Abstract
We have developed an effective method that converts a variety of mono- and disaccharides into formic acid predominantly. Our recyclable NHC-amidate palladium(II) catalyst facilitated oxidative degradation of carbohydrates without using excess oxidant. Stoichiometric amounts of hydrogen peroxide and sodium hydroxide were employed at ambient temperatures.
The conversion of biomass into valuable chemical feedstock products is an emerging theme in the field of chemistry.1 Notably, sugars comprise a majority of biomass, of which glucose is the primary monosaccharide by mass.2 Therefore, being abundant carbon sources, it is of paramount importance to discover novel ways to degrade such monosaccharides and convert them into valuable chemical feedstock products such as 5-hydroxymethylfurfural (HMF), furfural, succinic acid, lactic acid, and formic acid.3 Numerous studies have shown that formic acid can be employed as a hydrogen source in direct formic acid fuel cells (DFAFC). Moreover, due to their ease of refueling, efficiency, and safety, DFAFC are considered to be an alternative to methanol and hydrogen fuel cells. Having such characteristics allows DFAFC to potentially be utilized in common consumer electronics in addition to being a power source to automobiles.4 Therefore it is of great value to be able to convert abundant and inexpensive biomass into formic acid efficiently and under mild conditions.
Various work has been performed in degrading sugars into useful chemical feedstock products. Such degradation techniques include the use of acid, high-temperature liquid water, singlet oxygen, and other oxidants. The most prominent protocols utilized acidic media and produced HMF in moderate yields, where large amounts of mineral acids such as hydrochloric and sulfuric acids were required.5 Further, though the use of high-temperature liquid water in degrading sugars excludes the use of catalysts, high pressures and temperatures of up to 10 MPa and 600 °C were required in addition to a 2-4 molar excess of oxidant.6 While diradical oxygen degradation of reducing sugars sensibly employed the use of an abundant and inexpensive oxidant, the lack of product selectivity hindered the practicality of this methodology.7 Solid-supported palladium catalysts have also been utilized in the oxidation of glucose into gluconic acid; however, these catalysts need to be activated between 300-500 °C in a hydrogen or argon atmosphere.8
Several studies have employed hydrogen peroxide in the presence of an alkali and transition metals in the degradation of monosaccharaides and oligosaccharides.9 In particular, Isbell et al. thoroughly examined such conditions in great detail.10 However, high yields were achieved using excess amounts of base and oxidant and/or only after long reaction times (>300 h). Described herein is the development of efficient catalytic methods for the oxidative degradation of common saccharides to formic acid. These conditions employ a novel Pd(II) complex 1 (Figure 1) and stoichiometric amounts of hydrogen peroxide under aqueous alkaline conditions at ambient temperatures. In sharp contrast to previous work, this simple methodology gives rise to the highly efficient formation of formic acid as an exclusive or predominant product under mild conditions using the minimal amount of oxidant.
Figure 1.

Structure of our NHC-amidate Pd(II) complex
In effect, this study is a continuation of our previously reported work on the direct conversion of glycerol into formic acid,11 which also utilized our NHC-amidate Pd(II) complex12 using hydrogen peroxide in aqueous media. Hydrogen peroxide continues to be the oxidant used in this procedure since it is convenient, accessible, and breaks down into environment-friendly side products unlike other oxidants. In an effort to perform these reactions in the most sustainable manner, water was also chosen as the sole solvent in the reaction mixture. Under these conditions, our NHC-amidate palladium complex remains stable, yet is highly active.
Utilizing NHC-amidate Pd(II) complex 1 in our initial reaction conditions (Scheme 1) provided inefficient yet promising results. Though over half of the starting material was consumed over the reaction time, there was only a 12 percent carbon mass balance to either formic acid or glycolic acid. We believed that the excess amount of hydrogen peroxide caused the overoxidation of formic acid into carbon dioxide.
Scheme 1.

Initial reaction conditions utilizing catalyst 1.
Therefore, in order to prevent this thermodynamically favorable reaction from taking place, base was added to the reaction mixture, consequently converting the formic acid produced into a more stable formate salt and preventing overoxidation of the product (Table 1).6b-d The addition of NaOH to the reaction mixture increased the turnover of formic acid 15-fold (entry 3) compared to a reaction in the absence of any base (entry 1). The addition of KOH was less effective (entry 2) and resulted in catalyst degradation. Lower amounts of base afforded proportionally less product despite longer reaction times (entry 4). It was found that overnight treatment with six equivalents of NaOH led to the highest yield of the formate salt (entry 5). This is reasonable since each molecule of glucose should theoretically produce six molecules of formic acid. Additional amounts of base did not change the outcome significantly (entry 6). A reaction was conducted with sodium chloride in the absence of base to determine any potential role of the alkali metal. This reaction did not produce any formic acid, suggesting the alkali metal did not catalyze the reaction.
Table 1.
Effects of varying amounts of base and time.a
| Entry | Base | Amount (μmol) | Time (h) | HCOOH (TON)b |
|---|---|---|---|---|
| 1 | - | - | 6 | 5.6 |
| 2 | KOH | 600 | 6 | 44.9 |
| 3 | NaOH | 600 | 6 | 85.2 |
| 4 | NaOH | 300 | 16 | 48.1 |
| 5 | NaOH | 600 | 16 | 119.8 |
| 6 | NaOH | 1,200 | 16 | 110.1 |
Reaction conditions: 100 μmol glucose, 5 μmol 1, and varying amounts of base were dissolved in 0.4 mL H2O. 1.0 mmol H2O2 was added and the mixture was stirred at 40 °C.
Determined by wet1D NMR with a DMSO standard.
In light of these impressive results, we decided to optimize conditions, and investigated the effect of hydrogen peroxide (Table 2). We observed a background reaction in the absence of hydrogen peroxide, which produced minimal amounts of formic acid (entry 1). Using less than stoichiometric amounts of the hydrogen peroxide furnished only a fraction of the formic acid expected (entry 2). Therefore, adding a stoichiometric amount of hydrogen peroxide was necessary to afford formic acid at over 100 TON (entry 3). Excess amounts of oxidant increased the yield marginally (entry 4), suggesting that stoichiometric amounts of hydrogen peroxide (six equivalents to each glucose; one equivalent to each carbon) would be optimal for practical use.
Table 2.
Effects of varying amount of hydrogen peroxide.a
| Entry | H2O2 (μmol) | HCOOH (TON)b |
|---|---|---|
| 1 | - | 6.2 |
| 2 | 250 | 76.4 |
| 3 | 600 | 109.7 |
| 4 | 1,000 | 114.6 |
Reaction conditions: 100 μmol glucose, 5 μmol 1, and 600 μmol NaOH were dissolved in a 0.5 mL H2O/ H2O2 solution and stirred at 25 °C for 16 hours.
Determined by wet1D NMR with a DMSO standard.
Comparing our palladium complex with other palladium salts produced interesting results (Table 3). While each reaction using palladium salts consumed all the starting material, catalyst 1 produced the highest TON of formic acid. Pd(MeCN)2Cl2, which was the palladium precursor of catalyst 1, generated a small amount of formic acid (entry 1) compared to catalyst 1 (entry 5). Pd(OAc)2 yielded twice the amount of formic acid as Pd(MeCN)2Cl2, yet the product carbon mass balance remained less than stoichiometric (entry 2). PdCl2 was the least effective of the palladium sources, degrading all of the glucose but only producing two TON of formic acid (entry 3). Interestingly, it was concluded that these palladium salts catalyzed the overoxidation of the formate salt into carbon dioxide, since the amount of formic acid observed was significantly higher in the absence of the palladium salts (entry 4). This hypothesis was verified by stirring sodium formate under the standard reaction conditions. A significant amount (up to 80%) of the formate was decomposed to carbon dioxide in the presence of the palladium salts used in entries 1 - 3, while catalyst 1 did not affect the amount of formate, thus offering high chemoselectivity. In addition, decreased catalyst 1 loading continued to produce significant amounts of formic acid with higher TON (entries 5-6) but did not completely convert the starting material to product.
Table 3.
Effects of palladium catalysts and loading.a
| Entry | Pd Catalyst | mol % | HCOOH (TON)b |
|---|---|---|---|
| 1 | Pd(MeCN)2Cl2 | 5 | 22.7 |
| 2 | Pd(OAc)2 | 5 | 40.0 |
| 3 | PdCl2 | 5 | 2.1 |
| 4 | - | - | 76.3 |
| 5 | 1 | 2.5 | 192.7 |
| 6 | 1 | 1.25 | 390.0 |
Standard conditions using varying amounts of a palladium source.
Most importantly, the greatest differentiating factor between our NHC-amidate Pd(II) catalyst and the other palladium salts tested was the fact that our catalyst could be recycled and reused through the simple means of extraction, while maintaining high reactivity (vide infra). In the absence of catalyst 1, various Lewis acids were also tested under standard reaction conditions, all of which produced lower TON than catalyst 1 (see Supplementary Information). These results suggest that our NHC-amidate Pd(II) complex has distinguished reactivity compared to commercially available Lewis acids as well as known palladium salts.
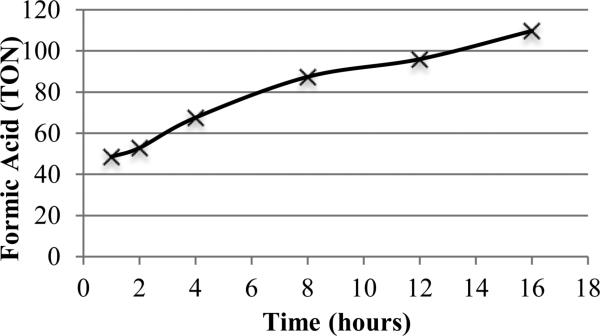
With optimal conditions in hand, we examined the time dependence of this transformation (Figure 2). A high initial rate of conversion was followed by a gradual degradation of the remaining starting material. Within the first three hours of the reaction, about half of the formic acid was produced, while it took an additional 13 hours for the reaction to go to completion.
Figure 2.
Time study. (Standard conditions using D-glucose from 1-16 hours.)
We then decided to examine the feasibility of catalyst reclamation. We found that due to the stable nature of the NHC-amidate ligand of catalyst 1, at the end of each reaction the catalyst could be extracted using methylene chloride and reprocessed in a sequential reaction while still maintaining its reactivity and selectivity. We were able to recover and utilize our catalyst three times and still produce formic acid at over 100 TON (Figure 3). This is of paramount value since it allows for the removal of the heavy metal from the aqueous media. Thus this procedure has the promise to be utilized in water purification and reclamation while simultaneously producing a chemical feedstock product, which can be used as an alternative fuel source.
Figure 3.
Effects of catalyst recycling. (Standard conditions using D-Glucose and recycled catalyst 1.)
We applied our developed conditions to various carbohydrates (Table 4). Other monosaccharide reducing substrates such as D-galactose, D-ribose, and D-xylose garnered high yields of formic acid selectively (entries 1-3), while D-fructose and D-tagatose furnished formic acid as a major product and significant amounts of glycolic acid as the minor product (entries 4-5). This was believed to be due to the difference between the structure and functionality of aldoses and ketoses. For disaccharide reducing substrates, such as D-maltose, D-lactose, and D-cellobiose, the reaction temperature was increased to 60 °C in order to achieve comparable amounts of formic acid overnight (entries 6-8). Alternatively, running the reactions at room temperature for longer periods of time (48 h) produced similar results.
Table 4.
Scope of reaction conditions with mono and disaccharide reducing substrates.a
| Entry | Substrate | HCOOH (TON) | Glycolic Acid (TON) |
|---|---|---|---|
| 1 | D-Galactose | 111.2 | - |
| 2 | D-Ribose | 119.7 | - |
| 3 | D-Xylose | 116.9 | - |
| 4 | D-Fructose | 83.1 | 14.5 |
| 5 | D-Tagatose | 89.3 | 16.3 |
| 6 | D-Maltoseb | 86.0 | - |
| 7 | D-Lactoseb | 98.7 | - |
| 8 | D-Cellobioseb | 97.4 | - |
Standard conditions.
Reaction was heated to 60 °C.
As expected, the conversion yields of formic acid with non-reducing substrates were poor (Table 5), due to their lack of hemiacetal and hemiketal groups. Sucrose, D-melezitose, and D-raffinose produced formic acid in the range of 15 – 30 TON (entries 1-3) compared to over 100 TON for reducing substrates. Moreover, increasing the amount of oxidant and reaction time did not render higher conversions, as it did with reducing substrates. Applying our reaction conditions to a sugar alcohol such as glycerol did not produce significant yields, either (entry 4). The low yields were not believed to be due to the overoxidation of these substrates to carbon dioxide. A large amount of starting material remained at the end of the reaction, indicating that the degradation of these substrates was less active.
Table 5.
Scope of reaction conditions with non-reducing substrates.a
| Entry | Substrate | HCOOH (TON) |
|---|---|---|
| 1 | Sucrose | 27.3 |
| 2 | D-Melezitose | 15.9 |
| 3 | D-Raffinose | 28.5 |
| 4 | Glycerol | 39.1 |
Standard conditions heated at 60 °C.
Mechanistically, there are two primary routes of the oxidative degradation of aldoses into formic acid (Scheme 2). As reported in previous work, initial oxidation of the aldehyde is most likely, followed by breaking of the C1-C2 bond (α-scission).10c, 13 This forms the first equivalent of formic acid and a successive aldehyde at C2. This process is continued until the complete degradation of the hexose and the formation of six molecules of formic acid is produced. Alternatively, rather than initially cleaving the C1-C2 bond, a β-scission cleaves the C2-C3 bond, yielding an equivalent of oxalic acid, which in turn degrades to CO2 and formic acid, as well as a shorter-chain aldose. Since ketoses are slower to oxidize than aldoses, harsher conditions and lower yields of formic acid were observed.
Scheme 2.
Proposed Pd(II) assisted α-oxidative degradation pathway of D-glucose with hydrogen peroxide.
Based on our results, we believe our procedure proceeds primarily through an α-oxidation mechanism. Our primary source of evidence is the nearly complete conversion of monosaccharides into formic acid. Since the carbon turnover of the monosaccharides used was 93% or higher, a mechanism entailing the loss of half of the carbon mass by decarboxylation of oxalic acid14 would not be viable. In addition, under alkaline aqueous conditions with catalyst 1 and stoichiometric amounts of hydrogen peroxide, oxalic acid did not produce formic acid. Lastly, only trace amounts of oxalic acid were detected by 13C NMR under our standard conditions after four hours, corroborating that this route was minor and the α-oxidation pathway would be predominant. As suggested in our previous work,11 catalyst 1 acts as a bidentate system, simultaneously activating an aldehyde and alcohol in close proximity of each other, forming a five-membered heterocycle glucose adduct. This enhances the nucleophilic attack of peroxide, promoting the α-oxidation in a systematic sequential order. While other palladium salts may activate saccharides in a similar fashion, catalyst 1 avoids the overoxidation of formate into carbon dioxide, setting it apart from other Pd(II) counterparts.
Conclusions
In summary, we have discovered a set of efficient and environmentally friendly conditions that oxidatively degraded saccharides using stoichiometric amounts of hydrogen peroxide in aqueous alkaline media at ambient temperatures. The NHC-amidate Pd(II) complex 1 catalyzed the reactions, likely acting as a Lewis acid and activating the aldehyde functional group in the aldoses. Contrary to other procedures, this method did not use excess amounts of oxidant and did not require the input of heat for aldoses, while the catalyst was recyclable and maintained its efficiency. This methodology can become of great value since sugars comprise a majority of biomass. Thus, our developed conditions contribute to converting this abundant carbon source into alternative fuels.
Supplementary Material
Standard conditions.
100 μmol of substrate, 5 μmol of 1, and 600 μmol NaOH were dissolved in 0.44 mL H2O. 60 μL 30% H2O2 was added and the mixture was stirred at 25 °C for 16 hours. 0.25 mL of D2O was then added to the reaction mixture with a sealed capillary DMSO standard. The solution was then analyzed using wet1D NMR.
Acknowledgements
The authors acknowledge Dr. Joo Ho Lee and Prasanna Pullanikat for their initial efforts in this project and Richard Giles for insightful discussions. We also acknowledge generous financial support from the Hydrocarbon Research Foundation and the National Institute of Health (S10 RR025432).
Footnotes
† Electronic Supplementary Information (ESI) available: formic acid calibration curve, glycolic acid calibration curve, wet1D NMR spectra. See DOI: 10.1039/c000000x/
References
- 1.Gallezot P. Chem. Soc. Rev. 2012;41:1538–1558. doi: 10.1039/c1cs15147a. [DOI] [PubMed] [Google Scholar]
- 2.Liu D, Nimlos MR, Johnson DK, Himmel ME, Qian X. J. Phys. Chem. A. 2010;114:12936–12944. doi: 10.1021/jp1078407. [DOI] [PubMed] [Google Scholar]
- 3.Tong X, Ma Y, Li Y. Appl. Catal., A. 2010;385:1–13. [Google Scholar]
- 4.a Rees NV, Compton RG. J. Solid State Electrocem. 2011;15:2095–2100. [Google Scholar]; b Uhm S, Chung ST, Lee J. J. Power Sources. 2008;178:34–43. [Google Scholar]; c Rice C, Ha S, Masel RI, Waszczuk P, Wieckowski A, Barnard T. J. Power Sources. 2002;111:83–89. [Google Scholar]
- 5.a Qi L, Mui YF, Lo SW, Lui MY, Akien GR, Horváth IT. ACS Catal. 2014;4:1470–1477. [Google Scholar]; b Román-Leshkov Y, Davis ME. ACS Catal. 2011;1:1566–1580. [Google Scholar]; c Bower S, Wickramasinghe R, Nagle NJ, Schell DJ. Bioresour. Technol. 2008;99:7354–7362. doi: 10.1016/j.biortech.2007.05.045. [DOI] [PubMed] [Google Scholar]; d Mehdi H, Fábos V, Tuba R, Bodor A, Mika LT, Horváth IT. Top. Catal. 2008;48:49–54. [Google Scholar]; e Jow J, Rorrer GL, Hawley MC. Biomass. 1987;14:185–194. [Google Scholar]
- 6.a Moreno T, Kouzaki G, Sasaki M, Goto M, Cocero MJ. Carbohydr. Res. 2012;349:33–38. doi: 10.1016/j.carres.2011.12.005. [DOI] [PubMed] [Google Scholar]; b Jin F, Enomoto H. Energy Environ. Sci. 2011;4:382–397. [Google Scholar]; c Yun J, Jin F, Kishita A, Tohji K, Enomoto H. J. Phys.: Conf. Ser. 2010;215:012126. [Google Scholar]; d Jin F, Yun J, Li G, Kishita A, Tohji K, Enomoto H. Green Chem. 2008;10:612–615. [Google Scholar]
- 7.Isbell HS. Carbohydr. Res. 1976;49:C1–C4. [Google Scholar]
- 8.a Witońska I, Frajtak M, Karski S. Appl. Catal., A. 2011;40:73–82. [Google Scholar]; b Liang X, Liu C, Kuai P. Green Chem. 2008;10:1318–1322. [Google Scholar]
- 9.a Eguchi H, Ikeda Y, Koyota S, Honke K, Suzuki K, Gutteridge JMC, Taniguchi N. J. Biochem. 2002;131:477–484. doi: 10.1093/oxfordjournals.jbchem.a003124. [DOI] [PubMed] [Google Scholar]; b Velarde AM, Bartl P, Nieβen TEW, Hoelderich WF. J. Mol. Catal. A: Chem. 2000;157:225–236. [Google Scholar]; c Arts SJHF, Mombarg EJM, van Bekkum H, Sheldon RA. Synthesis. 1996;6:597–613. [Google Scholar]
- 10.a Isbell HS, Frush HL. Carbohydr. Res. 1987;161:181–193. [Google Scholar]; b Isbell HS, Parks EW, Naves RG. Carbohydr. Res. 1975;45:197–204. [Google Scholar]; c Isbell HS, Naves RG. Carbohydr. Res. 1974;36:C1–C4. [Google Scholar]; d Isbell HS, Frush HL, Martin ET. Carbohydr. Res. 1973;26:287–295. [Google Scholar]
- 11.Pullanikat P, Lee JH, Yoo KS, Jung KW. Tetrahedron Lett. 2013;54:4463–4466. doi: 10.1016/j.tetlet.2013.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JH, Yoo KS, Park CP, Olsen JM, Sakaguchi S, Prakash GKS, Mathew T, Jung KW. Adv. Synth. Catal. 2009;351:563–568. doi: 10.1002/adsc.200800698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.a Jin F, Zhou Z, Moriya T, Kishida H, Higashijima H, Enomoto H. Environ. Sci. Technol. 2005;39:1893–1902. doi: 10.1021/es048867a. [DOI] [PubMed] [Google Scholar]; b McGinnis GD, Prince SE, Biermann CJ, Lowrimore JT. Carbohydr. Res. 1984;128:51–60. [Google Scholar]
- 14.Mantzavinos D, Livingston AG, Hellenbrand R, Metcalfe IS. Chem. Eng. Sci. 1996;51:4219–4235. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.