Synopsis
Despite the relative frequency of Chiari-associated spinal deformities, this disease process remains poorly understood. Syringomyelia is often present; however, this is not a necessary situation and scoliosis has been described in the absence of a syrinx. Decompression of the hindbrain is recommended. In young patients (<10 years old) and/or those with smaller coronal Cobb measurements (<40 degrees), decompression of the hindbrain may lead to resolution of the spinal deformity. Spinal fusion is reserved for those curves that progress to deformities greater than 50 degrees. These procedures may be associated with a higher risk of postoperative deficit and intraoperative neurological monitoring may be difficult. Patients, families, and surgeons should be prepared for this possibility. Further research is needed to understand the underlying pathophysiology to improve prognostication and treatment of this patient population.
Keywords: scoliosis, Chiari malformation, syrinx, syringomyelia, early-onset
Introduction
Scoliosis is frequently associated with the presence of a Chiari I malformation (CM), up to 20% of patients, and even more frequently associated with CM in the setting of syringomyelia, with rates as high as 60%. While this clinical entity is not exceedingly rare in practice, there is a relative paucity of published research regarding spinal deformities associated with CM. Thus, the pathophysiology of the spinal deformity, and the effects of the CM with or without syringomyelia, remain poorly understood. Some have proposed that the formation of a syrinx may cause anterior horn cell dysfunction, with scoliosis as a result.1,2 As syringomyelia is not necessary for concomitant scoliosis, others have proposed that cerebellar tonsil compression is the inciting event.3 Identifying those patients with this constellation of diagnoses is essential, however, as there may be some neurological risk to corrective surgery for the spinal deformity performed in the setting of an untreated syrinx or CM. Furthermore, early diagnosis of scoliosis associated with CM may allow for nonoperative management of the spinal deformity, thus avoiding a spinal fusion.4-6 The purpose of this article is to review the current literature regarding scoliosis associated with CM, with and without syringomyelia.
Clinical Presentation
The presentation of scoliosis associated with CM varies significantly and, as such, it may remain undiagnosed and spine surgeons should have some clinical suspicion when evaluating idiopathic scoliosis that exhibits any atypical features. Charry et al reported a series of patient with syringomyelia where a paucity had abnormal exams (10/25, 40%) and the majority were normal.7 In fact, up to 10% of patients with suspected idiopathic scoliosis were found to have some abnormality on a preoperative magnetic resonance imaging (MRI), the majority of which were syringomyelia (67%) followed by Chiari malformations (31%).8 A prospective, observational cohort of patients ages 10 to 19 did not support these findings, however, and the clinical concern from disease presentation and physical exam findings are likely the most important factors.9,10 The diagnosis of scoliosis made in childhood is categorized by age, with children diagnosed as infantile (birth – 3 years old), juvenile (4-10 years), and adolescent (>10 years old) scoliosis. The age of the child is important to consider, as younger cases (infantile and juvenile, early onset scoliosis) of scoliosis are more frequently associated with some neural axis abnormality and an MRI study of the entire spine must be ordered.11-13 Age and skeletal maturity are important considerations, not only as predictors of the presence of a neural axis abnormality, but also because there are implications for prognosis and potential for resolution with management of the CM and syrinx.4,5,14-16 Menarchal status is assessed in girls, as this may correlate with curve progression and allows for a more informed conversation with patient and parents.17
In some cases, neurological symptoms may precede the diagnosis of the spinal deformity. Perhaps the most common neurological complaint is headaches, however sensory or motor disturbances may exist.18 The presence of any of these in an orthopedic clinic should elicit an MRI study of the entire spinal column; with referral to a neurosurgeon should the MRI find abnormalities. A complete neurological examination must be performed at the initial visit for any child presenting with a spinal deformity. In many cases the exam will be normal; however, in some instances a motor deficit, hyperreflexia, or a pathologic reflex may be present. Amongst the reflexes that must be examined are the abdominal reflexes, as these have been correlated with neural axis abnormalities, including syringomyelia.19,20 Gait should be examined and clonus should be checked, to ensure symmetry and no more than 2-3 beats per side, if present at all. Those patients presenting as adults may have more profound neurological abnormalities, which should prompt an MRI prior to any surgical intervention for the spinal deformity.21
Radiographic Evaluation
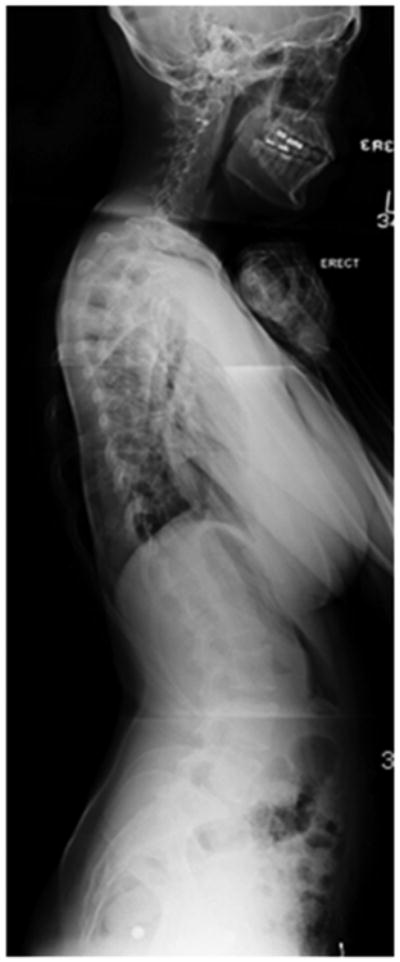
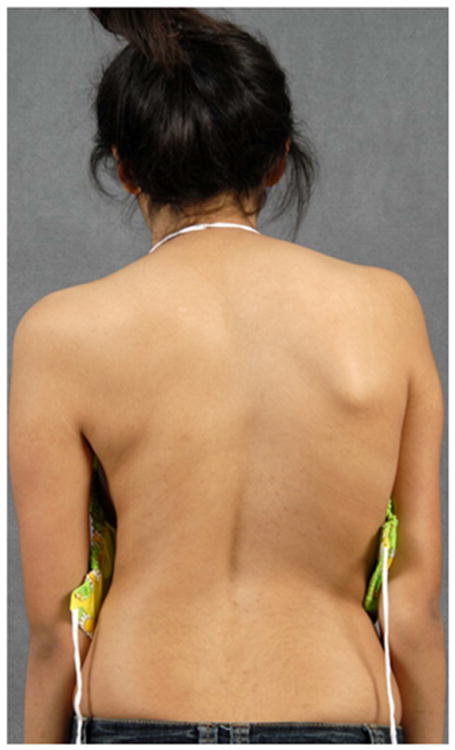
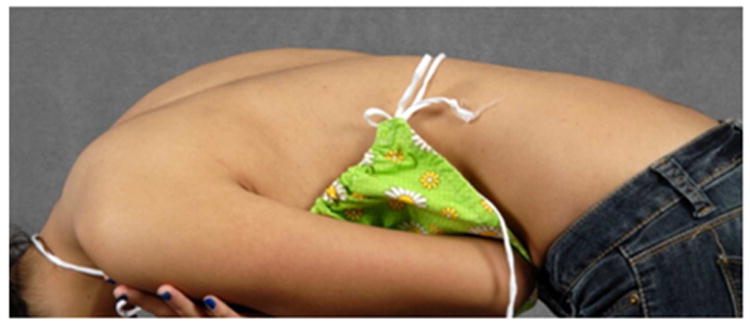
A standard radiographic evaluation of spinal deformity include upright, posteroanterior and lateral, full spine images. The most commonly used classification system for adolescent idiopathic scoliosis (AIS) is the Lenke Classification.22 The “usual” AIS deformity is an apex right, main thoracic curve, with a loss of thoracic kyphosis at the apex of the deformity (Figure 1a, b, c, d). Atypical findings on posteroanterior (coronal plane) radiographs include and apex left thoracic deformity and sharp, angular deformities(Figure 2a, b).23 Perhaps the most important detail, related to identification of CM, is the measurement required of the sagittal Cobb angles. In our practice, a Lenke “+” modifier, indicating hyperkyphosis, will undergo MRI to ensure that there is no underlying neurological abnormality (Figure 2c).5,23,24 In the case of adolescent scoliosis, any atypia to the curve morphology or appearance warrants an MRI examination (Figure 2d). As previously noted, early onset scoliosis is more frequently associated with neural axis abnormalities, such as CM.11,12 One must remember that a large deformity presenting as AIS was almost certainly present as a juvenile and, in such cases, MRI may be indicated.
Figure 1.
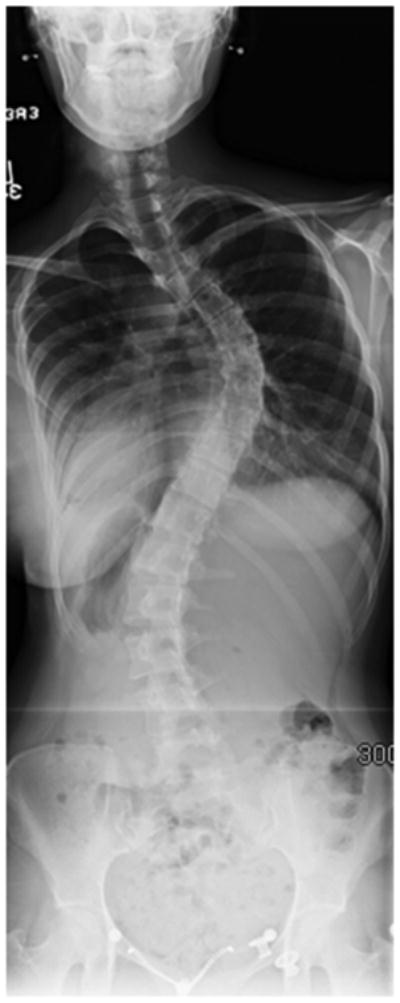
a – Standing postero-anterior radiograph of a 17 year old girl with typical radiographic findings of adolescent idiopathic scoliosis: apex right, no angular deformity
b – Standing lateral radiograph showing thoracic hypokyphosis, typical of adolescent idiopathic scoliosis.
c – Standing clinical image showing a loss of thoracic kyphosis.
d - Lateral forward bending clinical image showing a loss of thoracic kyphosis.
Figure 2.
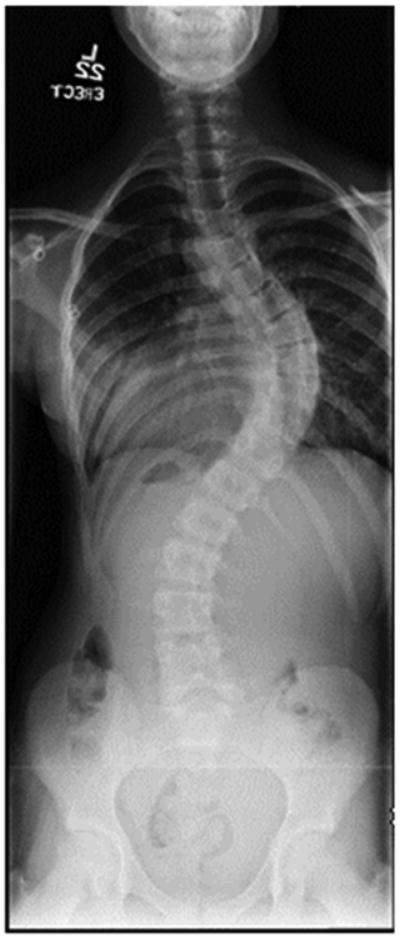
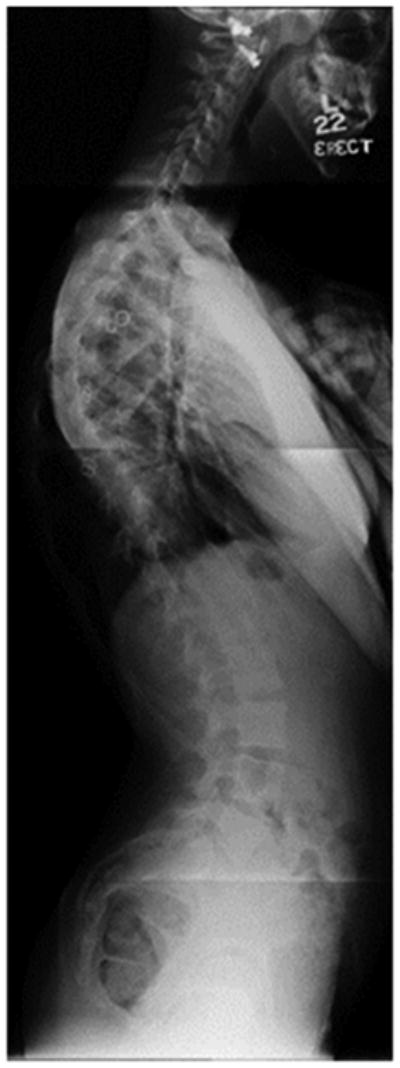
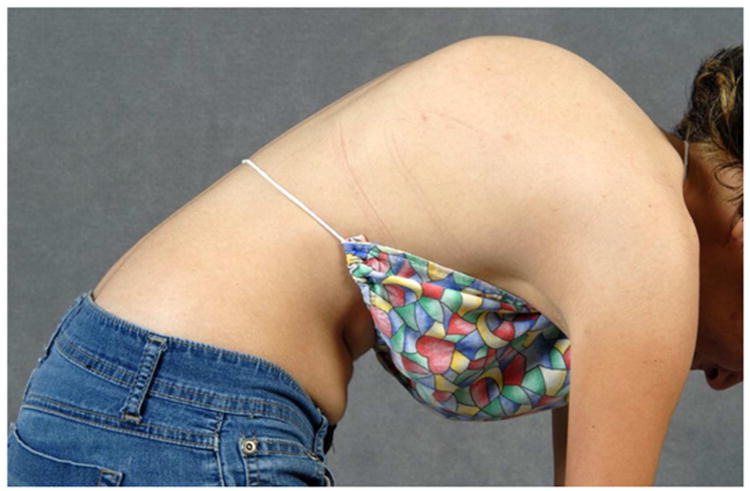
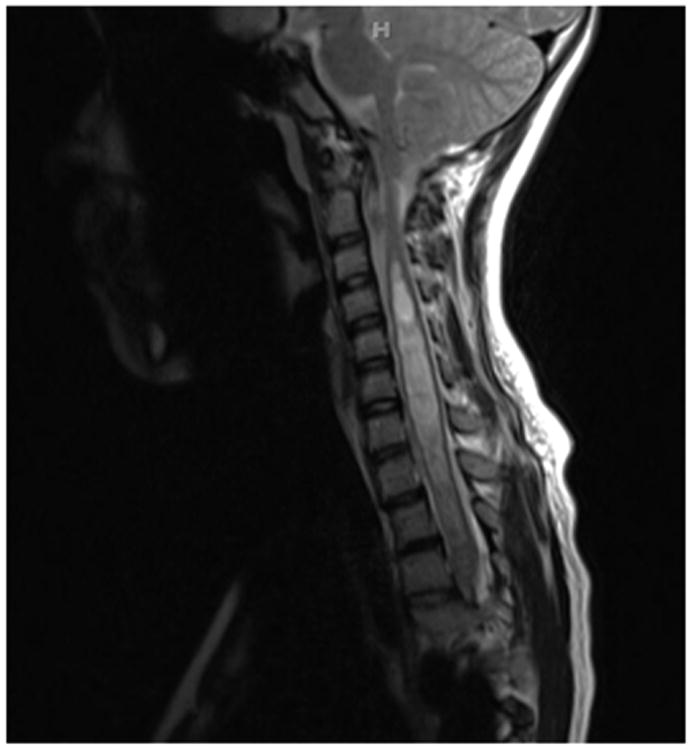
a - Standing postero-anterior radiograph of a 13 year old girl with “typical” coronal plane findings of adolescent idiopathic scoliosis.
b – Standing lateral radiograph showing proximal thoracic kyphosis, “atypical” for adolescent idiopathic scoliosis.
c – Lateral clinical image of 13 year old girl with proximal thoracic kyphosis.
d – T2-Weighted magnetic resonance imaging of this young woman, showing Chiari-I with syringomyelia.
Measurement of the coronal Cobb is important for prognostic factors, prior to decompression of the Chiari malformation. In general terms, the smaller the deformity at the time of presentation, the more likely the patient is to avoid surgery for the spinal deformity.4,5,15,16 Risk of spinal deformity progression is multifactorial, however, and also related to skeletal maturity of the patient, which is related to future growth. The Risser score is a reasonable proxy for remaining growth potential and it should be noted on the posteroanterior radiograph, in addition to the descriptives of the spinal deformity itself.25 Given the varied presentations of these deformities, it should not be surprising that relationships between the spinal deformity and the underlying CM and syringomyelia have been difficult to describe. Yeom et al and Qiu et al found no relationships between curve magnitude or character and descriptives of tonsillar descent or syrinx character.16,23 Conversely, Godzik et al found that larger syringes (maximum diameter > 6 mm) were more commonly associated with the presence of a scoliosis.26 Those patients were severe tonsillar descent (>12 mm) were less likely to have a scoliosis than those with moderate tonsillar descent (5-12mm). That these findings were not consistent emphasizes the varied nature of presentation and the relatively poorly understood pathophysiology of this disease process.
Management
Decompression of Chiari
Decompression of the CM may be offered, for both younger and older patients, to treat any CM-related symptoms, potentially minimize the need for future spinal deformity surgery, and to potentially decrease the risk of any future spinal deformity surgery. In younger patients (10 years old), there is evidence that decompression of the CM may result in resolution of the spinal deformity, without any further orthopedic intervention.4,14-16,27,28 Özerdemoglu, Transfeldt, and Denis described their experience, in one of the largest series today, with 59 patients. They found that decompression of the hindbrain offers potential benefit to the patients, whereas drainage of a syrinx did not offer any improvements in spinal deformity.29 While the pathophysiology remains a debate, there is consistency to the reports of improvement after CM decompression; the deformities tend to be smaller (less than 30 degrees) and the patients tend to be younger. Brockmeyer, Gollogly, and Smith reported curves as large as 56 degrees improving, though improvement of these larger deformities is an exception rather than the rule.15 As it is entirely uncommon for spontaneous resolution of an idiopathic case of juvenile scoliosis, the processes driving these spinal deformities are different in these two types of scoliosis. Improvements in the deformity are not consistent, however, and Farley et al reported no improvements in a small series of patients treated with suboccipital decompression and bracing, with eight of nine patients requiring spinal fusions.5
Decompression is often considered for CM with syringomyelia to potentially reduce the risk of neurological deficits with surgery. In the absence of a syrinx, the protective benefits of decompression are less compelling, and clinical judgment must be excercised. The presence of a syrinx is more concerning, as lengthening the spinal column through a reduction in the deformity could increase pressure within the fluid column, injuring the neural tissue. The protective benefits of decompression are not known. It is advisable, however, that given the potential for catastrophe that the CM be decompressed. Reduction in the syrinx is preferable and reimaging of the spinal column six months after the CM decompression is performed to evaluate the status of the syringomyelia. The spine surgeon must know whether a large syrinx exists, both for the preoperative informed decision-making process and for preparation for surgery.
Nonoperative Management
Nonoperative management of scoliosis associated with a CM is similar to management of idiopathic scoliosis, with the exception of the aforementioned neurosurgical intervention. Cases of severe, early onset scoliosis may be managed initially with casting, with transition to bracing or surgical intervention with a growing construct. Nonoperative management of a juvenile or adolescent scoliosis associated with CM is treated with observation for curves smaller than 20 degrees. In skeletally immature patients (e.g. Risser Stage < 4, Premenarchal girls) we will monitor at six month intervals, with a physical examination and standing posteroanterior and lateral full spine radiographs. Major Cobb angles are compared for evidence of progression. Deformities measuring between 20 and 45 degrees are often treated with a custom molded TLS orthosis. As with AIS, compliance with brace wear is essential, with a goal of 20+ hours in the brace per day. Brace wear is continued until the patient has reached skeletal maturity or the curve progresses despite bracing. There is no evidence to support particular nonoperative physical therapy modalities or manipulations in the management of scoliosis associated with CM.
Surgical Management of Spinal Deformity
Indications for surgical intervention in early-onset scoliosis (EOS) associated with CM are similar to those for idiopathic spinal deformities. Unfortunately, the heterogeneity of spinal deformity makes “hard and fast” rules for intervention impossible. In cases where curves are progressing in spite of casting and bracing, control of the deformity must be obtained to prevent detrimental changes to pulmonary function. A small number of “guided-growth” techniques exist, including Shilla constructs and growing-rod constructs. CM-associated deformities form a subset of EOS worthy of discussion because of the “typical” curve, hyperkyphotic, as opposed to the hypokyphotic scoliosis commonly seen with idiopathic deformities. A growing construct, then, must have control of the deformity in two planes, controlling both the progressive kyphosis and scoliosis. In the case of hypokyphosis, control of the scoliosis gives the surgeon adequate control of the sagittal plane deformity, through distraction, and a Shilla procedure may be a reasonable option. In the case of hyperkyphosis, stresses at the proximal end of construct may be more inclined to loosen, though given the paucity of literature on EOS with CM in general, there is no peer-reviewed proof of this. For this reason, it may be advisable to performing growing-rod procedures, to obtain control of the proximal segment via fusion with six pedicle screws, not four, again emphasizing a solid grasp of the progressively kyphotic, and growing, proximal segment.
Indications for definitive fusion in older patients are in line with idiopathic recommendations as well. Deformities measuring greater than 50 degrees on upright radiographs are generally considered for surgery. Early reports of spinal fusion in cases of scoliosis associated with CM reported higher rates of perioperative complications.1 More recent reports have minimized the risk associated with surgery in this patient population, likely due to advances in techniques, instrumentation, imaging, and intraoperative neurological monitoring. Several series have reported no new neurological deficits in these surgeries; however the number of patients examined in these studies is small and the studies may be underpowered to make definitive conclusions regarding safety.30,31 Each series was complicated by some deformities progressing above or below the fusion levels, stressing the need for careful preoperative evaluation of appropriate construct length. Ferguson et al. noted two patients developing proximal junctional kyphosis after posterior spinal fusion when the fusion construct was stopped caudal to a prior laminectomy level.30 We recommend strongly against this and would recommend including previously decompressed levels in the fusion construct.
Xie proposed that larger, more severe, spinal deformities may not need posterior fossa decompression prior to spine surgery if a vertebral column resection (VCR) is performed. The VCR shortens the vertebral column and, thus, relaxes the spinal cord. This decreases tension and pressure within the cord and any syringomyelia that may exist, likely minimizing the risk of iatrogenic injury to the still compressed hindbrain. However, one must acknowledge that the VCR is amongst the most dangerous and technically challenging procedures performed by pediatric spinal deformity surgeons, with intraoperative neurological event rates (spinal cord monitoring change or new neurological deficit) approaching 30% in one series.32 Thus, one must weigh the potential risks and benefits of VCR versus a staged decompression and posterior spinal fusion in scoliosis associated with CM.
The consideration of VCR for management of scoliosis associated with CM raises an important technical consideration, which is the use intra-operative neurophysiologic monitoring (IOM). IOM is mandatory in any spinal deformity surgery. Commonly employed modalities include somatosensory evoked potentials (SSEP) and transcranial motor evoked potentials (TcMEP). These methods monitor the posterior column sensory tracts and anterior column motor tracts, respectively. A less common modality is neurogenic motor evoked potentials, which monitor sensory pathways in an antidromic fashion and may serve as an alternative to TcMEP. As IOM is imperfect modality, patients should be instructed on the Stagnara intraoperative wake-up test and be prepared to perform a rehearsed examination should difficulties with IOM data arise.33 Our experience emphasizes this, as we have found more frequent IOM difficulties with CS than with AIS (27% vs 3%, p=0.007). Fortunately, Xie et al did not sustain any neurological deficits in their VCR series, though these surgeries were performed without IOM and this is unadvisable.
Conclusions
Despite the relative frequency of Chiari-associated spinal deformities, this disease process remains poorly understood. Syringomyelia is often present; however, this is not a necessary situation and scoliosis has been described in the absence of a syrinx. Decompression of the hindbrain may be considered but the protective benefits remain unclear. In young patients (<10 years old) and/or those with smaller coronal Cobb measurements (<40 degrees), decompression of the hindbrain may lead to resolution of the spinal deformity. Spinal fusion is reserved for those curves that progress to deformities greater than 50 degrees. These procedures may be associated with a higher risk of postoperative deficit and intraoperative neurological monitoring may be difficult. Patients, families, and surgeons should be prepared for this possibility. Further research is needed to understand the underlying pathophysiology to improve prognostication and treatment of this patient population.
Key Points.
Scoliosis commonly occurs in the setting of Chiari I malformation (CM) and even more frequently in the setting of CM with syringomyelia.
Decompression of the CM is often recommended as may lead to resolution of the spinal deformity and may make any subsequent spinal deformity surgery safer.
Spinal deformities are more likely to improve after CM decompression in younger patients (<10 years) with smaller coronal Cobb measurements (<30 degrees).
Spinal deformity surgery may be more challenging in these patients, due in part to difficulties with intraoperative, neurological monitoring challenges.
Despite the relative frequency of this disease process, the pathophysiology remains poorly understood.
Acknowledgments
Disclosure: Dr. Kelly receives research funding from AO Spine, Orthopedic Research and Education Foundation, Barnes Jewish Foundation, and the Cervical Spine Research Society. Dr. Lenke shares numerous patents with Medtronic (unpaid). He receives substantial royalties from Medtronic and modest royalties from Quality Medical Publishing. Dr. Lenke also receives or has received reimbursement related to meetings/courses from AMCICO, AOSpine, COA, BroadWater, DePuy, Dubai Spine Society, Medtronic, SOSORT, The Spinal Research Foundation, SRS and SSF. This project was supported by the Clinical and Translational Science Award (CTSA) program of the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) under Award Number UL1 TR000448.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huebert HT, MacKinnon WB. Syringomyelia and scoliosis. The Journal of bone and joint surgery British volume. 1969;51:338–43. [PubMed] [Google Scholar]
- 2.Isu T, Iwasaki Y, Akino M, Abe H. Hydrosyringomyelia associated with a Chiari I malformation in children and adolescents. Neurosurgery. 1990;26:591–6. doi: 10.1097/00006123-199004000-00006. discussion 6-7. [DOI] [PubMed] [Google Scholar]
- 3.Brockmeyer DL. Editorial. Chiari malformation Type I and scoliosis: the complexity of curves. J Neurosurg Pediatr. 2011;7:22–3. doi: 10.3171/2010.9.PEDS10383. discussion 3-4. [DOI] [PubMed] [Google Scholar]
- 4.Eule JM, Erickson Ma, O'Brien MF, Handler M. Chiari I malformation associated with syringomyelia and scoliosis: a twenty-year review of surgical and nonsurgical treatment in a pediatric population. Spine. 2002;27:1451–5. doi: 10.1097/00007632-200207010-00015. [DOI] [PubMed] [Google Scholar]
- 5.Farley Fa, Puryear A, Hall JM, Muraszko K. Curve progression in scoliosis associated with Chiari I malformation following suboccipital decompression. Journal of spinal disorders & techniques. 2002;15:410–4. doi: 10.1097/00024720-200210000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Krieger MD, Falkinstein Y, Bowen IE, Tolo VT, McComb JG. Scoliosis and Chiari malformation Type I in children. Journal of neurosurgery Pediatrics. 2011;7:25–9. doi: 10.3171/2010.10.PEDS10154. [DOI] [PubMed] [Google Scholar]
- 7.Charry O, Koop S, Winter R, Lonstein J, Denis F, Bailey W. Syringomyelia and scoliosis: a review of twenty-five pediatric patients. J Pediatr Orthop. 1994;14:309–17. doi: 10.1097/01241398-199405000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Diab M, Landman Z, Lubicky J, et al. Use and outcome of MRI in the surgical treatment of adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2011;36:667–71. doi: 10.1097/BRS.0b013e3181da218c. [DOI] [PubMed] [Google Scholar]
- 9.Do T, Fras C, Burke S, Widmann RF, Rawlins B, Boachie-Adjei O. Clinical value of routine preoperative magnetic resonance imaging in adolescent idiopathic scoliosis. A prospective study of three hundred and twenty-seven patients. The Journal of bone and joint surgery American volume. 2001;83-A:577–9. doi: 10.2106/00004623-200104000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Davids JR, Chamberlin E, Blackhurst DW. Indications for magnetic resonance imaging in presumed adolescent idiopathic scoliosis. The Journal of bone and joint surgery American volume. 2004;86-A:2187–95. doi: 10.2106/00004623-200410000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Gupta P, Lenke LG, Bridwell KH. Incidence of neural axis abnormalities in infantile and juvenile patients with spinal deformity. Is a magnetic resonance image screening necessary? Spine (Phila Pa 1976) 1998;23:206–10. doi: 10.1097/00007632-199801150-00011. [DOI] [PubMed] [Google Scholar]
- 12.Dobbs MB, Lenke LG, Szymanski DA, et al. Prevalence of neural axis abnormalities in patients with infantile idiopathic scoliosis. The Journal of bone and joint surgery American volume. 2002;84-A:2230–4. doi: 10.2106/00004623-200212000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Koc T, Lam KS, Webb JK. Are intraspinal anomalies in early onset idiopathic scoliosis as common as once thought? A two centre United Kingdom study. Eur Spine J. 2013;22:1250–4. doi: 10.1007/s00586-012-2599-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Attenello FJ, McGirt MJ, Atiba A, et al. Suboccipital decompression for Chiari malformation-associated scoliosis: risk factors and time course of deformity progression. J Neurosurg Pediatr. 2008;1:456–60. doi: 10.3171/PED/2008/1/6/456. [DOI] [PubMed] [Google Scholar]
- 15.Brockmeyer D, Gollogly S, Smith JT. Scoliosis associated with Chiari 1 malformations: the effect of suboccipital decompression on scoliosis curve progression: a preliminary study. Spine (Phila Pa 1976) 2003;28:2505–9. doi: 10.1097/01.BRS.0000092381.05229.87. [DOI] [PubMed] [Google Scholar]
- 16.Yeom JS, Lee CK, Park KW, et al. Scoliosis associated with syringomyelia: analysis of MRI and curve progression. Eur Spine J. 2007;16:1629–35. doi: 10.1007/s00586-007-0472-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Little DG, Song KM, Katz D, Herring JA. Relationship of peak height velocity to other maturity indicators in idiopathic scoliosis in girls. The Journal of bone and joint surgery American volume. 2000;82:685–93. doi: 10.2106/00004623-200005000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Hida K, Iwasaki Y, Koyanagi I, Abe H. Pediatric syringomyelia with chiari malformation: its clinical characteristics and surgical outcomes. Surgical neurology. 1999;51:383–90. doi: 10.1016/s0090-3019(98)00088-3. discussion 90-1. [DOI] [PubMed] [Google Scholar]
- 19.Nakahara D, Yonezawa I, Kobanawa K, et al. Magnetic resonance imaging evaluation of patients with idiopathic scoliosis: a prospective study of four hundred seventy-two outpatients. Spine (Phila Pa 1976) 2011;36:E482–5. doi: 10.1097/BRS.0b013e3181e029ed. [DOI] [PubMed] [Google Scholar]
- 20.Zadeh HG, Sakka SA, Powell MP, Mehta MH. Absent superficial abdominal reflexes in children with scoliosis. An early indicator of syringomyelia. The Journal of bone and joint surgery British volume. 1995;77:762–7. [PubMed] [Google Scholar]
- 21.Ono A, Suetsuna F, Ueyama K, et al. Surgical outcomes in adult patients with syringomyelia associated with Chiari malformation type I: the relationship between scoliosis and neurological findings. J Neurosurg Spine. 2007;6:216–21. doi: 10.3171/spi.2007.6.3.216. [DOI] [PubMed] [Google Scholar]
- 22.Lenke LG, Betz RR, Harms J, et al. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. The Journal of bone and joint surgery American volume. 2001;83-A:1169–81. [PubMed] [Google Scholar]
- 23.Qiu Y, Zhu Z, Wang B, Yu Y, Qian B, Zhu F. Radiological presentations in relation to curve severity in scoliosis associated with syringomyelia. Journal of pediatric orthopedics. 2008;28:128–33. doi: 10.1097/bpo.0b013e31815ff371. [DOI] [PubMed] [Google Scholar]
- 24.Whitaker C, Schoenecker PL, Lenke LG. Hyperkyphosis as an indicator of syringomyelia in idiopathic scoliosis: a case report. Spine (Phila Pa 1976) 2003;28:E16–20. doi: 10.1097/00007632-200301010-00027. [DOI] [PubMed] [Google Scholar]
- 25.Noordeen MH, Haddad FS, Edgar MA, Pringle J. Spinal growth and a histologic evaluation of the Risser grade in idiopathic scoliosis. Spine (Phila Pa 1976) 1999;24:535–8. doi: 10.1097/00007632-199903150-00006. [DOI] [PubMed] [Google Scholar]
- 26.Godzik J, Kelly MP, Radmanesh A, et al. Relationship of syrinx size and tonsillar descent to spinal deformity in Chiari malformation Type I with associated syringomyelia. J Neurosurg Pediatr. 2014;13:368–74. doi: 10.3171/2014.1.PEDS13105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sengupta DK, Dorgan J, Findlay GF. Can hindbrain decompression for syringomyelia lead to regression of scoliosis? Eur Spine J. 2000;9:198–201. doi: 10.1007/s005860000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hwang SW, Samdani AF, Jea A, et al. Outcomes of Chiari I-associated scoliosis after intervention: a meta-analysis of the pediatric literature. Childs Nerv Syst. 2012 doi: 10.1007/s00381-012-1739-3. [DOI] [PubMed] [Google Scholar]
- 29.Ozerdemoglu Ra, Transfeldt EE, Denis F. Value of treating primary causes of syrinx in scoliosis associated with syringomyelia. Spine. 2003;28:806–14. [PubMed] [Google Scholar]
- 30.Ferguson RL, Devine J, Stasikelis P, Caskey P, Allen BL. Outcomes in Surgical Treatment of “Idiopathic-Like” Scoliosis Associated With Syringomyelia. Journal of spinal disorders & techniques. 2002;15:301–6. doi: 10.1097/00024720-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Bradley LJ, Ratahi ED, Crawford Ha, Barnes MJ. The outcomes of scoliosis surgery in patients with syringomyelia. Spine. 2007;32:2327–33. doi: 10.1097/BRS.0b013e3181557989. [DOI] [PubMed] [Google Scholar]
- 32.Lenke LG, Newton PO, Sucato DJ, et al. Complications after 147 consecutive vertebral column resections for severe pediatric spinal deformity: a multicenter analysis. Spine (Phila Pa 1976) 2013;38:119–32. doi: 10.1097/BRS.0b013e318269fab1. [DOI] [PubMed] [Google Scholar]
- 33.Wilson-Holden TJ, Padberg AM, Lenke LG, Larson BJ, Bridwell KH, Bassett GS. Efficacy of intraoperative monitoring for pediatric patients with spinal cord pathology undergoing spinal deformity surgery. Spine (Phila Pa 1976) 1999;24:1685–92. doi: 10.1097/00007632-199908150-00010. [DOI] [PubMed] [Google Scholar]


