Abstract
Objective: The purpose of this study was to evaluate the feasibility of performing MR Elastography (MRE) as a screening tool for elevated liver stiffness in patients’ status-post Fontan procedure.
With Fontan patients now reaching adulthood, one of the factors that is increasingly affecting long-term prognosis is the presence of hepatic congestion and fibrosis. When the liver fibrosis progresses to cirrhosis, these patients may be candidates for liver transplantation. However, if detected early, steps can be taken to potentially slow or halt the progression of hepatic fibrosis. Currently, liver biopsy is the gold-standard for assessing liver fibrosis. Magnetic Resonance Elastography (MRE) is a relatively new, non-invasive imaging technique that quantitatively measures liver stiffness and provides an estimate of fibrosis extent. A retrospective study was performed evaluating liver stiffness with MRE in patients with a history of Fontan procedure. The MRE of the liver was performed in the same session as a clinical cardiac MRI. Liver stiffness values were calculated by drawing regions-of-interest on the stiffness maps. The mean liver stiffness and its correlation with the length of time since Fontan surgery were studied. Sixteen patients with 17 MRE exams were included in this study. All patients had elevated liver stiffness values by MRE, suggesting the presence of mild to severe fibrosis, and there was a trend towards higher liver stiffness with greater duration of time with the Fontan circulation. MRE is a feasible method for evaluating the liver in patients status-post Fontan procedure who are undergoing surveillance cardiac MRI. Our preliminary study shows that duration of hepatic congestion following Fontan procedure may be related to liver stiffness. Further investigation with histologic correlation is needed to determine the etiology and long-term sequela of elevated liver stiffness in this population.
Keywords: Fontan, Elastography, Pediatric, MRE, liver biopsy
Introduction
The Fontan operation (introduced by Francis Fontan in 1968) is used to palliate complex congenital heart defects with functionally univentricular physiology [1]. Studies estimate that survival rates for single ventricle congenital heart disease (CHD) have improved significantly over the past 20 years with many centers reporting greater than 90% survival after Stage 1 in recent years, even for hypoplastic left heart syndrome [2, 3]. After Stage 2 (bidirectional Glenn or hemi-Fontan), 98% of patients survive in the current era [4]. Utilization of staged palliation (i.e., an intervening Stage II operation), surgical innovations (e.g. lateral tunnel and extracardiac conduit modifications, and Fontan baffle fenestration placement), as well as technological improvements (e.g. modified ultrafiltration during the operation) have steadily increased surgical survival rates from 60% in the 1970’s to more than 98% currently [5]. This has resulted in a large population of Fontan survivors who are now aging from adolescence into adulthood [6].
After the Fontan procedure, the circulation of blood depends upon higher central venous pressures (CVP) to maintain an effective transpulmonary gradient, thus generating adequate blood flow through the pulmonary vascular bed in the absence of a pumping chamber [7, 8]. This elevated CVP is directly transmitted to the hepatic sinusoids. In the early stages post Fontan procedure, the liver becomes congested, enlarged and edematous. Evidence of hepatic fibrosis, confirmed via autopsy studies, has been shown to develop as soon as the early post-operative Fontan timeframe [9]. Over the long term, cellular atrophy, necrosis and fibrosis ensue, commonly referred to as congestive hepatopathy. The end-stage of congestive hepatopathy is cardiac cirrhosis, a result of irreversible hepatocyte damage and scarring. The exact mechanism for development of hepatic fibrosis in this patient population appears to be multi-factorial and is an area of active research [9, 10]. The reduction in Fontan mortality allows for additional morbidities to be manifestedfrom secondary organ injury due to the abnormal Fontan circulation, including an increased prevalence of hepatic insufficiency [11]. Recent MRI studies have shown that a high percentage of Fontan patients display abnormal parenchymal liver morphology and enhancement characteristics, as well as frequently demonstrating hypervascular liver nodules consistent with focal nodular hyperplasia (FNH) [12]. The incidence of FNH increases as the duration since Fontan procedure increases [12].
In pediatric liver disease, as in adults, management choices for these patients may depend upon the stage of fibrosis at diagnosis and the rate of progression [13]. While liver biopsy is the gold standard for diagnosing and assessing the presence and degree of fibrosis, disadvantages include: the potential for sampling error, the risk of complications to include hemorrhage and infection, need for anesthesia or sedation in children, a relatively high cost, and general poor acceptance by pediatric patients and their parents [14]. Magnetic Resonance Elastography (MRE) is a relatively new imaging technique with the potential for allowing a safe, rapid, cost-effective and non-invasive evaluation of a wide variety of hepatic diseases by quantitatively evaluating the stiffness of the liver parenchyma. MRE assesses tissue stiffness by measuring the speed of shear waves propagating within the parenchyma. This technique is FDA approved, and in adults has been shown to accurately detect and stage hepatic fibrosis and detect steatohepatitis [15, 16]. To our knowledge, a detailed description of the application of liver MRE in Fontan patients has not been published. The purpose of this article is to present our initial clinical experience with MRE in the Fontan population and illustrate our technique for the application of liver MRE in patients scanned for non-invasive post-Fontan evaluation.
Methods
Study Design
A HIPAA compliant, retrospective case series was assembled after approval was obtained from the institutional review boards at two tertiary care medical centers. The radiology databases at both institutions were searched to identify patients treated with Fontan palliation. After patients were identified, the PACS were searched to select those who had a MR scan. All Fontan patients that underwent MRE of the liver between November 2010 and March 2013 were included. Clinical, laboratory and histologic data regarding hepatic and cardiac abnormalities obtained within six months of imaging were recorded when available.
MRE technique
All studies were performed on a 1.5T GE HDx MRI scanner (General Electric, Waukesha, WI) using the 8-channel Cardiac/Torso coil. MRE equipment consists of an active and a passive driver system. The active driver is kept in the MR equipment room and the passive driver is placed on the patient’s right lower chest and upper abdomen at the level of xyphoid and the receiver surface RF coil is placed over the driver (Figure 1). The passive driver (approximately 19 cm in diameter) is connected to the active driver in the equipment room with a 25 feet long hollow plastic tube via a wave-guide. The passive driver and the connecting plastic tubing do not have any metal components. The audio subwoofer, part of the active driver, is programmed to generate low amplitude 60 Hz vibrations which are passed via pneumatic pressure to the passive driver. The start and end of the vibrations are controlled by the MR pulse sequence programmed and embedded as part of the scanner software. The MRE pulse sequence does not bypass any scanner safety standards as specified by the manufacturer. MRE in adult patients was performed as previously described by Ehman et al. [15, 16]. The MRE protocol for children was adapted and modified from adult MRE scan methods to better fit the younger age population, and has been previously detailed [17]. To help reduce anxiety and sudden movements in pediatric patients, we performed a pre-scan simulation mimicking the vibration during scanning. This adjustment enabled more successful scanning during the actual MRE sequence. For this investigation, MRE was performed as part of the routine clinical cardiac MRI exam in all patients.
Figure 1.
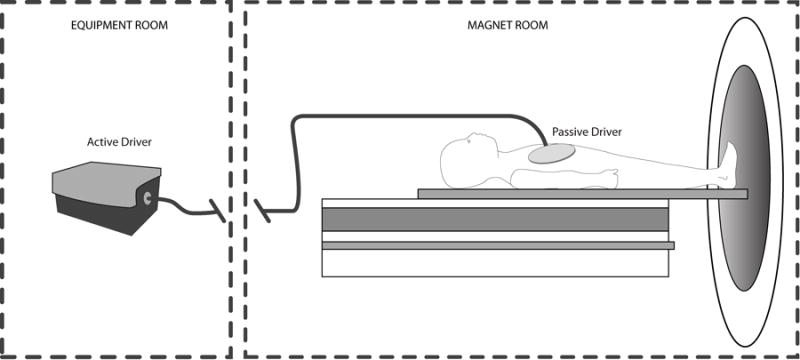
A schematic diagram of patient set-up with the MRE hardware. The active driver is placed in the MR computer room and the passive driver, connected via a hollow plastic tube through a wave-guide, is positioned on the anterior body approximately over the liver region [14]. MRE techniques modified and adapted for pediatric imaging.
For the MRE sequence, 4 axial slices through the liver, each 10 mm thick, were prescribed from the coronal localizer images. The clinical protocol and typical acquisition parameters for the MRE exam are outlined in Table 1. The initial coil coverage is set up in such a way that it covers the heart and liver. When prescribing the axial slices, the technologist chooses a location inferior to the heart at the widest portion of the liver (Figure 2). A new localizer was obtained at end expiration after the cardiac study so that the prescribed slices for the MRE sequence are placed in a reproducible location. MRE was performed in end-expiration for reproducibility of the position of the liver. One breath hold of 14–18 seconds was required for single MRE slice acquisition. Total MRE scan duration was determined.
Table 1.
MRE Sequence Acquisition Parameters
| Sequence | Plane | Approx. Scan time (seconds) | TR/TE (msec) | Slice thickness (mm) | Matrix size | Protocol |
|---|---|---|---|---|---|---|
| MRE FGRE | Axial | 50 | 50/min | 10 | 256×64 | Limited Liver MRE |
MRE= MR Elastography, FGRE= fast gradient recalled echo, min= minimum, TR= repetition time, TE= echo time, Coil used= 8 channel cardiac/torso coil
Figure 2.
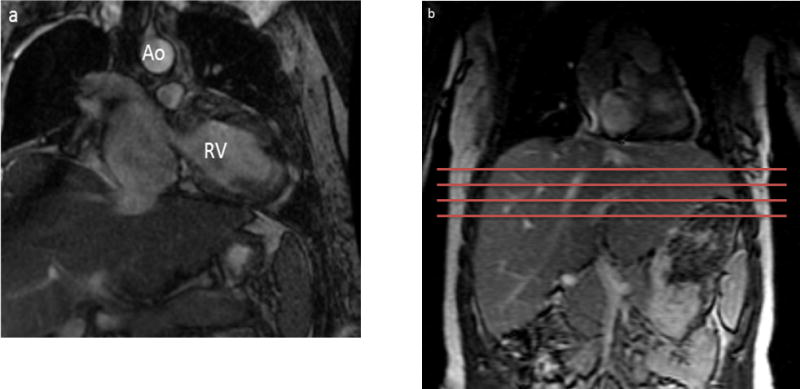
(a) 18 year old female with a history of hypoplastic left heart syndrome post Fontan procedure performed 17 years ago. MRE exam was performed as part of a clinical cardiac MR study. Patient had no visible Fontan baffle fenestration.
(b) MRE slice selection on the patient: The Torso coil is positioned such that the anterior portion of the coil can be used for cardiac imaging and the posterior region can be used for liver imaging. The four axial slices are prescribed so that the liver is imaged in its widest portion, inferior to the heart.
Image analysis
Post-processing of MRE images was performed using MRE Wave software (Mayo Clinic, Rochester, MN). Using this software, the image data is converted into quantitative stiffness maps, referred to as elastograms, displaying the stiffness of the liver parenchyma. The user can then draw a region-of-interest (ROI) around the liver carefully excluding all large blood vessels.
Imaging examinations were reviewed by a board certified pediatric radiologist with additional expertise in cardiac and body imaging and a pediatric cardiologist with cardiac MRI experience. MRE examinations were performed as part of a cardiac MRI and additional dedicated liver sequences were not performed routinely though images were reviewed for liver abnormalities. Standard sequences included multiplanar balanced steady state free precession (bSSFP) and multiphase magnetic resonance angiongrapy (MRA) which generally included the majority of the liver within the field of view. Prior and subsequent cross-sectional imaging of the liver was also reviewed for comparison.
Statistical analysis
Comparisons were made between current patient age and number of years since Fontan completion. Statistical tests were performed on the entire group using standard Mann-Whitney test. Non-parametric tests were chosen to keep the observations from the two groups independent from each other. For the sample size of n=17, Spearman’s correlation coefficients were derived for the two groups. The level of statistical significance was set at p < 0.05. Intraclass correlation coefficient results were interpreted according to the guidelines by Fleiss, with excellent at R > 0.75, fair to good at 0.40 ≤ R ≤ 0.75, and poor at R < 0.40 [18].
Results
Patients
Sixteen post-Fontan patients who underwent liver MRE as part of their clinical cardiac MRI examination were included in this study (17 study cases; 1 patient was scanned twice in the time period). The age range of the cohort was 12 to 42 years (mean age: 23.3 yrs. and median age: 21 yrs.) and the time since Fontan surgery (Fontan duration) ranged from 5 to 26 years (mean Fontan duration: 18.2 yrs. and median Fontan duration: 19 yrs.). The study included 7 patients with an atriopulmonary Fontan connection and 9 patients with a total cavopulmonary connection.
MRE/MRI findings
MRE added less than five minutes to total scan duration in all cases, including preparation time. All patients had an elevated liver stiffness value, with a mean of 5.1 ± 1.0 kPa and ranging from 3.4 kPa to 8.2 kPa (normal adult value is approximately 2.3 kPa) [15]. In a patient with normal circulation, these values suggest the presence of mild to severe fibrosis [15–17, 19]. Representative MRE magnitude images and elastogram stiffness maps are shown in Figures 3 and 4. In the overall population, liver stiffness values trended upwards with increasing years since Fontan palliation (Spearman’s correlation coefficient equals 0.546 with p-value of 0.023) (Figure 5). Also, a statistically significant difference was found in the liver stiffness between total cavopulmonary and atriopulmonary connection patients with a mean of 4.8 (3.4–5.8) kPa versus 5.7 (4.0–8.2) kPa respectively. This may primarily be due to the difference in the median Fontan duration between the two populations of 15.5 (5–19.8) years versus 25 (19–27) years respectively.
Figure 3.
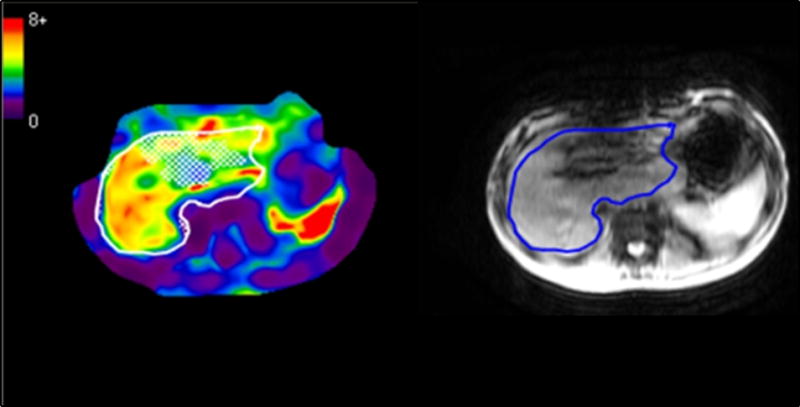
13 year old female with a history of tetralogy of Fallot with pulmonary atresia and hypoplastic right ventricle. Patient had an extracardiac fenestrated Fontan surgery at age 4. MRE was performed as part of the follow up cardiac MR exam. MRE images show increased liver stiffness (5.0 kPa), represented by the red, orange, and yellow areas. The sternotomy wires in the patient cause susceptibility artifact through the left lobe of the liver, but did not affect the ability to obtain liver stiffness values in the right lobe of the liver. The red structure in the left upper quadrant is the spleen.
Figure 4.
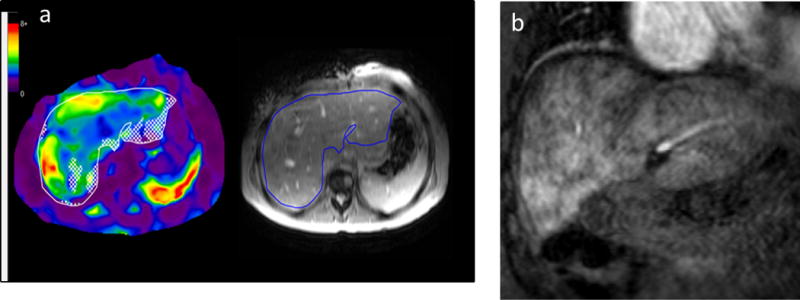
(a) 31 year old female with a history of pulmonary atresia with tricuspid stenosis, hypoplastic right ventricle, and normally related great vessels, status post neonatal Waterston shunt followed by classic Fontan procedure at age 5 years. A follow-up cardiac MRI was performed to evaluate her Fontan pathway, cardiac chamber sizes, and ventricular function and liver MRE was performed as part of this exam. Mean liver stiffness was 4.0 kPa, well above the accepted normal mean for an adult of 2.3 kPa.
(b) Coronal post-contrast MRI image obtained in the portal venous phase shows diffuse heterogeneous “cloud-like” enhancement of the liver parenchyma, a finding that is seen with liver fibrosis [21].
Figure 5.
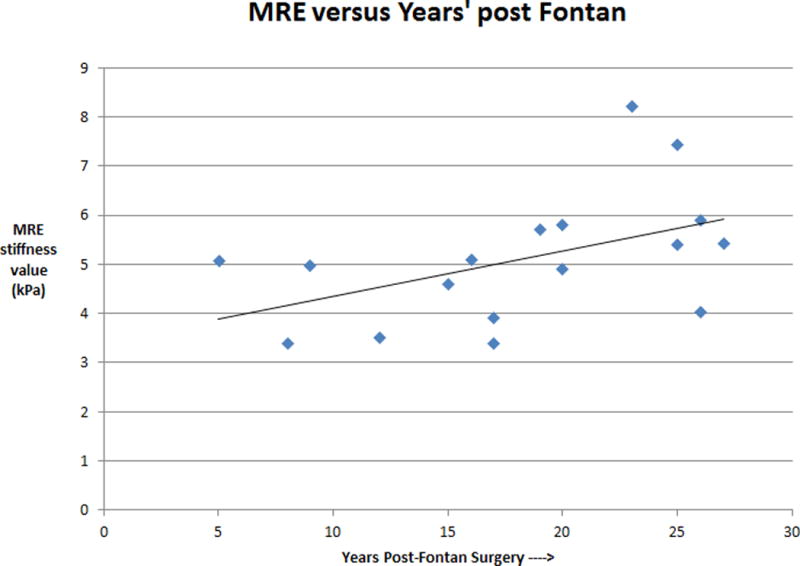
A graph of MRE liver stiffness versus years status post Fontan procedure (n=17). There is a trend towards increasing liver stiffness with increasing duration of Fontan physiology (correlation coefficient of 0.5 and p < 0.05).
On review of anatomic imaging, there were abnormalities of the liver parenchyma present in 14 of the 16 patients. These abnormalities ranged from heterogeneous enhancement of the liver, and other findings indicative of congestive hepatopathy [20], to frank cirrhosis by imaging criteria [21]. Using a cutoff of 4.89 kPa between normal or mildy fibrotic livers (F0-1) and moderate to severe fibrosis (F2–4) [15], 11 of the 16 patients (69%) demonstrated evidence of fibrotic livers in the moderate to severe range. Nine of those patients also had imaging features of frank cirrhosis including abnormal surface contour, scarring, parenchymal atrophy, caudate or left hepatic lobe hypertrophy and extrahepatic findings of portal hypertension. Additionally, four patients had hypervascular liver nodules, which are often associated with Fontan patients and thought to most likely represent focal nodular hyperplasia. One of these patients had a follow-up dedicated liver MRI scan using gadoxetate disodium (Eovist®) [22] and multiple small well-circumscribed nodules were observed throughout the liver that had imaging characteristics typical of focal nodular hyperplasia. The presence of nodules did not correlate with the degree of liver stiffness or Fontan duration.
Two patients in our study group developed hepatocellular carcinoma (HCC), one of which was biopsy-proven while the other was an imaging diagnosis. Both of these patients had METAVIR stage 3 fibrosis on biopsy taken from parenchyma not involved by tumor. In the first patient, a nodule was present which had imaging features suggestive of HCC, showed interval growth, and was subsequently confirmed as HCC by biopsy. This patient had a mean liver stiffness of 8.2 kPa and no other risk factors for the development of HCC. The other patient was diagnosed with HCC based on imaging features. This patient had a mean liver stiffness of 5.9 kPa and also had a history of chronic hepatitis C.
Hematological and biochemical profiles
Due to the lack of a standard surveillance approach for the development of liver complications in this population and the lack of a standard diagnostic work-up for causes of identified liver abnormalities, the clinical data available on our patients was quite variable. Historical liver function tests (LFTs), including aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, and direct/conjugated bilirubin, were available for 8 patients. The tests were normal in five out of these patients. A single patient had a history of an elevated gamma glutamyltransferase (GGT) several years prior to MRE with no follow-up value obtained.
Three patients had been screened for viral hepatitis, and two were negative for Hepatitis B and C while one patient was positive for chronic hepatitis C. Three of the patients had been exposed to medications with potential hepatotoxicity. All three were receiving, or had received, angiotensin converting enzyme (ACE) inhibitors and one patient had previously received procainamide and was taking amiodarone at the time of the study. However, the likelihood of drug induced hepatotoxicity seems small, given that all three patients had documented concurrent and historically normal serum aminotransferases.
Discussion
In our early experience, MRE has been useful as a noninvasive screening tool for assessing the liver in Fontan patients. The MRE was performed in a short period of time and revealed increased liver stiffness in all patients. Increased liver stiffness was associated with increased duration of time as a Fontan and atriopulmonary Fontan. The vast majority of Fontan survivors had abnormalities of the liver parenchyma present and a majority demonstrated evidence of liver fibrosis. A minority of the Fontan survivors had actual cirrhosis of the liver.
From a logistical standpoint, since most post-Fontan patients undergo surveillance cardiac MRI exams at our institution, and in many similar specialized centers, an MRE is easily added to the study. The MRE sequences add less than five minutes of preparation and actual imaging time to the standard cardiac MRI, which itself takes approximately one hour.
Our preliminary data show a high prevalence of liver abnormalities in this patient population, as evidenced by the elevated liver stiffness and anatomic imaging abnormalities in nearly all patients. Anatomic imaging, however, has not been shown to correlate with the degree of hepatic dysfunction or underlying histologic abnormality. Studies have been performed showing good correlation between the liver stiffness measured by MRE and stage of fibrosis, exceeding that of conventional imaging, specific biochemical laboratory tests or serum fibrosis panels [12, 15, 17, 23, 24]. MRE is able to quantitatively evaluate for liver disease and monitor for progression in conjunction with the clinical exam and biochemical analyses. Our results suggest that MRE should be further investigated as a possible screening and diagnostic tool for liver diseases in Fontan patients. A high number of studied Fontan patients had elevated liver stiffness in the moderate to severe fibrosis range. Additionally, we detected a trend of elevated liver stiffness values in patients whose livers were exposed to the high central venous pressure of the Fontan circulation for a longer duration. We believe these observations indicate a greater degree of congestive liver changes and probably ultimately fibrosis. Similarly, Friedrich-Rust et al. evaluated liver stiffness in Fontan patients using transient ultrasound elastography and found them to be at increased risk of having liver fibrosis and cirrhosis based on ultrasound and biochemical markers [25]. Consistent with our results with MRE, the authors report an increase in risk of liver fibrosis with the age of the patient and the Fontan duration.
Transient ultrasound elastography (Fibroscan, Echosens, France) is another non-invasive tool for assessment of liver fibrosis by measuring liver stiffness with ultrasound. Transient elastography is an easy and rapid procedure, however, strict adherence to quality criteria need to be followed to ensure the reliability of the results obtained. While using transient elastography to monitor liver disease in Fontan patients is also an option, MRE has several advantages comparatively. The ultrasound technique interrogates a selective, relatively small tissue sample with the transducer placed between the ribs, is operator dependent, has sampling errors due to heterogeneity of the liver in advanced fibrosis, and has somewhat poor performance in obese patients and in the presence of ascites [26, 27]. In addition, transient elastography with a pediatric size probe is not currently FDA approved for clinical use on children.
The prolonged elevation of CVP transmitted to the liver in patients with Fontan circulation results in congestive hepatopathy, which may lead to fibrosis and ultimately cirrhosis [11]. These changes are present in the majority of Fontan patients, with evidence of fibrosis found less than two years after surgery and its progression to cirrhosis reportedly observed within 10 years of the initial Fontan surgery [28]. Ghaferi et al reviewed autopsy specimens of nine patients who had prior Fontan operation and found that all had some degree of characteristic histologic changes along the spectrum of fibrosis to cirrhosis. Four of these nine patients were autopsied very soon after their Fontan, indicating that their hepatic pathology may have originated pre-Fontan surgery or perioperatively [29]. While worsening post-Fontan cardiac physiology can be expected to result in more severe liver pathology, no evidence exists that the presence of congestive liver disease worsens the prognosis of patients with heart failure, whose mortality rates are dominated by the severity of their cardiac disease. A better understanding of the natural course in patients with Fontan circulation and greater attention to liver complications is needed. Undoubtedly, a hepatologist will increasingly be required to care for this patient population as survival rates continue to improve [11]. These patients could be candidates for early intervention and more aggressive therapy. Although congestive hepatopathy secondary to Fontan circulation is an expected and well known consequence of long term elevation of CVP, the liver may not be routinely evaluated as patients usually remain relatively asymptomatic from liver disease until late in their clinical course. Chronically elevated CVP and ventricular dysfunction substantially increase the risk of hepatic dysfunction and may worsen over time in patients with Fontan circulation. With increased survival of post-Fontan CHD patients, physicians will face new management challenges with a shift in focus to long-term morbidity and quality of life issues which will include a greater emphasis on monitoring, treating and preventing liver disease.
While describing the anatomic imaging abnormalities associated with Fontan palliation was not a primary goal of this investigation, the presence of two patients with HCC highlights the importance of liver surveillance from an oncologic standpoint once cirrhosis has developed. Admittedly, one of these patients had chronic hepatitis C, which may have played a primary role in carcinogenesis. The other patient, however, had no additional risk factors for hepatic malignancy. The hypervascular nodules seen in these patients are generally benign and most commonly reported as FNH in the literature [12]. However, when nodules are large, have atypical imaging features, or show interval growth, further workup is warranted.
The current study was limited by its retrospective nature and small number of subjects. We acknowledge that the identified trend of increasing liver stiffness with increased Fontan duration is a tenuous one, based on a small number of subjects, with a wide range of values detected in patients at similar Fontan durations. In addition, none of the patients were less than 5 years post-Fontan. This study did not include prospective or longitudinal data to track liver stiffness through the stages of disease progression in individual patients, which would require many years of patient monitoring. Also, at present, liver biopsy is still considered the reference standard for the assessment of liver fibrosis, and only two of our patients had liver biopsies, though both biopsies correlated well with MRE results. With the ultimate goal of utilizing MRE to enhance current diagnostic capabilities used in the care of Fontan patients, this proof-of-principle study provided us with valuable information that will be used for future investigations. We did not attempt to correlate the liver stiffness with cardiac function but feel this is an important area of future investigation. A decrease in the cardiac output would be expected to affect the CVP, hence, resulting in increased liver stiffness when the pressure is elevated over a longer period of time.
Additionally, while it has been demonstrated that the simple presence of hepatic venous congestion can result in elevated liver stiffness estimates in a canine model [30], and admittedly, in the absence of histopathology in our patient population it is unknown whether the elevated liver stiffness values in this investigation are due to fibrosis alone, a combination of fibrosis and venous congestion, or venous congestion alone, we believe that fibrosis is a major underlying pathology in Fontan livers. This is supported by prior pathologic studies on Fontan patients which demonstrated liver fibrosis even in the very early post-operative period [9, 10] as well as by the presence of classic morphologic features of liver fibrosis on routine anatomic CT and MR imaging in these patients [12]. Future longitudinal liver elastography studies with histopathologic correlation will ultimately determine what contribution simple elevated hepatic venous pressure versus underlying fibrosis results in elevated liver stiffness in Fontan patients.
Conclusion
As the earliest cohort of patients to have undergone the Fontan procedure have now reached adulthood, it is becoming increasingly important to recognize, understand, and treat sources of morbidity in at-risk survivors. While progressive liver disease is known to occur in Fontan patients, there is currently a relative dearth of literature specifically investigating its monitoring, treatment and prevention. In this feasibility study, MRE was used as a screening tool as part of the clinical cardiac MRI exam to evaluate liver stiffness in Fontan patients. The liver stiffness values were elevated in all patients, and trended towards increasing values with greater duration of Fontan circulation.
MRE is a non-invasive technique that can potentially be used as a rapid tool to identify and stage liver fibrosis and can potentially be used as a practical alternative to liver biopsy in many cases. Because MRE visually quantifies and localizes the extent of tissue stiffness throughout the liver, it provides the opportunity to create a visual map of the extent of fibrosis in the whole liver. This pilot investigation has prompted us to begin the process of more formally evaluating MRE as a diagnostic tool in this patient population. Larger, prospective studies are needed to compare MRE with biopsy findings in these patients. As with any emerging technology, cost, set-up and available expertise will play important roles in determining the utility of MRI based technologies for detecting and quantifying hepatic fibrosis.
Acknowledgments
We wish to acknowledge Rhonda Strunk, RT, who helped with post-processing of MRE maps.
Footnotes
- Daniel J. Podberesky – travel reimbursement by GE Healthcare.
- Richard L. Ehman and the Mayo Clinic hold patents and have a financial interest through royalties related to MRE technology.
- All other authors – none.
- No funding or any grant was available for this research.
Author contributions
SDS, DBW & SKV: concept, data analysis, results and preparation of the manuscript; KMC, JS: result interpretation; RLE: MRE technique development; DJP: image interpretation, revision of the article and approval of the article; BSM: critical review of the article.
References
- 1.Gates RN, et al. The Fontan procedure in adults. Ann Thorac Surg. 1997;63(4):1085–90. doi: 10.1016/s0003-4975(96)01256-8. [DOI] [PubMed] [Google Scholar]
- 2.Mahle WT, et al. Survival after reconstructive surgery for hypoplastic left heart syndrome: A 15-year experience from a single institution. Circulation. 2000;102(19 Suppl 3):III136–41. doi: 10.1161/01.cir.102.suppl_3.iii-136. [DOI] [PubMed] [Google Scholar]
- 3.Tweddell JS, et al. Mixed venous oxygen saturation monitoring after stage 1 palliation for hypoplastic left heart syndrome. Ann Thorac Surg. 2007;84(4):1301–10. doi: 10.1016/j.athoracsur.2007.05.047. discussion 1310–1. [DOI] [PubMed] [Google Scholar]
- 4.Mainwaring RD, et al. Effect of accessory pulmonary blood flow on survival after the bidirectional Glenn procedure. Circulation. 1999;100(19 Suppl):II151–6. doi: 10.1161/01.cir.100.suppl_2.ii-151. [DOI] [PubMed] [Google Scholar]
- 5.Khairy P, et al. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation. 2008;117(1):85–92. doi: 10.1161/CIRCULATIONAHA.107.738559. [DOI] [PubMed] [Google Scholar]
- 6.Gersony WM. Fontan operation after 3 decades: what we have learned. Circulation. 2008;117(1):13–5. doi: 10.1161/CIRCULATIONAHA.107.748566. [DOI] [PubMed] [Google Scholar]
- 7.Mair DD, et al. Early and late results of the modified Fontan procedure for double-inlet left ventricle: the Mayo Clinic experience. J Am Coll Cardiol. 1991;18(7):1727–32. doi: 10.1016/0735-1097(91)90511-7. [DOI] [PubMed] [Google Scholar]
- 8.Burkhart HM, et al. The modified Fontan procedure: early and late results in 132 adult patients. J Thorac Cardiovasc Surg. 2003;125(6):1252–9. doi: 10.1016/s0022-5223(03)00117-x. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz MC, et al. Hepatic pathology may develop before the Fontan operation in children with functional single ventricle: an autopsy study. J Thorac Cardiovasc Surg. 2012;143(4):904–9. doi: 10.1016/j.jtcvs.2011.08.038. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz MC, et al. Portal and sinusoidal fibrosis are common on liver biopsy after Fontan surgery. Pediatr Cardiol. 2013;34(1):135–42. doi: 10.1007/s00246-012-0402-9. [DOI] [PubMed] [Google Scholar]
- 11.Shah H, Kuehl K, Sherker AH. Liver disease after the Fontan procedure: what the hepatologist needs to know. J Clin Gastroenterol. 2010;44(6):428–31. doi: 10.1097/MCG.0b013e3181d476fc. [DOI] [PubMed] [Google Scholar]
- 12.Wallihan DB, Podberesky DJ. Hepatic pathology after Fontan palliation: spectrum of imaging findings. Pediatr Radiol. 2013;43(3):330–8. doi: 10.1007/s00247-012-2531-y. [DOI] [PubMed] [Google Scholar]
- 13.Pariente D, Franchi-Abella S. Paediatric chronic liver diseases: how to investigate and follow up? Role of imaging in the diagnosis of fibrosis. Pediatr Radiol. 2010;40(6):906–19. doi: 10.1007/s00247-010-1600-3. [DOI] [PubMed] [Google Scholar]
- 14.Castera L, Pinzani M. Biopsy and non-invasive methods for the diagnosis of liver fibrosis: does it take two to tango? Gut. 2010;59(7):861–6. doi: 10.1136/gut.2010.214650. [DOI] [PubMed] [Google Scholar]
- 15.Yin M, et al. Assessment of hepatic fibrosis with magnetic resonance elastography. Clin Gastroenterol Hepatol. 2007;5(10):1207–1213 e2. doi: 10.1016/j.cgh.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mariappan YK, Glaser KJ, Ehman RL. Magnetic resonance elastography: a review. Clin Anat. 2010;23(5):497–511. doi: 10.1002/ca.21006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serai SD, Towbin AJ, Podberesky DJ. Pediatric liver MR elastography. Dig Dis Sci. 2012;57(10):2713–9. doi: 10.1007/s10620-012-2196-2. [DOI] [PubMed] [Google Scholar]
- 18.Fleiss JL. The design and analysis of clinical experiments. xiv. New York: Wiley; 1986. p. 432. (Wiley series in probability and mathematical statistics Applied probability and statistics). [Google Scholar]
- 19.Yin M, et al. Abdominal magnetic resonance elastography. Top Magn Reson Imaging. 2009;20(2):79–87. doi: 10.1097/RMR.0b013e3181c4737e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berrada O, El Mouhadi S, Arrive L. Congestive hepatopathy. Clin Res Hepatol Gastroenterol. 2012;36(4):314–5. doi: 10.1016/j.clinre.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Brancatelli G, et al. Cirrhosis: CT and MR imaging evaluation. Eur J Radiol. 2007;61(1):57–69. doi: 10.1016/j.ejrad.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Meyers AB, et al. Characterization of pediatric liver lesions with gadoxetate disodium. Pediatr Radiol. 2011;41(9):1183–97. doi: 10.1007/s00247-011-2148-6. [DOI] [PubMed] [Google Scholar]
- 23.Carey E, Carey WD. Noninvasive tests for liver disease, fibrosis, and cirrhosis: Is liver biopsy obsolete? Cleve Clin J Med. 2010;77(8):519–27. doi: 10.3949/ccjm.77a.09138. [DOI] [PubMed] [Google Scholar]
- 24.Yin M, et al. Quantitative assessment of hepatic fibrosis in an animal model with magnetic resonance elastography. Magn Reson Med. 2007;58(2):346–53. doi: 10.1002/mrm.21286. [DOI] [PubMed] [Google Scholar]
- 25.Friedrich-Rust M, et al. Noninvasive assessment of liver fibrosis in patients with Fontan circulation using transient elastography and biochemical fibrosis markers. J Thorac Cardiovasc Surg. 2008;135(3):560–7. doi: 10.1016/j.jtcvs.2007.09.039. [DOI] [PubMed] [Google Scholar]
- 26.Castera L, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128(2):343–50. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 27.Huwart L, et al. Liver fibrosis: non-invasive assessment with MR elastography. NMR Biomed. 2006;19(2):173–9. doi: 10.1002/nbm.1030. [DOI] [PubMed] [Google Scholar]
- 28.Pike NA, et al. Clinical profile of the adolescent/adult Fontan survivor. Congenit Heart Dis. 2011;6(1):9–17. doi: 10.1111/j.1747-0803.2010.00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghaferi AA, Hutchins GM. Progression of liver pathology in patients undergoing the Fontan procedure: Chronic passive congestion, cardiac cirrhosis, hepatic adenoma, and hepatocellular carcinoma. J Thorac Cardiovasc Surg. 2005;129(6):1348–52. doi: 10.1016/j.jtcvs.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Rotemberg V, et al. The impact of hepatic pressurization on liver shear wave speed estimates in constrained versus unconstrained conditions. Phys Med Biol. 2012;57(2):329–41. doi: 10.1088/0031-9155/57/2/329. [DOI] [PMC free article] [PubMed] [Google Scholar]


