227.67 ± 28.91 |
| Control | 252.22 ± 12.28 | 246.56 ± 11.48 | 247.22 ± 12.13 | 249.11 ± 12.35 | 251.11 ± 12.65 | 251.89 ± 12.94 | 253.22 ± 12.35 | 253.22 ± 12.35 |
a Data are presented as Mean ± Standard Deviation.
b Values are presented as grams (g).
Table 2. Mean Paw Volume of the Studied Rats in Different Occasions a,b.
| Group | Baseline | Hour 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 |
|---|---|---|---|---|---|---|---|---|---|
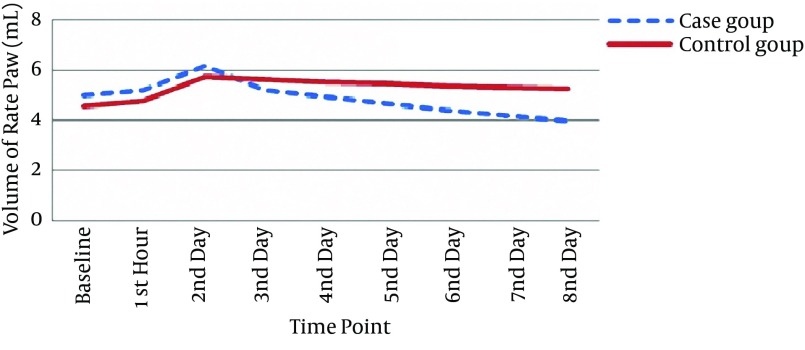
| Case | 4.97 ± 0.67 | 5.22 ± 0.66 | 6.17 ± 0.48 | 5.22 ± 0.54 | 4.92 ± 0.43 | 4.67 ± 0.44 | 4.42 ± 0.43 | 4.18 ± 0.40 | 3.95 ± 0.39 |
| Control | 4.56 ± 0.58 | 4.77 ± 0.59 | 5.74 ± 0.78 | 5.63 ± 0.73 | 5.53 ± 0.74 | 5.45 ± 0.73 | 5.37 ± 0.73 | 5.31 ± 0.72 | 5.25 ± 0.71 |
a Data are presented as Mean ± Standard Deviation.
b Values are presented as milliliter (mL).
Figure 1. Changes of Rat Weight on Different Occasions in the Study Groups.
Table 3. Percentage Change of Weight of the Studied Rats in Different Occasions a,b.
| Group | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 |
|---|---|---|---|---|---|---|---|
| Case | -2.12 (0.30) | -9.40 (0.52) | -9.4 (0.63) | -11.10 (0.92) | -11.64 (0.33) | -9.45 (0.68) | -8.32 (0.77) |
| Control | 0.45 (1.10) | -2.11 (1.70) | -1.34 (1.12) | -0.54 (1.03) | -0.27 (1.12) | -0.01 (1.32) | 0.12 (2.67) |
| P Value | < 0.001 b | < 0.001 b | < 0.001 b | < 0.001 b | < 0.001 b | < 0.001 b | < 0.001 b |
a Data are presented as median (interquartile range).
b P Value < 0.05 is statistically significant.
A trend similar to that for rat weight was also observed in this regard. Accordingly, mean paw volume decreased significantly and more saliently in the case group, particularly after day 3 (repeated measures analysis P = 0.001) (Figure 2). Percentage change of paw edema was significant from the third day (Table 4).
Figure 2. Changes of Rat Paw Volume on Different Occasions in the Study Groups.
Table 4. Percentage Change of Mean Paw Volume of the Studied Rats in Different Occasions a, b.
| Group | Hour 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 |
|---|---|---|---|---|---|---|---|---|
| Case | 4.12 (0.80) | 21.08 (6.12) | 3.59 (7.29) | -0.12 (4.22) | -4.16 (7.01) | -9.98 (0.03) | -13.18 (0.28) | -16.87 (0.17) |
| Control | 3.33 (0.01) | 21.45 (7.34) | 20.04 (5.79) | 19.24 (5.23) | 17.23 (6.45) | 16.22 (7.29) | 14.12 (5.67) | 12.01 (6.13) |
| P value | 0.18 | 0.39 | < 0.001 b | < 0.001 b | < 0.001 b | < 0.001 b | < 0.001 b | < 0.001 b |
a Data are presented as median (interquartile range).
b P value < 0.05 is statistically significant.
Serum parameters of the studied rats at baseline and at endpoint are summarized in Table 5. Between the two groups, however, only mean ESR was significantly higher in the control than in the case group at the endpoint (Independent samples t test, P = 0.001). While the frequency of cases with abnormal serum CRP was higher in controls than in cases on day 8, the difference did not reach a statistically significant level (Fisher exact test, P = 0.58). Other variables, including serum platelets, RBC and WBC numbers, and serum hemoglobin were not significantly different between the two groups on day 8.
Table 5. Serum Parameters of Studied Rats at Baseline and at Endpoint a,b,c.
| Serum parameters | CRP (+) | ESR | PLT | Hb | RBC | WBC |
|---|---|---|---|---|---|---|
| Baseline | 0 (0) | 7.00 ± 1.75 | 2234.00 ± 558.50 | 56.50 ± 14.12 | 30.15 ± 7.53 | 28.50 ± 7.12 |
| Endpoint | ||||||
| Cases | 1 (11.1) | 14.00 ± 1.55 | 5023.00 ± 558.11 | 132.60 ± 14.73 | 74.69 ± 8.30 | 58.80 ± 6.53 |
| Controls | 3 (33.3) | 29.00 ± 2.89 | 4963.22 ± 551.43 | 124.50 ± 13.83 | 70.01 ± 7.77 | 65.20 ± 7.24 |
| P Value | 0.58 | 0.001 c | 0.38 | 0.42 | 0.59 | 0.19 |
a Abbreviations: CRP, c-reactive protein; ESR, erythrocyte sedimentation rate (h); Hb, Hemoglobin (mg/dL); PLT, platelets (× 103/μL); RBC, red blood cell (× 106/μL); WBC, white blood cell (× 106/μL).
b Data are presented as frequency (%) or Mean ± standard Deviation.
c P value (Case vs. controls) < 0.05 is statistically significant.
5. Discussion
In the present study, we showed that a short-term, 30% reduction in daily calorie intake significantly hastened reversal of formalin-induced inflammation and serum ESR decline in rats. Acute and chronic inflammatory conditions are among the most important health problems all over the world. In many cases, the last choice is using medications such glucocorticoids and non-steroidal anti-inflammatory drugs. Many of these medications, however, are accompanied with serious complications and side-effects, particularly when they are used for a long time (17). This is where traditional medicine may assist the modern medicine or even play a pivotal role.
Anti-inflammatory agents have been widely discussed in traditional Iranian medicine. Arnebia euchroma (16), Urtica pilulifera L. seeds extracts (15), Berberis vulgaris L. (18), and Anethum graveolens L. (13) are some available examples in the literature.
Although according to the published literature, evidence regarding CR and inflammation was first presented in 1930s by McCay et al. (19), similar debates can be found in traditional Iranian medicine texts since a long time ago. “Food reduction” in the form of diminishing food quality, food quantity or both has been discussed as a way of dealing with inflammation (20). We can describe this role by relating Imtila and inflammation in TIM references (5).
Low-grade inflammation, as indicated by higher circulating levels of inflammatory mediators such as C-reactive protein, interleukin-6 and tumor necrosis factor-α, is a strong risk factor for several chronic diseases. There are data indicating that decreasing energy intake may be an effective therapy for reducing overall inflammation. Moreover, very-low-energy dietary weight loss reduces both circulating markers of inflammation and adipose-tissue cytokine production (21, 22).
Short-term dietary intervention resulted in reductions in proinflammatory cytokines and metabolic risk factors, including IL-6, IL-8 and TNF-α (45, 27, 28).
Beneficial effects of CR have been previously proposed in many health-related conditions such as aging, metabolic disorders, cancers, cardiovascular disease, and neurodegenerative disorders (23).
Although some studies have shown that long- and mid-term CR may enhance immune functions through improving responses of T cells to mitogens, cytotoxic T lymphocyte activity, natural kill cell activity, and the capability of mononuclear cells in releasing pro-inflammatory cytokines and markers (1, 24), possible effects of short-term CR on immune system and inflammation are not clear.
We showed that short-term CR for only 8 days was significantly effective in alleviating of inflammation. At the same time, weight loss was significantly more prominent in case group compared to the control rats.
The simplest justification for the association between calorie intake and inflammation is based on the inflammatory changes in adiposity. As obesity is associated with the changes in number, distribution, and function of immune cells (25) a low-grade inflammation is usually present in obese individuals and animals. On the other hand, CR in obesity has been associated with enhanced cell-mediated immune function and consequently, curtailed inflammatory responses (11, 26, 27).
We also found that the number of rats with “positive” serum CRP was higher in the control than in case rats. However, this difference did not reach a statistically significant level. Small sample size may be the reason of this finding. Further studies with larger sample size and employment of quantitative, high sensitivity measurement of serum CRP are recommended in this regard.
According to some other reports, however, beneficial effects of CR against inflammation are not restricted to obese or over-weight cases (28), indicating that decreased adiposity is possibly not the only mediator of these effects of CR. In line with this notion, some studies have shown that the protecting effect of CR against inflammation through reducing its markers such as CRP, IL-6 and plasminogen activator inhibitor type 1 is independent of obesity (29). In some recent studies, CR favorably affected both innate and acquired immunities (30). Other studies have reported that CR results in lower systemic inflammation (31) with reduced inflammatory cytokine expression in various tissues (32). Genomic profiling examinations in rodents have revealed that CR may also inhibit the release of pro-inflammatory mediators from macrophages (33).
Enhanced T cell proliferation and IL-2 production, as well as decreased prostaglandin (PG) E2 production have been reported in CR experimental animals and humans (30, 34). It should be noted that PGE2 is a potent suppressor of T cell proliferation and IL-2 production and thus, a very potent lipid inflammatory mediator, which is involved in the pathogenesis of many inflammatory diseases (35). In addition, it has been shown that in CR rats, the activity of cyclooxygenase (COX), a rate-limiting enzyme for eicosanoid synthesis, was lower compared to the control rats (36).
Another possible mechanism that justifies the association between diet and inflammation is the role of orexigenic hormone, ghrelin. Dixit et al. showed that this hormone inhibits pro-inflammatory cytokine production by acting on various immune cell subsets (37).
According to available data, it seems that the exact mechanism underlying anti-inflammatory effect of CR has not been exactly defined. Because of its complexity, it seems that CR exerts its anti-inflammatory role through multiple mechanisms. Based on a recent concept, energy accumulation in the body is the origin of chronic inflammation. According to this theory, energy expenditure induced by inflammatory response is a feedback to fight against energy surplus (38).
Whatever the exact physiopathology of the association between CR and anti-inflammation, from a clinical viewpoint, its consequences are very important (39). As, there is a solid connection between inflammation and many pathological conditions such as metabolic disease, type 2 diabetes, hypertension, atherosclerosis, fatty liver, cancer, asthma, etc. (22), findings regarding the beneficial effects of CR on inflammation are critically important (40).
In conclusion, this study showed that rats fed with a 30% calorie-restricted diet in comparison with ad libitum fed controls for 8 days had significantly more prominent regression of inflammation and this finding is reported for the first time in the literature. Small sample size and using conventional method of serum CRP measurement and one observer are limitations that are acknowledged.
References
References
- 1.Nikolich-Zugich J, Messaoudi I. Mice and flies and monkeys too: caloric restriction rejuvenates the aging immune system of non-human primates. Exp Gerontol. 2005;40(11):884–93. doi: 10.1016/j.exger.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 2.Tsuchiya T, Higami Y, Komatsu T, Tanaka K, Honda S, Yamaza H, et al. Acute stress response in calorie-restricted rats to lipopolysaccharide-induced inflammation. Mech Ageing Dev. 2005;126(5):568–79. doi: 10.1016/j.mad.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Longo VD, Finch CE. Evolutionary medicine: from dwarf model systems to healthy centenarians? Science. 2003;299(5611):1342–6. doi: 10.1126/science.1077991. [DOI] [PubMed] [Google Scholar]
- 4.Dixit VD, Yang H, Sayeed KS, Stote KS, Rumpler WV, Baer DJ, et al. Controlled meal frequency without caloric restriction alters peripheral blood mononuclear cell cytokine production. J Inflamm (Lond). 2011;8:6. doi: 10.1186/1476-9255-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghods R, Gharooni M, Amin G, Nazem E, Nikbakht Nasrabadi A. Hypertension from the perspective of Iranian traditional medicine. Iran Red Crescent Med J. 2014;16(3):e22590. doi: 10.5812/ircmj.16449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HJ, Jung KJ, Yu BP, Cho CG, Choi JS, Chung HY. Modulation of redox-sensitive transcription factors by calorie restriction during aging. Mech Ageing Dev. 2002;123(12):1589–95. doi: 10.1016/s0047-6374(02)00094-5. [DOI] [PubMed] [Google Scholar]
- 7.Matarese G, La Cava A. The intricate interface between immune system and metabolism. Trends Immunol. 2004;25(4):193–200. doi: 10.1016/j.it.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Kumar VL, Basu N. Anti-inflammatory activity of the latex of Calotropis procera. J Ethnopharmacol. 1994;44(2):123–5. doi: 10.1016/0378-8741(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 9.Fereidoni M, Ahmadiani A, Semnanian S, Javan M. An accurate and simple method for measurement of paw edema. J Pharmacol Toxicol Methods. 2000;43(1):11–4. doi: 10.1016/s1056-8719(00)00089-7. [DOI] [PubMed] [Google Scholar]
- 10.Morgan TE, Wong AM, Finch CE. Anti-inflammatory mechanisms of dietary restriction in slowing aging processes. Interdiscip Top Gerontol. 2007;35:83–97. doi: 10.1159/000096557. [DOI] [PubMed] [Google Scholar]
- 11.Huang P, Li S, Shao M, Qi Q, Zhao F, You J, et al. Calorie restriction and endurance exercise share potent anti-inflammatory function in adipose tissues in ameliorating diet-induced obesity and insulin resistance in mice. Nutr Metab (Lond). 2010;7:59. doi: 10.1186/1743-7075-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295(13):1539–48. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirkwood TL, Kapahi P, Shanley DP. Evolution, stress, and longevity. J Anat. 2000;197 Pt 4:587–90. doi: 10.1046/j.1469-7580.2000.19740587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naseri M, Mojab F, Khodadoost M, Kamalinejad M, Davati A, Choopani R, et al. The Study of Anti-Inflammatory Activity of Oil-Based Dill (Anethum graveolens L.) Extract Used Topically in Formalin-Induced Inflammation Male Rat Paw. Iran J Pharm Res. 2012;11(4):1169–74. [PMC free article] [PubMed] [Google Scholar]
- 15.Abbasian A, Naseri M, Masoud A. Anti-inflammatory effects of Urtica pilulifera l. seeds extracts in the rat. Iran J Pharm Res. 2010;3(2):49. [Google Scholar]
- 16.Soltanian A, Faghihzadeh S, Mehdibarzi D, Gerami A, Nasery M, Cheng J. Assessment of marhame-mafasel pomade effect on knee osteoarthritis with non-compliance. J Res Health Sci. 2009;9(2):19–24. [PubMed] [Google Scholar]
- 17.Fauci AS, Braunwald E, Kasper DL. Harrison's principles of internal medicine. 18th ed. USA: McGraw-Hill; 2012. [Google Scholar]
- 18.Fouladi RF. Aqueous extract of dried fruit of Berberis vulgaris L. in acne vulgaris, a clinical trial. J Diet Suppl. 2012;9(4):253–61. doi: 10.3109/19390211.2012.726702. [DOI] [PubMed] [Google Scholar]
- 19.McCay CM, Maynard LA, Sperling G, Barnes LL. The Journal of Nutrition. Volume 18 July--December, 1939. Pages 1--13. Retarded growth, life span, ultimate body size and age changes in the albino rat after feeding diets restricted in calories. Nutr Rev. 1975;33(8):241–3. doi: 10.1111/j.1753-4887.1975.tb05227.x. [DOI] [PubMed] [Google Scholar]
- 20.Avicenna H. The Canon of Medicine. 1th ed. Beirut Alaalami Library; 2005. [Google Scholar]
- 21.Nicklas BJ, Ambrosius W, Messier SP, Miller GD, Penninx BW, Loeser RF, et al. Diet-induced weight loss, exercise, and chronic inflammation in older, obese adults: a randomized controlled clinical trial. Am J Clin Nutr. 2004;79(4):544–51. doi: 10.1093/ajcn/79.4.544. [DOI] [PubMed] [Google Scholar]
- 22.Nicklas BJ, You T, Pahor M. Behavioural treatments for chronic systemic inflammation: effects of dietary weight loss and exercise training. CMAJ. 2005;172(9):1199–209. doi: 10.1503/cmaj.1040769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin B, Mattson MP, Maudsley S. Caloric restriction and intermittent fasting: two potential diets for successful brain aging. Ageing Res Rev. 2006;5(3):332–53. doi: 10.1016/j.arr.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Messaoudi I, Warner J, Fischer M, Park B, Hill B, Mattison J, et al. Delay of T cell senescence by caloric restriction in aged long-lived nonhuman primates. Proc Natl Acad Sci U S A. 2006;103(51):19448–53. doi: 10.1073/pnas.0606661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Q, Leeman SE, Amar S. Signaling mechanisms involved in altered function of macrophages from diet-induced obese mice affect immune responses. Proc Natl Acad Sci U S A. 2009;106(26):10740–5. doi: 10.1073/pnas.0904412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, Vanegas SM, Du X, Noble T, Zingg JM, Meydani M, et al. Caloric restriction favorably impacts metabolic and immune/inflammatory profiles in obese mice but curcumin/piperine consumption adds no further benefit. Nutr Metab (Lond). 2013;10(1):29. doi: 10.1186/1743-7075-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milner JJ, Beck MA. The impact of obesity on the immune response to infection. Proc Nutr Soc. 2012;71(2):298–306. doi: 10.1017/S0029665112000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith JV, Heilbronn LK, Ravussin E. Energy restriction and aging. Curr Opin Clin Nutr Metab Care. 2004;7(6):615–22. doi: 10.1097/00075197-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Heilbronn LK, Noakes M, Clifton PM. Energy restriction and weight loss on very-low-fat diets reduce C-reactive protein concentrations in obese, healthy women. Arterioscler Thromb Vasc Biol. 2001;21(6):968–70. doi: 10.1161/01.atv.21.6.968. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed T, Das SK, Golden JK, Saltzman E, Roberts SB, Meydani SN. Calorie restriction enhances T-cell-mediated immune response in adult overweight men and women. J Gerontol A Biol Sci Med Sci. 2009;64(11):1107–13. doi: 10.1093/gerona/glp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cefalu WT, Wagner JD, Wang ZQ, Bell-Farrow AD, Collins J, Haskell D, et al. A study of caloric restriction and cardiovascular aging in cynomolgus monkeys (Macaca fascicularis): a potential model for aging research. J Gerontol A Biol Sci Med Sci. 1997;52(1):B10–9. doi: 10.1093/gerona/52a.1.b10. [DOI] [PubMed] [Google Scholar]
- 32.Flachs P, Rossmeisl M, Bryhn M, Kopecky J. Cellular and molecular effects of n-3 polyunsaturated fatty acids on adipose tissue biology and metabolism. Clin Sci (Lond). 2009;116(1):1–16. doi: 10.1042/CS20070456. [DOI] [PubMed] [Google Scholar]
- 33.Cao SX, Dhahbi JM, Mote PL, Spindler SR. Genomic profiling of short- and long-term caloric restriction effects in the liver of aging mice. Proc Natl Acad Sci U S A. 2001;98(19):10630–5. doi: 10.1073/pnas.191313598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang H, Youm YH, Dixit VD. Inhibition of thymic adipogenesis by caloric restriction is coupled with reduction in age-related thymic involution. J Immunol. 2009;183(5):3040–52. doi: 10.4049/jimmunol.0900562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012;188(1):21–8. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim YJ, Kim HJ, No JK, Chung HY, Fernandes G. Anti-inflammatory action of dietary fish oil and calorie restriction. Life Sci. 2006;78(21):2523–32. doi: 10.1016/j.lfs.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 37.Dixit VD, Schaffer EM, Pyle RS, Collins GD, Sakthivel SK, Palaniappan R, et al. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004;114(1):57–66. doi: 10.1172/JCI21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ye J, Keller JN. Regulation of energy metabolism by inflammation: a feedback response in obesity and calorie restriction. Aging (Albany NY). 2010;2(6):361–8. doi: 10.18632/aging.100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gabriely I, Ma XH, Yang XM, Atzmon G, Rajala MW, Berg AH, et al. Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine-mediated process? Diabetes. 2002;51(10):2951–8. doi: 10.2337/diabetes.51.10.2951. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282(48):35279–92. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]