Abstract
Purpose
Colorectal cancer (CRC) survivors who remain fatigued during long-term follow-up are at risk for worse health outcomes and need relevant interventions most. The aim of this study is to prospectively assess cancer-related fatigue (CRF) and four categories of CRF correlates (clinical characteristics, demographic characteristics, behavior/well-being, functional status).
Methods
CRC survivors diagnosed between 2000 and 2009, as registered in the population-based Eindhoven Cancer Registry, completed the Fatigue Assessment Scale at three annual time points. Linear mixed models were used to assess the course of CRF and identify its correlates.
Results
CRF levels were relatively stable over time. Being female, young (≤65 years of age), and single; having a low educational level; treatment with chemotherapy; and having one or more comorbid conditions were associated with higher CRF scores. Years since diagnosis, radiotherapy, and disease stage were not related to CRF over time.
Significant between- and within-subject effects were found for all well-being factors (social, emotional, and cognitive functioning, and global quality of life), symptoms (anxiety, depression, pain, and insomnia), and functional status (physical and role functioning, physical activity levels) in relation to CRF.
The differences in CRF levels could, for a large part, be attributed to differences in behavior/well-being (59 %), functional status (37 %), and, to a lesser extent, to sociodemographic (4 %) and clinical characteristics (8 %).
Conclusion
This study showed that sociodemographic and clinical factors were associated with CRF levels over time among CRC survivors; however, behavior/well-being and functional status explained a larger part of the variance in levels of CRF.
Keywords: Behavior, Cancer, Fatigue, Functional status, Survivorship, Well-being
Introduction
Improvements in early detection and treatment have increased colorectal cancer (CRC) survival rates [1]. In the Netherlands, there were about 77,000 CRC survivors in 2009, which is expected to increase to 121,000 in 2020 [2]. The majority (>56 %) of the CRC patients survive relatively long (>10 years after diagnosis) [3]. Despite these advancements, cancer treatments may put cancer survivors at risk for long-term side effects. With more patients surviving longer, the long-term effects of cancer and its treatment on the survivors’ well-being are of increasing importance.
An important long-term effect of cancer and its treatment is cancer-related fatigue (CRF). CRF is defined as a subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion that is not proportional to recent activity and interferes with usual functioning [4]. CRF is the most common symptom experienced by cancer survivors, with prevalence rates of up to 99 % among survivors directly after diagnosis and during treatment [5]. Among CRC survivors, prevalence rates range from 41 % among short-term survivors (<5 years since diagnosis) and 35 % among long-term survivors (≥5 years since diagnosis) [6]. Cancer survivors indicate that CRF is more troublesome and has a greater negative impact on health-related quality of life (HRQoL) and daily activities than other distressing cancer symptoms, like pain and depression [7]. Given the high prevalence and impact of CRF, it is an important target for identification and treatment.
The causes underlying CRF are still not well understood. However, it is hypothesized that CRF is rooted in both biology and behavior [8]. In a recent recommendation paper, a conceptual framework was proposed whereby five categories of CRF correlates were identified: clinical characteristics (e.g., disease stage, treatment), demographic characteristics (e.g., age, sex), behavior/well-being (e.g., anxiety, pain), functional status (e.g., performance status), and biologic status (e.g., cytokine function) [8]. Although studies show that prevalence rates of CRF diminish during post-treatment follow-up, more insight is needed into the longitudinal course of CRF during long-term survivorship and the correlates that contribute to CRF over time. Survivors who remain fatigued during long-term follow-up are at risk for worse health outcomes and need relevant interventions most [8]. To achieve personalized management of CRF, insight into the correlates of CRF among long-term survivors is needed to identify subgroups of survivors at risk of remaining fatigued and also the correlates for intervention. Therefore, the aim of this study is to prospectively assess CRF and its correlates among CRC survivors. An adapted version of the conceptual CRF correlates framework will be used for our study (Fig. 1). We have not included the biological status dimension of the original model in our study, as the Patient Reported Outcomes Following Initial treatment and Long term Evaluation of Survivorship (PROFILES) registry currently does not collect biological data.
Fig. 1.
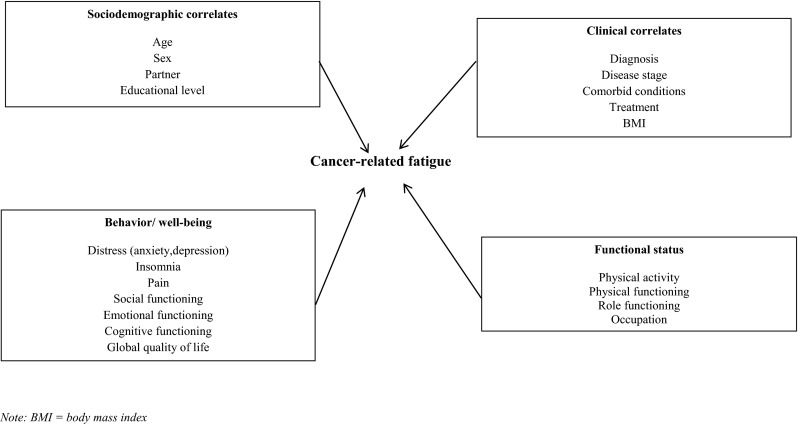
Correlates of cancer-related fatigue
Methods
Setting and population
This study is part of a longitudinal, population-based survey among CRC survivors registered within the Eindhoven Cancer Registry (ECR) of the Comprehensive Cancer Centre Netherlands. The ECR records data on all individuals who are newly diagnosed with cancer in the southern part of the Netherlands, an area with 2.3 million inhabitants, 18 hospital locations, and two large radiotherapy institutes. All individuals diagnosed with CRC between 2000 and 2009 as registered in the ECR were eligible for participation. Survivors who had died prior to start of the study (according to the Central Bureau for Genealogy which collects information on all deceased Dutch citizens via the civil municipal registries and hospital records) or had unverifiable addresses were excluded. Also, survivors with severe cognitive impairment (e.g., dementia) were excluded because it was expected that they would have difficulties completing the questionnaires without assistance. The study started in December 2010 (T1), and respondents received a subsequent questionnaire in 2011 (T2) and 2012 (T3). A complete overview of the selection of survivors can be found in Fig. 2. Ethical approval for the study was obtained from a local certified Medical Ethics Committee of the Maxima Medical Centre Veldhoven, the Netherlands.
Fig. 2.
Flow chart of the study
Data collection
Data collection was done within PROFILES. PROFILES is a registry for the study of the physical and psychosocial impact of cancer and its treatment from a dynamic, growing population-based cohort of both short- and long-term cancer survivors. PROFILES contains a large web-based component and is linked directly to clinical data from the ECR. Details of the data collection method were previously described [9].
Study measures
Fatigue
CRF was assessed with the Fatigue Assessment Scale (FAS), a questionnaire consisting of ten items. The response scale is a five-point scale (1 never to 5 always), and total scores can range from 10 to 50. Survivors can be divided into two groups based on total FAS scores [10]: not fatigued (as defined by a score of 10 to 21) and fatigued (22 to 50). The psychometric properties are good [11].
Clinical characteristics
Clinical information was available from the ECR that routinely collects data on tumor characteristics, including date of diagnosis, tumor stage, primary treatment, and survivors’ background characteristics. Comorbidity at the time of survey was assessed with the adapted Self-Administered Comorbidity Questionnaire (SCQ) [12]. Questions on height and weight were added to the questionnaire to calculate body mass index (BMI).
Sociodemographic characteristics
Questions on marital status and educational level were added to the questionnaire.
Behavior/well-being
Well-being (or HRQoL) was measured by the Dutch version of the validated European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (EORTC QLQ-C30) [13]. This 30-item HRQoL questionnaire consists of five functional scales, of which only the emotional, social, and cognitive functioning scales were used in this study, a global health status scale, three symptom scales, and single symptom items (only the insomnia and pain items will be used). Answer categories range from one (not at all) to four (very much). All scales are linearly transformed according to the guidelines of the EORTC [13], to reach a scale range of 0 to 100. A higher score on the functional scales and global quality of life implies better HRQoL, while a higher score on the symptom scales and items implies more symptoms.
Symptoms of anxiety and depression were assessed with the Hospital Anxiety and Depression Scale (HADS), with seven items each for assessing both anxiety and depression [14]. All items were scored on a zero- to three-point scale, with higher scores indicating more symptoms. A sum score was calculated for both scales, which can range from 0 to 21.
Functional status
Functional status was assessed with the physical and role functioning scales of the EORTC QLQ-C30 [13] and a self-designed question on employment status which was added to the questionnaire.
Physical activity level as a dimension of functional status was assessed with questions derived from the validated European Prospective Investigation into Cancer (EPIC) Physical Activity Questionnaire [15]. Survivors were asked how much time they spend on the following activities (average number of hours per week (h/week), in summer and winter separately): walking, bicycling, gardening, housekeeping, and sports. Six separate sports could be specified. Total PA was calculated by summing hours/week of all activities. To include an estimate of intensity, metabolic equivalent intensity values (MET) were assigned to each activity, according to the compendium of physical activities [16, 17]. The duration of moderate to vigorous physical activity (MVPA) was assessed as time (h/week) spent on walking, bicycling, gardening, and sports (≥3 MET), excluding housekeeping and light intensity sports (<3 MET).
Statistical analyses
Differences in sociodemographic and clinical variables between respondents and non-respondents were examined with analysis of variance (ANOVA) for continuous variables and chi-square tests for categorical variables, where appropriate. Potential non-response bias during follow-up was assessed by comparing the characteristics of survivors who responded to all three waves (full respondent), with those who responded to either two or one data wave. All further analyses were based on survivors who responded to all three waves.
The course of CRF and the individual associations between each independent variable and CRF over time were analyzed using linear mixed models (covariance pattern model with an unstructured error covariance matrix and maximum likelihood estimation) [18]. This technique uses data efficiently by also including incomplete cases in the analyses. As a result of this, bias is limited and statistical power is preserved. Linear mixed models were used to adjust for the dependence of observations. In order to correctly interpret all model parameters, all continuous variables were grand mean centered [18, 19]. Time was analyzed as a regular categorical predictor with three levels (i.e., three time points). Sociodemographic and clinical variables were analyzed as time-invariant predictors (i.e., baseline characteristics were used).
In the first step, we developed a longitudinal linear mixed model by putting CRF as a dependent variable in the regression equation and one variable of interest, time, and the possible confounders as independent variables. Confounders were chosen based on a priori assumptions: age, gender, having a partner, educational level, years since diagnosis, disease stage, treatment, and number of comorbidities. The confounders were also assessed for their association with CRF.
In the second step of the longitudinal data analyses, we examined the between-subject and within-subject effects for each continuous independent variable separately. Differences in CRF can occur because survivors differ in the independent variable (between-subject) or because a survivor differs over time in the independent variable (within-subject). Therefore, the between-subject estimate determined if differences in the independent variable between survivors resulted in differences in CRF. This estimate was represented by survivors’ average amount of CRF reported during the study across the three measurements. The within-subject estimate determined causal relations by assessing if changes in the independent variable within a participant were related to changes in CRF and was represented by the difference between survivors’ CRF at a certain point in time and his/her average CRF during the study. The between-subject and within-subject estimates were simultaneously entered in the linear mixed models together with the possible confounders and two dummies for time, with T1 as reference category, as independent variables and CRF as dependent variable.
Furthermore, cross-sectional multivariate linear regression analyses were used to assess the conjoint association between multiple independent variables and CRF at T1. The explained variance at T1 was assessed for the following domains: sociodemographic factors, clinical factors, behavior/well-being, and functional status. Linear regression analyses were more appropriate than linear mixed models to assess the explained variance, and therefore, we examined the conjoint association at one time point instead of over time.
Analyses were performed in IBM SPSS 22.0, using significance level of α = 0.05.
Results
Sociodemographic and clinical characteristics of respondents and non-respondents at T1
The questionnaire was completed by 73 % of survivors (n = 2625) at T1, 83 % (n = 1643) at T2, and 82 % (n = 1458) at T3. Respondents at T1 were significantly younger, were more often male, had a longer time since diagnosis, were more often diagnosed with stage I disease, and were more often treated with radiotherapy compared to non-respondents at T1 (all p < 0.05; data not shown). Furthermore, respondents at T1 more often received radiotherapy and were more often male compared to survivors with unverifiable addresses at T1 (p < 0.05; data not shown).
Differences between CRC survivors who completed one or more than one questionnaire
CRC survivors who completed only one questionnaire were older at time of first enrollment, were more often female, were less likely to have a partner, were less likely to have a job, were not meeting the physical activity guidelines, and were more fatigued compared to those who completed two or more questionnaires. In addition, they were more often diagnosed with disease stage IV and, thus, less likely to receive radiotherapy and surgery as primary treatment.
CRC survivors who completed only one or two questionnaires had a lower educational level and reported more comorbid conditions, more symptoms (anxiety, depression, pain), less functioning (physical, role, emotional, cognitive, and social), and lower quality of life compared to those who completed all three questionnaires.
No differences were found in time since diagnosis, receiving chemotherapy in addition to surgery as primary treatment, BMI, and insomnia (Table 1).
Table 1.
Sociodemographic and clinical characteristics of CRC survivors at T1
| One wave N = 891 | Two waves N = 281 | Full response (three waves) N = 1453 | p value | |
|---|---|---|---|---|
| Sociodemographic characteristics | ||||
| Sex | 0.02 | |||
| Male | 458 (51 %) | 157 (56 %) | 833 (57 %) | |
| Female | 433 (49 %) | 124 (44 %) | 620 (43 %) | |
| Age mean (SD) | 71.3 (9.4) | 69.4 (9.9) | 68.2 (9.3) | <0.001 |
| Age (years) | <0.001 | |||
| ≤ 65 | 234 (26 %) | 85 (30 %) | 536 (37 %) | |
| > 65 | 657 (74 %) | 196 (70 %) | 917 (63 %) | |
| Partner | ||||
| Yes | 626 (71 %) | 222 (80 %) | 1136 (79 %) | <0.001 |
| Educational level | <0.001 | |||
| Low | 235 (27 %) | 67 (24 %) | 218 (15 %) | |
| Middle | 510 (59 %) | 159 (58 %) | 899 (62 %) | |
| High | 125 (14 %) | 50 (18 %) | 333 (23 %) | |
| Clinical characteristics | ||||
| Years since diagnosis mean (SD) | 5.3 (2.8) | 5.1 (2.8) | 5.1 (2.8) | 0.46 |
| Years since diagnosis | 0.55 | |||
| ≤ 5 | 514 (58 %) | 170 (61 %) | 828 (57 %) | |
| > 5 | 377 (42 %) | 111 (39 %) | 625 (43 %) | |
| Stage | <0.001 | |||
| I | 262 (30 %) | 77 (28 %) | 441 (31 %) | |
| II | 335 (38 %) | 101 (36 %) | 511 (36 %) | |
| III | 220 (25 %) | 86 (31 %) | 418 (30 %) | |
| IV | 59 (7 %) | 14 (5 %) | 41 (3 %) | |
| Chemotherapy | ||||
| Yes | 255 (29 %) | 88 (31 %) | 428 (30 %) | 0.68 |
| Radiotherapy | ||||
| Yes | 243 (27 %) | 85 (30 %) | 474 (33 %) | 0.02 |
| Surgery | ||||
| Yes | 872 (98 %) | 279 (99 %) | 1447 (99 %) | <0.001 |
| Number of comorbid conditions | 0.01 | |||
| None | 192 (24 %) | 61 (23 %) | 361 (26 %) | |
| One | 208 (26 %) | 69 (26 %) | 432 (31 %) | |
| Two or more | 390 (49 %) | 139 (52 %) | 600 (43 %) | |
| Comorbid conditions (yes) | ||||
| Heart disease | 176 (22 %) | 53 (20 %) | 234 (17 %) | 0.01 |
| Stroke | 29 (4 %) | 13 (5 %) | 24 (2 %) | 0.01 |
| Hypertension | 267 (34 %) | 94 (35 %) | 502 (36 %) | 0.57 |
| Asthma/COPD | 104 (13 %) | 40 (15 %) | 123 (9 %) | 0.001 |
| Diabetes | 128 (16 %) | 52 (19 %) | 176 (13 %) | 0.004 |
| Stomach disease | 15 (2 %) | 6 (2 %) | 20 (1 %) | 0.54 |
| Kidney disease | 41 (5 %) | 19 (7 %) | 40 (3 %) | 0.001 |
| Liver disease | 34 (4 %) | 19 (7 %) | 25 (2 %) | <0.001 |
| Thyroid disease | 49 (6 %) | 12 (5 %) | 56 (4 %) | 0.07 |
| Osteoarthritis | 223 (28 %) | 72 (27 %) | 343 (25 %) | 0.18 |
| Rheumatoid arthritis | 68 (9 %) | 16 (6 %) | 80 (6 %) | 0.03 |
| Back pain | 223 (28 %) | 66 (25 %) | 378 (27 %) | 0.50 |
| BMI | 26.6 (4.8) | 26.5 (3.8) | 26.8 (4.1) | 0.41 |
| Behavior/well-being | ||||
| Anxiety (0–21) | 4.9 (4.0) | 5.1 (3.8) | 4.4 (3.7) | <0.001 |
| Depressive symptoms (0–21) | 5.3 (4.2) | 4.6 (3.7) | 3.8 (3.3) | <0.001 |
| Pain (0–100) | 18.1 (25.2) | 18.0 (25.1) | 15.2 (23.6) | 0.01 |
| Insomnia (0–100) | 21.9 (29.8) | 23.1 (29.1) | 19.9 (27.6) | 0.11 |
| Social functioning (0–100) | 85.3 (23.9) | 84.0 (23.9) | 87.6 (21.2) | 0.01 |
| Emotional functioning (0–100) | 84.1 (20.8) | 83.5 (20.1) | 87.4 (18.2) | <0.001 |
| Cognitive functioning (0–100) | 84.1 (20.5) | 82.7 (22.2) | 85.8 (19.9) | 0.03 |
| Global quality of life (0–100) | 73.4 (21.1) | 75.1 (18.1) | 79.7 (17.6) | <0.001 |
| Functional status | ||||
| Physical functioning (0–100) | 75.1 (22.8) | 78.0 (20.1) | 83.1 (18.7) | <0.001 |
| Role functioning (0–100) | 74.9 (29.9) | 76.2 (28.6) | 83.1 (25.3) | <0.001 |
| Occupation | <0.001 | |||
| Yes | 82 (10 %) | 51 (18 %) | 280 (19 %) | |
| Moderate or vigorous physical activity (h/week) | 9.1 (8.9) | 10.2 (8.4) | 12.3 (9.0) | <0.001 |
| Meeting physical activity guidelines | <0.001 | |||
| Yes | 664 (75 %) | 239 (86 %) | 1314 (91 %) | |
| Mean cancer-related fatigue | 21.8 (7.6) | 21.7 (7.0) | 19.9 (6.4) | <0.001 |
| Cancer-related fatigue | <0.001 | |||
| Yes | 352 (43 %) | 127 (46 %) | 485 (34 %) | |
Factors longitudinally associated with CRF
Figure 3 shows that the CRF levels were relatively stable over time. Mixed model analysis also showed no significant effect of time (Table 2).
Fig. 3.

Longitudinal changes in CRF over time for different correlates. Vertical axis represents CRF total scores (0–50); horizontal axis represents time points (T1–T3)
Table 2.
Adjusted linear mixed models estimating the individual associations between each independent variable and CRF over time
| B | 95 %CI | p value | |
|---|---|---|---|
| Time | |||
| T1 | Ref | Ref | |
| T2 | 0.10 | −0.15–0.35 | 0.44 |
| T3 | 0.12 | −0.13–0.38 | 0.34 |
| Sociodemographic characteristics | |||
| Sex | <0.001 | ||
| Male | −1.24 | −1.86 to −0.62 | |
| Female | Ref | Ref | |
| Age (years) | 0.02 | ||
| ≤ 65 | 0.62 | 0.12–1.14 | |
| > 65 | Ref | Ref | |
| Partner | 0.01 | ||
| Yes | −0.82 | −1.46 to −0.17 | |
| No | Ref | Ref | |
| Educational level | |||
| Low | 1.03 | 0.23–1.84 | 0.01 |
| Middle | 1.13 | 0.55–1.72 | <0.001 |
| High | Ref | Ref | |
| Clinical characteristics | |||
| Years since diagnosis | 0.20 | ||
| ≤ 5 | 0.30 | −0.16–0.75 | |
| > 5 | Ref | Ref | |
| Stage | |||
| I | −1.20 | −3.01–0.61 | 0.20 |
| II | −0.83 | −2.64–0.98 | 0.37 |
| III | −1.25 | −3.07–0.57 | 0.18 |
| IV | Ref | Ref | |
| Chemotherapy | |||
| Yes | Ref | Ref | |
| No | −0.86 | −1.72 to −0.01 | 0.05 |
| Radiotherapy | |||
| Yes | Ref | Ref | |
| No | −0.44 | −1.09–0.20 | 0.18 |
| Number of comorbid conditions | |||
| None | −1.63 | −2.07 to −1.19 | <0.001 |
| One | −0.80 | −1.15 to −0.44 | <0.001 |
| Two or more | Ref | Ref | |
| BMI | 0.12 | 0.07–0,17 | <0.001 |
| Behavior/well-being | |||
| Pain | |||
| Between | 0.15 | 0.14–0.17 | <0.001 |
| Within | 0.03 | 0.03–0.04 | <0.001 |
| Insomnia | |||
| Between | 0.10 | 0.09–0.11 | <0.001 |
| Within | 0.02 | 0.01–0.03 | <0.001 |
| Social functioning | |||
| Between | −0.21 | −0.23 to −0.20 | <0.001 |
| Within | −0.04 | −0.05 to −0.03 | <0.001 |
| Emotional functioning | |||
| Between | −0.23 | −0.24 to −0.21 | <0.001 |
| Within | −0.07 | −0.08 to −0.06 | <0.001 |
| Cognitive functioning | |||
| Between | −0.22 | −0.23 to −0.21 | <0.001 |
| Within | −0.06 | −0.07–0.05 | <0.001 |
| Global quality of life | |||
| Between | −0.27 | −0.28 to −0.25 | <0.001 |
| Within | −0.07 | −0.08 to −0.06 | <0.001 |
| Anxiety | |||
| Between | 1.04 | 0.97–1.12 | <0.001 |
| Within | 0.50 | 0.43–0.57 | <0.001 |
| Depressive symptoms | |||
| Between | 1.31 | 1.24–1.38 | <0.001 |
| Within | 0.60 | 0.54–0.67 | <0.001 |
| Functional status | |||
| Physical functioning | |||
| Between | −0.21 | −0.23 to −0.20 | <0.001 |
| Within | −0.08 | −0.09 to −0.06 | <0.001 |
| Role functioning | |||
| Between | −0.17 | −0.18 to −0.16 | <0.001 |
| Within | −0.05 | −0.05 to −0.04 | <0.001 |
| Occupation | |||
| Yes | Ref | Ref | |
| No | 0.64 | −0.21–1.51 | 0.14 |
| Moderate to vigorous physical activity level | |||
| Between | −0.14 | −0.18 to −0.10 | <0.001 |
| Within | −0.03 | −0.05 to −0.01 | <0.01 |
All sociodemographic factors were significantly associated with CRF levels over time (Table 2). Male CRC survivors reported, on average, 1.24 points lower levels of CRF than female survivors. Furthermore, survivors ≤65 years of age reported, on average, 0.62 points higher CRF levels compared to survivors >65 years of age. CRC survivors who have a partner scored 0.82 points lower on CRF than survivors without a partner, and survivors with a high educational level score 1 point lower on CRF compared to those with a medium or low educational level.
Regarding clinical characteristics, survivors who received chemotherapy as primary treatment scored 0.86 points higher on CRF compared to their counterparts. Survivors with two or more comorbid conditions reported higher levels of CRF compared to those with one (0.80 points) and no comorbid conditions (1.63 points). BMI was positively associated with levels of CRF (B = 0.12). Years since diagnosis, disease stage, and radiotherapy as primary treatment were not associated with levels of CRF over time.
Significant between- and within-subject effects were found for all well-being (social, emotional, cognitive functioning, and global quality of life) and symptom factors (anxiety, depression, pain, and insomnia).
In addition, significant between- and within-subject effects were found for all functional status variables (physical and role functioning, MVPA levels), except for occupational status which was not related to CRF levels over time among CRC survivors (Table 2).
Conjoint associations with CRF
At T1, 4 % of the differences in levels of CRF could be explained by sociodemographic factors, 8 % by clinical factors, 59 % by behavior/well-being, and 37 % by functional status. Of the behavior/well-being correlates, the highest variance in CRF could be attributed to differences in levels of depression (14 %), cognitive functioning (15 %), and global quality of life (12 %). Of the functional status correlates, the highest variance in CRF could be explained by physical (17 %) and role functioning (15 %). The total model explained 63 % of the variance in CRF levels.
Discussion
This study examined the course of CRF over time and its correlates among CRC survivors using a model adapted from a previously proposed conceptual framework. According to our results, multiple factors were associated with CRF over time: sociodemographic (sex, age, partner, educational level), clinical (chemotherapy, comorbid conditions, BMI), behavior/well-being (insomnia, pain, anxiety, depression, social, cognitive, and emotional functioning), and functional status (physical and role functioning, being employed, physical activity). The total model explained 63 % of the variance in CRF. The differences in CRF levels could, for a large part, be attributed to differences in behavior/well-being and functional status and, to a lesser extent, sociodemographic and clinical characteristics. The CRF levels were relatively stable over time, although significant within-person effects were found for behavior/well-being and functional status variables, which could be due to disease progression. All demographic characteristics were associated with levels of CRF, which is in accordance with the current literature and identifies groups at high risk for CRF. Younger cancer survivors, females, and lower educated survivors were more likely to feel fatigued [20–22]. Survivors with a partner were less likely to be fatigued, indicating the emotionally and instrumental supportive role of social support in addressing CRF [23].
Several studies among short-term cancer survivors found strong associations between clinical characteristics and levels of CRF [20, 24]. Our study among longer-term cancer survivors did not find significant temporal associations between disease stage, type of primary treatment, time since diagnosis, and levels of CRF, indicating that these factors become less important over time. Nevertheless, we found that survivors with two or more comorbid conditions were more likely to feel fatigued than those with either only one or without comorbidity. Similarly, other studies have reported that increasing numbers of comorbid conditions were associated with CRF [25]. We previously found that comorbidity explained a greater proportion of the variance in CRF scores among cancer survivors than clinical or sociodemographic variables [26].
Our results show that behavioral factors and well-being are significantly associated with CRF levels over time, which is consistent with previous literature demonstrating significant influences of physical distress factors like pain and insomnia and psychological distress factors like depression and anxiety on CRF [27]. It may be that these symptoms arise through a common pathway as previous research on inflammation and CRF suggests that tumors and the treatments used to treat them activate proinflammatory cytokines, leading to CRF and other symptoms [28].
Several previously conducted studies found significant associations between physical fitness and physical activity and levels of CRF among cancer survivors [27], which is in accordance with our finding of a negative association between functional status and CRF. Physical activity can counteract physical deconditioning and directly influence levels of CRF, or it can reduce CRF indirectly by its beneficial effects on mood, immune functioning, or sleep [27].
Most sociodemographic characteristics remain stable over time. On the other hand, behavioral/well-being factors and functional status may be subject to change. Although the CRF levels remained relatively stable over time in our study, we did find within-subject effects for all behavioral/well-being and functional status factors. This indicates that levels of CRF can change by influencing these factors by means of interventions. Some interventions have shown efficacy in reducing CRF. Meta-analyses showed that psychosocial interventions (cognitive behavioral therapy, supportive-expressive therapy, education/counseling, behavioral/relaxation therapy) had a small to moderate effect on CRF [29–31], exercise interventions had a near moderate effect in reducing CRF [29, 31–36], and pharmacological interventions with methylphenidate, a sympathomimetic psychostimulant, was more effective in reducing CRF than a placebo [37]. Nevertheless, it remains unclear which intervention (or combination of interventions) is best for each individual. A factor that makes the treatment of CRF complicated is that other symptoms such as pain, insomnia, and psychological distress frequently co-occur with CRF, as our results also indicate. The exact mechanisms of this co-occurrence are unclear: are all symptoms caused by cancer and its treatment? Are high pain levels causing high CRF levels or the other way around? And, which symptom(s) do we need to treat? It can be hypothesized that survivors with high levels of CRF and psychological distress will benefit most from a psychosocial intervention, while survivors with lower physical fitness will benefit from an exercise intervention. To personalize treatment of CRF, it may be an option for future studies to take a network approach. Instead of interpreting symptoms as a function of a set of underlying/latent factors, the network approach conceptualizes symptoms as mutually interacting, often reciprocally reinforcing elements of a complex network. Thus, rather than interpreting symptoms as measurements of a latent variable, symptoms are viewed as part of a causal system [38]. These causal systems can be different for each individual depending on centrality (which symptom is most important in one’s network), connectivity (which symptoms are connected and how well are they connected), and distance (how long does it take for one symptom to influence another symptom). By studying the network of an individual cancer survivor, it becomes possible to target interventions at particular part of a person’s network (e.g., the central symptom such as CRF) [39].
Some limitations of the present study should be mentioned. First, although information was present regarding demographic and clinical characteristics of the respondents and non-respondents, it remains unknown why non-respondents declined to participate. In addition, the differences found between respondents and non-respondents limit the generalizability of our results. Second, although our study has a longitudinal study design, it is still not possible to determine causality among the study variables, since the associations could also be influenced by variables that were not measured in the study. Further, we used the EORTC QLQ-C30 to assess different aspects of well-being; however, it could be argued that some constructs (e.g., cognitive functioning) could better be assessed with more specific questionnaires or even objective measures. Because all participants were CRC survivors, we can only generalize our results to this group of survivors. Last, we assessed an adapted model, as we do not have biological data. Nevertheless, this large population-based longitudinal study with good response rates provides a comprehensive view on several factors related to levels of CRF over time.
To conclude, this study showed that sociodemograpic and clinical factors were associated with CRF levels over time among CRC survivors; however, well-being, cancer-related symptoms, and functional status explained a larger part of the variance in levels of CRF.
Acknowledgments
Conflict of interest
None of the authors has a conflict of interest.
Funding
The present research is supported in part by a Veni grant (#451-10-041) from the Netherlands Organization for Scientific Research (The Hague, The Netherlands) awarded to Floortje Mols, a Cancer Research Award from the Dutch Cancer Society (#UVT-2009-4349) to Lonneke van de Poll-Franse, and a Social Psychology Fellowship from the Dutch Cancer Society to Melissa Thong (#UVT2011-4960). Data collection for this study was funded by the Comprehensive Cancer Centre Netherlands, Eindhoven, The Netherlands, the Center of Research on Psychology in Somatic diseases (CoRPS), Tilburg University, The Netherlands, and an investment subsidy (#480-08-009) of the Netherlands Organization for Scientific Research, The Hague, The Netherlands. The funding sources were involved neither in the collection, interpretation, and analysis of the data, nor in the decision for the writing and submission of this report for publication. The authors have full control over all primary data.
Footnotes
This manuscript has been prepared in accordance with the style of the journal, and all authors have approved of its contents. This manuscript is not being considered for publication elsewhere, and the findings of this manuscript have not been previously published.
References
- 1.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 2.Meulepas JM, Kiemeney LALM, Benraadt J (2011) Cancer in The Netherlands until 2020. Trends and prognoses (Kanker in Nederland Tot 2020: Trends En Prognoses). Kanker in Nederland Tot 2020: Trends En PrognosesSignaleringscommissie Kanker van KWF Kankerbestrijding, Vereniging van Integrale Kankercentra, Amsterdam, the Netherlands, 39
- 3.Cijfers over kanker. 2014. Available: http://www.cijfersoverkanker.nl. Accessed 19 December 2014
- 4.Berger AM, Abernethy AP, Atkinson A, et al. Cancer-related fatigue. J Natl Compr Cancer Netw. 2010;8:904–931. doi: 10.6004/jnccn.2010.0067. [DOI] [PubMed] [Google Scholar]
- 5.Servaes P, Verhagen C, Bleijenberg G. Fatigue in cancer patients during and after treatment: prevalence, correlates and interventions. Eur J Cancer. 2002;38:27–43. doi: 10.1016/S0959-8049(01)00332-X. [DOI] [PubMed] [Google Scholar]
- 6.Thong MSY, Mols F, Wang XS, et al. Quantifying fatigue in (long-term) colorectal cancer survivors: a study from the population-based patient reported outcomes following initial treatment and long term evaluation of survivorship registry. Eur J Cancer. 2013;49:1957–1966. doi: 10.1016/j.ejca.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hofman M, Ryan JL, Figueroa-Moseley CD, et al. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(Suppl 1):4–10. doi: 10.1634/theoncologist.12-S1-4. [DOI] [PubMed] [Google Scholar]
- 8.Barsevick AM, Irwin MR, Hinds P, et al. Recommendations for high-priority research on cancer-related fatigue in children and adults. J Natl Cancer Inst. 2013;105:1432–1440. doi: 10.1093/jnci/djt242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van de Poll-Franse LV, Horevoorts N, Eenbergen MV, et al. The patient reported outcomes following initial treatment and long term evaluation of survivorship registry: scope, rationale and design of an infrastructure for the study of physical and psychosocial outcomes in cancer survivorship cohorts. Eur J Cancer. 2011;47:2188–94. doi: 10.1016/j.ejca.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 10.Michielsen HJ, Drent M, Peros-Golubicic T, De Vries J. Fatigue is associated with quality of life in sarcoidosis patients. Chest. 2006;130:989–994. doi: 10.1378/chest.130.4.989. [DOI] [PubMed] [Google Scholar]
- 11.Michielsen HJ, De Vries J, Van Heck GL. Psychometric qualities of a brief self-rated fatigue measure: the fatigue assessment scale. J Psychosom Res. 2003;54:345–352. doi: 10.1016/S0022-3999(02)00392-6. [DOI] [PubMed] [Google Scholar]
- 12.Sangha O, Stucki G, Liang MH, et al. The self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49:156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 13.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 14.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 15.Pols MA, Peeters PH, Ocke MC, et al. Estimation of reproducibility and relative validity of the questions included in the EPIC Physical Activity Questionnaire. Int J Epidemiol. 1997;26(Suppl 1):S181–189. doi: 10.1093/ije/26.suppl_1.S181. [DOI] [PubMed] [Google Scholar]
- 16.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 18.Hedeker DR, Gibbons RD. Longitudinal data analysis. Chapter 6. Hoboken: Wiley; 2006. [Google Scholar]
- 19.Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. Chapter 7. Mahwah: Erlbaum; 2003. [Google Scholar]
- 20.Jansen L, Herrmann A, Stegmaier C, et al. Health-related quality of life during the 10 years after diagnosis of colorectal cancer: a population-based study. J Clin Oncol. 2011;29:3263–3269. doi: 10.1200/JCO.2010.31.4013. [DOI] [PubMed] [Google Scholar]
- 21.Baldwin CM, Grant M, Wendel C, et al. Gender differences in sleep disruption and fatigue on quality of life among persons with ostomies. J Clin Sleep Med. 2009;5:335–343. [PMC free article] [PubMed] [Google Scholar]
- 22.Wu H-S, Harden JK. Symptom burden and quality of life in survivorship: a review of the literature. Cancer Nurs. 2015;38:E29–54. doi: 10.1097/NCC.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 23.Soares A, Biasoli I, Scheliga A, et al. Association of social network and social support with health-related quality of life and fatigue in long-term survivors of Hodgkin lymphoma. Support Care Cancer. 2013;21:2153–2159. doi: 10.1007/s00520-013-1775-x. [DOI] [PubMed] [Google Scholar]
- 24.Goedendorp MM, Andrykowski MA, Donovan KA, et al. Prolonged impact of chemotherapy on fatigue in breast cancer survivors: a longitudinal comparison with radiotherapy-treated breast cancer survivors and noncancer controls. Cancer. 2012;118:3833–3841. doi: 10.1002/cncr.26226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbera L, Seow H, Howell D, et al. Symptom burden and performance status in a population-based cohort of ambulatory cancer patients. Cancer. 2010;116:5767–5776. doi: 10.1002/cncr.25681. [DOI] [PubMed] [Google Scholar]
- 26.Vissers PAJ, Thong MS, Pouwer F, et al. The impact of comorbidity on Health-Related Quality of Life among cancer survivors: analyses of data from the PROFILES registry. J Cancer Surviv. 2013;7:607–13. doi: 10.1007/s11764-013-0299-1. [DOI] [PubMed] [Google Scholar]
- 27.Seo Y, Oh H, Seo W. Causal relationships among factors associated with cancer-related fatigue. Eur J Oncol Nurs. 2010;14:380–386. doi: 10.1016/j.ejon.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Bower JE, Lamkin DM. Inflammation and cancer-related fatigue: mechanisms, contributing factors, and treatment implications. Brain Behav Immun. 2013;30(Suppl):S48–57. doi: 10.1016/j.bbi.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duijts SF, Faber MM, Oldenburg HS, et al. Effectiveness of behavioral techniques and physical exercise on psychosocial functioning and health-related quality of life in breast cancer patients and survivors–a meta-analysis. Psychooncology. 2011;20:115–126. doi: 10.1002/pon.1728. [DOI] [PubMed] [Google Scholar]
- 30.Jacobsen PB, Donovan KA, Vadaparampil ST, Small BJ. Systematic review and meta-analysis of psychological and activity-based interventions for cancer-related fatigue. Health Psychol. 2007;26:660–667. doi: 10.1037/0278-6133.26.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kangas M, Bovbjerg DH, Montgomery GH. Cancer-related fatigue: a systematic and meta-analytic review of non-pharmacological therapies for cancer patients. Psychol Bull. 2008;134:700–741. doi: 10.1037/a0012825. [DOI] [PubMed] [Google Scholar]
- 32.Arnold M, Taylor NF. Does exercise reduce cancer-related fatigue in hospitalised oncology patients? A systematic review. Onkologie. 2010;33:625–630. doi: 10.1159/000321145. [DOI] [PubMed] [Google Scholar]
- 33.Brown JC, Huedo-Medina TB, Pescatello LS, et al. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20:123–133. doi: 10.1158/1055-9965.EPI-10-0988. [DOI] [PubMed] [Google Scholar]
- 34.McMillan EM, Newhouse IJ. Exercise is an effective treatment modality for reducing cancer-related fatigue and improving physical capacity in cancer patients and survivors: a meta-analysis. Appl Physiol Nutr Metab. 2011;36:892–903. doi: 10.1139/h11-082. [DOI] [PubMed] [Google Scholar]
- 35.Cramp F, Daniel J (2008) Exercise for the management of cancer-related fatigue in adults. The Cochrane database of systematic reviews. CD006145 [DOI] [PMC free article] [PubMed]
- 36.Velthuis MJ, Agasi-Idenburg SC, Aufdemkampe G, Wittink HM. The effect of physical exercise on cancer-related fatigue during cancer treatment: a meta-analysis of randomised controlled trials. Clin Oncol (R Coll Radiol) 2010;22:208–221. doi: 10.1016/j.clon.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Minton O, Richardson A, Sharpe M, et al. A systematic review and meta-analysis of the pharmacological treatment of cancer-related fatigue. J Natl Cancer Inst. 2008;100:1155–1166. doi: 10.1093/jnci/djn250. [DOI] [PubMed] [Google Scholar]
- 38.Borsboom D. Psychometric perspectives on diagnostic systems. J Clin Psychol. 2008;64:1089–1108. doi: 10.1002/jclp.20503. [DOI] [PubMed] [Google Scholar]
- 39.Borsboom D, Cramer AO. Network analysis: an integrative approach to the structure of psychopathology. Annu Rev Clin Psychol. 2013;9:91–121. doi: 10.1146/annurev-clinpsy-050212-185608. [DOI] [PubMed] [Google Scholar]