Abstract
Background
Systematic investigations are needed identifying how variability in the biomechanical characteristics of spinal manipulation affects physiological responses. Such knowledge may inform future clinical practice and research study design.
Objective
To determine how contact site for high velocity, low amplitude spinal manipulation (HVLA-SM) affects sensory input to the central nervous system.
Design
HVLA-SM was applied to 4 specific anatomic locations using a no-HVLA-SM control at each location randomized in an 8×8 Latin square design in an animal model.
Methods
Neural activity from muscle spindles in the multifidus and longissimus muscles were recorded from L6 dorsal rootlets in 16 anesthetized cats. A posterior to anterior HVLA-SM was applied through the intact skin overlying the L6 spinous process, lamina, inferior articular process and L7 spinous process. HVLA-SMs were preceded and followed by simulated spinal movement applied to the L6 vertebra. Change in mean instantaneous discharge frequency (ΔMIF) was determined during the thrust and the simulated spinal movement.
Results
All contact sites increased L6 muscle spindle discharge during the thrust. Contact at all L6 sites significantly increased spindle discharge more than at the L7 site when recording at L6. There were no differences between L6 contact sites. For simulated movement, the L6 contact sites but not the L7 contact site significantly decreased L6 spindle responses to a change in vertebral position but not to movement to that position.
Conclusions
This animal study showed that contact site for an HVLA-SM can have a significant effect on the magnitude of sensory input arising from muscle spindles in the back.
Keyword Indexing Terms: manual therapy, spinal manipulation, specificity, dose, muscle spindles
INTRODUCTION
Spinal manipulation is a form of manual therapy used most frequently to treat musculoskeletal complaints (Hawk et al., 2001; Sorensen et al., 2006). It is most readily differentiated from spinal mobilization by use of an applied thrust and there is rationale to think that these two forms of treatment may not be equivalent either clinically (Cleland et al., 2009; Rubinstein et al., 2013; but see Cook et al., 2013) or in their mechanisms of action (Bolton and Budgell, 2006) and therefore should be studied individually. Utilization data indicate most patients who receive spinal manipulation receive a manual procedure relatively high in velocity and low in amplitude (HVLA-SM) (Shekelle et al., 1992; Eisenberg et al., 1998; Christensen et al., 2005). Following preloading of the spinal tissues, the clinician rapidly delivers a thrust to a target vertebra through a short lever arm by manually contacting the skin overlying that vertebra’s lamina, spinous, transverse or mammillary process, with the intent of displacing the vertebra, gapping its facet joints, and creating mechanical, neurological and biological effects (Greenman, 1989; Hooper, 2005; Bergmann, 2005; Cramer et al., 2013; Leach, 2004).
The biomechanical parameters that characterize an HVLA-SM are considered fundamental to its application (Triano, 2000; Bergmann, 2005), yet they can vary greatly. For example in the low back, thrust forces reach a peak ranging from 220 to 889N within 75 to 225ms (Hessell et al., 1990; Conway et al., 1993; Herzog et al., 1993; Triano and Schultz, 1997). Even when an individual clinician delivers similar HVLA-SMs, biomechanical characteristics vary (Cambridge et al., 2012). In addition, an HVLA-SM may not be as targeted to a specific vertebra as intended. By the time a thrust is delivered, the actual contact site may have migrated up to 10mm from the originally intended site (Herzog et al., 2001). How this variability affects the biological and therapeutic outcomes of HVLA-SM has yet to be determined and may be important to both clinical practice and research design.
Several groups using electromechanical devices to deliver controlled, repeatable HVLA-SMs (Pickar and Wheeler, 2001; Vaillant et al., 2010; Descarreaux et al., 2013) have been systematically investigating how variations in an HVLA-SM’s biomechanical characteristics affect neuromuscular, biomechanical and neurophysiological responses. In healthy humans Descarreaux and colleagues found that increasing thrust force but decreasing either thrust duration or preload force produces linear increases in the magnitudes of EMG responses evoked during and following the manipulative thrust (Nougarou et al., 2013; Page et al., 2014; Francois et al., 2014). In a feline model, Pickar and colleagues found that as thrust duration approaches a value previously shown to be used clinically, a threshold increase in the sensory input from paraspinal muscle spindles occurs during the thrust (Pickar et al., 2007; Reed et al., 2013). While preload magnitude and duration interact to modulate muscle spindle activity during the thrust (Reed et al., 2014), preload, thrust duration, and thrust amplitude all appear to have minimal effect on changing the responsiveness of muscle spindles to spinal movement following the thrust (Cao et al., 2013; Reed et al., 2014). Also using a feline model Kawchuk and colleagues found that thrust duration interacts with thrust amplitude toward changing spinal stiffness (Vaillant et al., 2012). In addition, the specific contact site through which the thrust is applied determines whether spinal stiffness changes (Edgecombe et al., 2013). Currently nothing is known about the relationship between an HVLA-SM’s contact site and any neural response.
The goal of the present study was to determine how the contact site through which the HVLA-SM’s thrust is applied affects the response of paraspinal muscle spindles. Although the mechanistic pathways underlying the effects of HVLA-SM are not yet known, muscle spindles were chosen because changes in neural input arising from co-activated paraspinal sensory receptors (Korr, 1978; Haldeman, 1983; Gillette, 1987; Greenman, 1989; Pickar, 2002; Leach, 2004; Henderson, 2005; Bialosky et al., 2009; Pickar and Bolton, 2012), including muscle spindles (Korr, 1975), have long been thought to contribute to HVLA-SM’s therapeutic effects. Studying sensory input from paraspinal tissues in humans has not been possible due to the invasive nature of these procedures. We used a feline model to determine the effect of contact site on the response of muscle spindles both during and following the HVLA-SM. Thinking that a lever’s mechanical advantage depends upon the length of its lever arm, we hypothesized that distinct clinically-relevant contact sites for delivering an HVLA-SM will produce significant differences in paraspinal muscle spindle response.
METHODS
Overview
A mechanical device (Fig. 1) was used to apply simulated HVLA-SMs to the lumbar spine of deeply anesthetized cats while recording sensory activity from individual muscle spindles in lumbar muscles attached to the L6 vertebra (cats have 7 lumbar vertebrae). An HVLA-SM was applied at each of 4 contact sites: lamina, mammillary process and spinous process of the target L6 vertebra and spinous process of the adjacent L7 vertebra. Two types of responses from muscle spindles were assessed: 1) their responses during the HVLA-SM thrust and 2) their responses to slow changes in vertebral position during simulated spinal movement using ramp and hold displacement of the L6 vertebra before and after the HVLA-SM.
Figure 1.
Schematic of the preparation for delivering spinal manipulation to the lumbar spine while recording sensory input from the dorsal roots. The motor was controlled by a programmable, electronic feedback system (not shown). Tissues of the vertebrae receiving a spinal manipulation (L6 and L7) remained intact including the skin. Rotary motion of the motor’s lever arm was converted to linear motion by a custom built converter. The converter in turn was attached to a rod terminating in a small, circular, plastic tip (5mm diameter) placed on the intact skin at the contact site. Micrometers attached to the 3-axis gantry allowed positioning of the manipulator’s tip to within 0.05mm of an intended contact site.
Preparation
Experiments were performed on 16 deeply anesthetized cats weighing between 2.6 and 4.1kg [mean 3.6 (SD 0.4)]. All experiments were approved by the Institutional Animal Care and Use Committee. All surgical and electrophysiological procedures have been previously established and described in detail (Pickar, 1999; Sung et al., 2005; Reed et al., 2013). Deep anesthesia was maintained with Nembutal [35mg/kg intravenous]. A laminectomy was performed at L5 exposing the L6 dorsal rootlets for electrophysiological recordings.
Muscle spindle activity
Finely teased filaments from the L6 dorsal rootlets were placed on a monopolar electrode until the recording contained a single unit that responded only to mechanical pressure applied directly to muscles of the low back and not the pelvis or leg. Standard neurophysiological techniques were used at the experiment’s end to confirm the afferent innervated a muscle spindle (see Appendix).
HVLA-SM and contact sites
The mechanical device used to load the spine (see Fig. 1) was identical to that used in previous investigations (Reed et al., 2013; Cao et al., 2013; Reed et al., 2014). The simulated HVLA-SM was applied in a posterior-anterior direction as commonly delivered to a patient in a prone position. Thrust duration was 100ms, similar to that used clinically and the peak thrust force was 21.3 N, scaled from a human to a cat. See Appendix for additional details.
Contact sites for applying the HVLA-SM were based upon those used in clinical practice: a lumbar vertebra’s lamina, spinous process, or mammillary process (Peterson and Bergmann, 1993). Three contact sites were located over the L6 vertebra. L6 was considered the target vertebra because the L6 dorsal roots, from which muscle spindle recordings were obtained, innervate muscles attaching to the L6 vertebra (Bogduk, 1980). The fourth contact site was located one vertebral segment from the L6 target, over the L7 spinous process. The process for locating these contact sites is described in the Appendix.
Ramp and hold
Simulated spinal movement before and after the HVLA-SM enabled us to determine how contact sites affected proprioceptive signaling. Movement was accomplished by placing the manipulator’s tip on the L6 spinous process and, from the vertebra’s preloaded resting position, the motor applied a ramp and hold displacement (see waveforms in Fig. 3) to L6. The ramp consisted of slowly (0.5mm/s) moving the L6 vertebra ventralward 2mm over 4s. The hold consisted of maintaining the vertebra at this new position for 4s.
Figure 3.
Schematic showing the timeline and protocols. At each contact site two protocols, identical except for the presence of an HVLA-SM, were used. The manipulation protocol always preceded the control protocol. Timeline not drawn to scale.
Experimental design and protocols
Two protocols (manipulation followed by control) were performed at each of the 4 contact sites for a total of 8 protocols. Each protocol consisted of three parts (Fig. 3): 1) preload followed by ramp and hold of the L6 vertebra; 2) followed 5min later by a preload and a 100ms intervention interval (HVLA-SM for the manipulation protocol; preload alone for the control protocol) at a contact site; 3) followed 2min later by preload and ramp and hold of the L6 vertebra. Sixteen afferents were studied using two replicates of an 8×8 Latin square design which controlled for each cat’s inherent level of muscle spindle activity and the order of protocols for the 4 contact sites.
Data analysis
Neural discharge was first quantified as instantaneous frequency (IF) by taking the reciprocal of the time interval between successive action potentials. IF during the first 12.5ms of the 100ms thrust was omitted in order to exclude the brief burst of activity that occurs at the start of movement associated with acceleration (Matthews, 1972; Hunt and Ottoson, 1976). The response during the 100ms intervention interval of the manipulation and control protocols was obtained by subtracting the mean IF (MIF) of the 2s baseline prior to the interval’s beginning from the MIF of the interval’s last 87.5ms yielding the response measure ΔMIFduring.
For the ramp and hold, the responsiveness to vertebral position was represented by 1) MIF measured over the 2s baseline prior to the onset of the ramp i.e., with the vertebra in its resting position (MIFresting), and by 2) MIF measured during the last 2s of the hold when the vertebra was moved into a new position (MIFnew position). By calculating MIFnew position over the last 2s of the 4s hold, mechanical contributions from the effects of tissue creep and spindle receptor adaptation were minimized (Matthews, 1972). The responsiveness to vertebral movement was represented by 1) the average IF calculated over the timecourse of the entire ramp (MIFaverage movement) and by 2) taking the average of the 3 largest IFs during the last half of the ramp (MIFpeak movement). MIFs before the HVLA-SM were subtracted from MIFs after the HVLA-SM, then the control protocol MIFs were subtracted from the manipulation protocol MIFs, yielding the response measures ΔMIFresting, ΔMIFnew position, ΔMIFaverage movement, or ΔMIFpeak movement.
An overall F-test was used to determine whether any contact site had an effect on each of the 5 response measures (SAS System for Windows v9.2, SAS Institute Inc., Cary, NC). F-tests and pre-planned contrasts were tested at the 0.05 level of significance. For statistically significant F-tests, six pre-planned contrasts of differences in ΔMIFs between the manipulation and control protocols at each site were compared for each pairwise combination of contact sites. Contrasts were performed on differences between the manipulation and control protocols at each site. Descriptive statistics are reported as means and standard deviations (SD). Inferential statistics are reported as means and 95% confidence intervals (lower, upper 95% CI) based on the ANOVA model.
RESULTS
Recordings were obtained from 16 single afferents. All afferents belonged to muscle spindles in the lumbar paraspinal muscles based upon criteria described in the Appendix. Succinylcholine injection increased the mean discharge frequency of each afferent. Mean maximum frequency increased by 66.7imp/s (4.4 to 173.5imp/s) and lasted at least one minute. A vibrator pressed onto the overlying skin or directly over the muscle belly caused all 16 afferents to follow the ~70 Hz vibration. Muscle twitch (stimulation amplitude = 0.2–0.5mA; duration = 50μs) silenced all but 2 of the 16 afferents. These latter two afferents could not be tested with muscle twitch because the recording was lost before completing the twitch protocol.
The most sensitive portion of each spindle afferent’s receptive field was often located near the level where the L6 paraspinal muscles crossed over the L6–7 facet joint (Fig. 2B). Six spindle afferents had receptive fields in the multifidus muscle (medial to the IAP/MP of Fig. 2B) and ten were in the longissimus muscle.
Figure 2.

Schematic of cat’s back showing overlying tissues and the underlying L6 and L7 vertebra. A) Contact sites located on the skin overlying the osseous landmarks. “X” indicates where the center of the manipulator’s tip was placed. See Appendix for standardized method of locating contact sites. B) Contact sites (circles), center of receptive fields (letters), and measurement grid depicting method for determining distance between contact sites and the receptive field for muscle spindle “J”. Distances from each contact site to the center of the afferent’s receptive field were calculated as the hypotenuse h (black lines) of a right triangle with legs a and b (dark gray lines) using h = √a2 + b2. Note x- and y- axes are scaled independently. SP, spinous process; LAM, lamina; IAP; inferior articular process, MP; mammillary process.
Effect of contact site on muscle spindle responses during the HVLA-SM thrust
All 4 contact sites increased muscle spindle activity during the manipulative thrust (Fig. 4). Contact at the L6 lamina produced the largest mean increase (104.3imp/s), followed by the L6 spinous process (85.4imp/s), L6 IAP/MP (79.9imp/s), and L7 spinous process (42.9imp/s). Contact site had a statistically significant effect on ΔMIFduring (F7, 98 = 24.88, p<0.001). Comparisons of ΔMIFduring between contact sites shown in Table 1 demonstrate that HVLA-SM given at any site over the target L6 vertebra always increased muscle spindle discharge significantly more at L6 than when applied one segment away at L7. There were no significant differences in ΔMIFduring recorded from L6 between contact sites given on the target L6 vertebra.
Figure 4.

Effect of 4 HVLA-SM contact sites on paraspinal muscle spindle during the manipulative thrust. Bars represent means accompanied by 95% confidence intervals. See Data Analysis section the Methods for description of ΔMIFduring.
Table 1.
Comparison between contact sites for their ability to change muscle spindle discharge during the HVLA-SM (ΔMIFduring).
| Contact Site | Mean difference in ΔMIFduring (imp/s) | Lower 95% Confidence Limit | Upper 95% Confidence Limit | p-value |
|---|---|---|---|---|
| L6 spinous process vs L7 spinous process | 42.5 | 6.8 | 78.3 | 0.02 |
| L6 lamina vs L7 spinous process | 61.5 | 25.6 | 97.3 | 0.001 |
| L6 IAP/MP vs L7 spinous process | 37.0 | 1.2 | 72.8 | 0.04 |
| L6 lamina vs L6 IAP/MP | 24.4 | −11.3 | −60.2 | 0.18 |
| L6 lamina vs L6 spinous process | 18.9 | −16.9 | 54.7 | 0.30 |
| L6 spinous process vs L6 IAP/MP | 5.5 | −30.3 | 41.3 | 0.76 |
MIF, mean instantaneous frequency; imp/s; impulses per second; IAP, inferior articular process; MP, mammillary process
It was important to assess the possibility that the relationships shown in figure 4 might have arisen simply from the proximity of each receptive field to a particular contact site. While muscle stretch is considered the most potent stimulus to muscle spindles because spindles lie parallel to the extrafusal fibers, transverse forces applied externally to a muscle can also deform the spindles (Bridgman and Eldred, 1964). Forces during the preload and thrust were applied transversely to the paraspinal muscles. If the largest increase in spindle discharge was simply related to physical proximity, discharge should decrease as the distance between the contact site and receptive field becomes greater. To assess this possibility we measured the distance (Fig. 2B) between the centers of each afferent’s receptive field and each contact site. Figure 5 plots the relationship between these distances and muscle spindle discharge during the HVLA-SM. Contact sites distant to the muscle spindle’s receptive field were just as effective at increasing spindle discharge as contact sites close to the receptive field.
Figure 5.
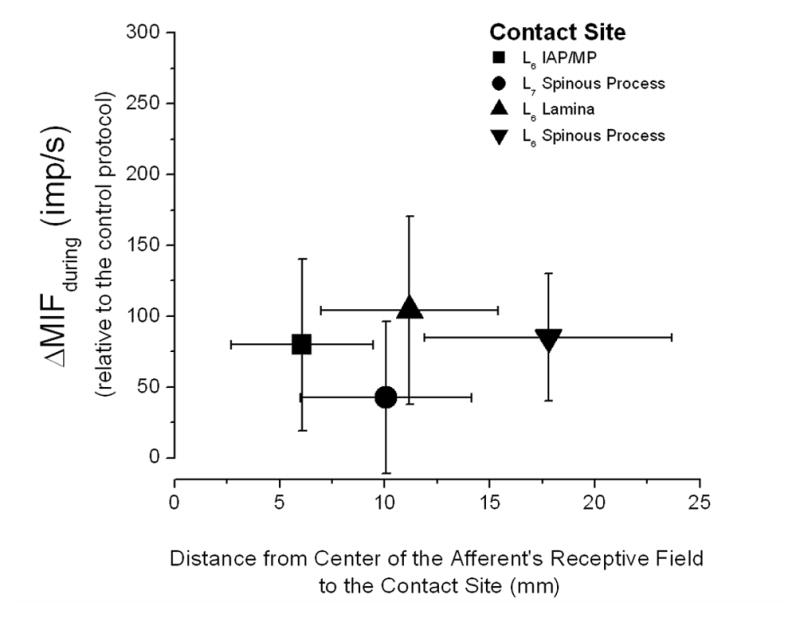
Relationship between the proximity of each receptive field to the 4 HVLA-SM contact sites and the magnitude of paraspinal muscle spindle discharge during the manipulative thrust. Each symbol represents the mean of 16 afferents and their standard deviations. Notice that the standard deviation of the discharge for only the L7 spinous process contact site crosses 0 indicating that only this site, one segment away from the segment innervating the muscle spindles, unloaded the spindles. See Data Analysis section the Methods for description of ΔMIFduring.
Effect of contact site on muscle spindle activity to vertebral position following the HVLA-SM
HVLA-SM at all contact sites produced a statistically significant decrease (F7, 98 = 3.57, p = 0.002) in resting muscle spindle activity (Fig. 6) that immediately preceded the ramp and hold. However there were no differences in ΔMIFresting for any of the pairwise contact site comparisons.
Figure 6.

Effect of 4 HVLA-SM contact sites on resting paraspinal muscle spindle activity following the manipulative thrust. Bars represent means accompanied by 95% confidence intervals. See Data Analysis section the Methods for description of ΔMIFresting.
HVLA-SM produced a statistically significant change (F7, 98 = 3.49, p = 0.002) in muscle spindle responsiveness to the change in L6’s position during the ramp and hold (Fig. 7). Pairwise comparisons showed that HVLA-SM given at any site over the specified L6 vertebra always decreased ΔMIFnew position more than when it was given one segment away at the L7 vertebra (Table 2). There were no differences in ΔMIFnew position between any of the L6 contact sites.
Figure 7.

Effect of 4 HVLA-SM contact sites on paraspinal muscle spindle responsiveness to a change in vertebral position following the manipulative thrust. Bars represent means accompanied by 95% confidence intervals. See Data Analysis section the Methods for description of ΔMIFnew position.
Table 2.
Comparison between contact sites for their ability to change muscle spindle responsiveness to a change in vertebral position following an HVLA-SM (ΔMIFΔposition).
| Contact Site | Mean difference in ΔMIFposition (imp/s) | Lower 95% Confidence Limit | Upper 95% Confidence Limit | p-value |
|---|---|---|---|---|
| L6 spinous process vs L7 spinous process | −2.6 | −5.0 | −0.3 | 0.03 |
| L6 lamina vs L7 spinous process | −2.4 | −4.8 | 0.0 | 0.05 |
| L6 IAP/MP vs L7 spinous process | −4.4 | −6.8 | −2.0 | <0.001 |
| L6 lamina vs L6 IAP/MP | 2.0 | −0.4 | 4.4 | 0.10 |
| L6 lamina vs L6 spinous process | 0.2 | −2.1 | 2.6 | 0.85 |
| L6 spinous process vs L6 IAP/MP | 1.8 | −0.6 | 4.1 | 0.15 |
MIF, mean instantaneous frequency; imp/s, impulses per second; IAP, inferior articular process; MP, mammillary process.
Effect of contact site on muscle spindle activity to vertebral movement following the HVLA-SM
HVLA-SM at all contact sites produced a statistically significant decrease (F7, 98 = 2.57, p = 0.02) in the responsiveness of muscle spindles to movement (ΔMIFaverage movement) of the L6 vertebra (Figure 8) but not (F7, 98 = 1.88, p = 0.08) to the peak of that movement (ΔMIFpeak movement) (Figure 9). There were no differences in ΔMIFaverage movement for any of the pairwise comparisons.
Figure 8.

Effect of 4 HVLA-SM contact sites on muscle spindle responsiveness to movement of the L6 vertebra following manipulation of the L6 vertebra. Bars represent means accompanied by 95% confidence intervals. See Data Analysis section the Methods for description of ΔMIFaverage movement.
Figure 9.

Effect of 4 HVLA-SM contact sites on the peak muscle spindle responsiveness during movement of the L6 vertebra following manipulation of the L6 vertebra. Bars represent means accompanied by 95% confidence intervals. See Data Analysis section the Methods for description of ΔMIFpeak movement.
DISCUSSION
The data show that during an HVLA-SM sensory input from paraspinal muscle spindles of a target vertebra is at least 186–243% greater when the HVLA-SM is applied to the target vertebra compared to an adjacent vertebra. In addition, using any of 3 traditionally-used contact sites on the target vertebra does not produce neural responses significantly different from each other. The data also show that all 3 contact sites on the target vertebra compared to the site on an adjacent vertebra produce larger decreases in spindle signaling of a new vertebral position during simulated spinal movement. Because we expected neural responses from the four contact sites to be different from each other, the data only partially support our hypothesis. Changes in sensory input from muscle spindles caused by an HVLA-SM applied to different contact sites on a target vertebra are not different from each other but are different from the changes caused by an HVLA-SM applied to an adjacent vertebra.
To our knowledge, only one previous study (Edgecombe et al., 2013) systematically investigated consequences related to an HVLA-SM’s contact site by examining changes in segmental spine stiffness. Contact at the spinous process of the target vertebra but not at an adjacent vertebra significantly decreases average spinal stiffness measured at the target vertebra (Edgecombe et al., 2013). In addition, contact at the spinous process but not at the lamina nor mammillary process of the target vertebra decreases average spinal stiffness measured at the target vertebra (Edgecombe et al., 2013). Edgecombe and colleagues speculated that differences in soft tissue thickness at the each contact site differentially attenuates or alters the transmission of thrust force to deeper connective tissues. In the present study, the similar neurophysiological effects arising from the 3 target vertebra contact sites suggest that different contact sites on the same vertebra similarly stretch paraspinal muscles to activate their spindles.
Previously using the animal model of the current study, Pickar and colleagues showed that during a spinous process thrust, the discharge rate of lumbar paraspinal muscle spindles during a spinous process thrust begins to increase several fold when thrust duration lasts less than 150ms (Pickar and Kang, 2006; Pickar et al., 2007; Reed et al., 2013). Clinically in the lumbar spine, HVLA-SM thrusts typically last less than 150ms (Hessell et al., 1990; Herzog et al., 1993; Triano, 2001). The consequent thrust rate achieved by this duration turns out to be close to the threshold rate for muscle stretch at which the velocity sensitivity of muscle spindles begins to increase non-linearly and predominate over length sensitivity (Matthews and Stein, 1969). Thus, the rapid thrust of an HVLA-SM appears to engage an inherent signaling property of the muscle spindle apparatus (for more extensive discussion see section 4.2 of Pickar and Bolton, 2012). While the barrage of sensory input to the central nervous system during the manipulative thrust is postulated as one neural mechanism contributing to HVLA-SM’s therapeutic effects of HVLA-SM (Korr, 1975; Korr, 1978; Haldeman, 1983; Gillette, 1987; Greenman, 1989; Pickar, 2002; Leach, 2004; Henderson, 2005; Bialosky et al., 2009; Pickar and Bolton, 2012), its contribution to these effects has not yet been established.
The decrease in spindle responsiveness to position and movement of the L6 vertebra in the current study were statistically significant but small (−0.6 to −3.2imps/s). Their biological significance is difficult to gauge. Muscle spindles in lumbar axial muscles are ~3.5× more sensitive to a change in the parent muscle length changes and ~3–10× more sensitive to the rate of change in their length when compared to muscle spindles in appendicular muscles (Cao et al., 2009a; Cao et al., 2009b). For example, whereas passive muscle spindles in soleus muscle discharge ~3imp/s for each 1mm of length change (Harvey and Matthews, 1961), paraspinal muscles would be expected to discharge 3imp/s for each 0.28mm of length change. While the small changes in spindle responsiveness may seem inconsequential, one might reason that a system highly sensitive to input would be impaired by even small reductions in that input. This latter conclusion is consistent with clinical findings from Clark and colleagues (Clark et al., 2011) who found that spinal manipulation with cavitation attenuates the stretch reflex in individuals with low back pain. Peripheral changes in the spindle apparatus that outlasted the thrust itself may have contributed. Further work is needed to clarify this issue.
The current study addresses issues related to contact “specificity” for delivering an HVLA-SM which is characterized by the number of vertebra over which contact for the manipulative thrust is applied (Greenman, 1989; Bergmann, 2005). Specific spinal manipulation is intended to predominately affect tissues connected to a target vertebra by using a relatively narrow contact area between the clinician and patient over a site on the target vertebra. Nonspecific spinal manipulation is meant to affect a region of the spine by using a substantially broader contact over multiple vertebrae.
Previous studies provide both insight and paradoxes associated with contact “specificity”. On the one hand several factors may interfere with the intent of applying a specific contact. As the clinician prepares to deliver the manipulation, the contact site’s location can migrate up to ~10mm (Herzog et al., 2001). In addition, the area of the clinician’s hand that contacts the patient’s skin can influence contact specificity because as the area increases, the pressures generated during the thrust may not develop at the location intended by the clinician (Perle and Kawchuk, 2005). Yet on the other hand if clinicians maintain contact specificity, vertebral kinematics likely differ between the target and adjacent levels. In a single human participant with no known spinal pathology, vertebral translations were greatest at the site of spinal manipulation and diminished linearly at successively contiguous vertebrae (Nathan and Keller, 1994). Similarly in human cadaveric spines with no evidence of spinal pathology, vertebral rotations were largest at the target vertebra and diminished in a graded fashion at successively contiguous vertebrae (Ianuzzi and Khalsa, 2005a), but vertebral translations and FJC strains were similar between the vertebrae (Ianuzzi and Khalsa, 2005a; Ianuzzi and Khalsa, 2005b). The larger biomechanical changes reported to occur at the vertebral level where the manipulative thrust is delivered to human spines are consistent with the larger neurophysiological responses we recorded at the target level in an animal model and with the biomechanical changes reported by Edgecombe et al (2013) in the same animal model.
Several limitations of this study should be noted. First, this study does not provide knowledge about whether contact with a target vertebra or with a specified site on a target vertebra during an HVLA-SM is optimal. A human study involving outcomes must inform this issue. Second, this study does not directly address the mechanism of action for HVLA-SM. Despite the dependence of muscle spindle discharge on contact with the target vertebra, additional work is needed to determine whether activation of this particular proprioceptor makes an important contribution to the mechanism(s) of HVLA-SM’s therapeutic action.
This mechanistic study in an animal model supports prior biomechanical evidence regarding the potential relevance of contact site to the effects of HVLA-SM. It showed that contact made with a target vertebra for an HVLA-SM affects sensory input from muscle spindles associated with that vertebral level more than contact made with an adjacent vertebra. This study is clinically informative as it suggests that the site of delivery may affect the physiological outcome of the HVLA-SM technique. Further studies now need to be undertaken investigating if similar physiological phenomena occur in humans as well as if differences in clinical outcomes are induced when subtle changes in contact site occur.
HIGHLIGHTS.
Contact site specificity for an HVLA-SM impacts neurophysiological responses
Contact on target vertebra increased muscle spindle activity more compared to adjacent vertebra
Different contact sites on same vertebra similarly influenced muscle spindle activity
Changes in muscle spindle responsiveness to simulated spinal movement were small
Acknowledgments
This work was supported by NIH grants U19 AAT004137 to JGP and GNK, and K01 AT005935 to WRR. GNK is supported by the Canada Research Chairs program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Complementary and Alternative Medicine or the National Institutes of Health. The work was conducted in a facility constructed with support from Research Facilities Improvement Grant Number C06 RR15433 from the National Center for Research Resources, NIH.
APPENDIX
Muscle spindle activity
Similar to previous studies (Pickar and Kang, 2006; Pickar et al., 2007; Ge and Pickar, 2012; Reed et al., 2013; Cao et al., 2013) one afferent was investigated per cat because following completion of all spinal manipulations, surgery of the intact spinal tissues was needed to confirm that afferent activity was from muscle spindles in the lumbar multifidus or longissimus muscles. Only afferents that responded to mechanical pressure applied to low back muscles and not to pelvic or leg muscles were studied. Von Frey hairs were used to characterize the location of the most sensitive portion of the afferent’s receptive field. Standard neurophysiological techniques were used to identify muscle spindles including: (a) their increased discharge to succinylcholine (100–400mg/kg intra-arterially; mean maximum frequency increased by 66.7imp/s [4.4 to 17.5imp/s] and lasted at least one minute); (b) decreased discharge to electrically induced muscle contraction (0.2–0.5mA; 50μs); and (c) sustained response to a fast vibratory stimulus (~70Hz) applied to the muscle’s surface close to the neuron’s receptive field. Spindle responses represented their passive activity because surgical anesthesia and cutting the dorsal roots abolished reflex gamma responses. Single unit action potentials were passed through a high impedance probe (HIP511, Grass, MA), amplified (P511K, Grass, MA), and then recorded and analyzed using a PC based data acquisition system (Spike 2, Cambridge Electronic Design, Cambridge, England).
HVLA-SM and contact sites
Because the thrust of an HVLA-SM is intended to impart movement to a vertebra (Bergmann, 2005) we first applied a preload force beyond which the L6 vertebra would be expected to move during the thrust using methods previously described (Vaillant et al., 2012; Reed et al., 2013; Cao et al., 2013). Thrust duration was fixed at 100ms based upon clinical findings (Hessell et al., 1990; Herzog et al., 1993; Triano, 2001) where thrust durations in the thoracic and lumbar spines are typically less than 150ms and upon animal studies (Pickar and Kang, 2006; Pickar et al., 2007; Reed et al., 2013) where 100ms represents the threshold duration at which a non-linear increase in muscle spindle discharge occurred. The peak applied force represented 55% of an average cat’s body based upon weights obtained from 112 cats in previous experiments (Reed et al., 2013; Cao et al., 2013). This relative force level is within the range used clinically after normalizing reported forces (Hessell et al., 1990; Suter et al., 1994) to a 70kg person. Following the thrust, the manipulator’s tip was withdrawn from the spine within 25ms.
Anatomical location of contact sites was standardized relative to the L6 spinous process based upon the average of measurements from 3 cadaveric cat spines. Because contact over the L6 mammillary process mechanically disrupted our neural recordings, the L6 mammillary contact occurred over the inferior articular process of L6 near the L7 mammillary process. Relative to the L6 spinous process, contact for the L6 lamina was 3mm lateral and 7mm caudal, L6 IAP/MP was 7mm lateral and 15mm caudal, and L7 was 21mm caudal.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- Bergmann TF. High-velocity low-amplitude manipulative techniques. In: Haldeman S, Dagenais S, Budgell B, Grunnet-Nilsson N, Hooper PD, Meeker WC, Triano J, editors. Principles and Practice of Chiropractic. New York: McGraw-Hill; 2005. pp. 755–66. [Google Scholar]
- Bialosky JE, Bishop MD, Price DD, Robinson ME, George SZ. The mechanisms of manual therapy in the treatment of musculoskeletal pain: A comprehensive model. Manual Therapy. 2009;14:531–8. doi: 10.1016/j.math.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogduk N. The dorsal lumbar muscles of the cat. Acta Anz, Jena. 1980;148:55–67. [PubMed] [Google Scholar]
- Bolton PS, Budgell BS. Spinal manipulation and spinal mobilization influence different axial sensory beds. Medical Hypotheses. 2006;66(2):258–62. doi: 10.1016/j.mehy.2005.08.054. [DOI] [PubMed] [Google Scholar]
- Bridgman CF, Eldred E. Hypothesis for a pressure-sensitive mechanism in muscle spindles. Science. 1964;143:481–2. doi: 10.1126/science.143.3605.481. [DOI] [PubMed] [Google Scholar]
- Cambridge ED, Triano JJ, Ross JK, Abbott MS. Comparison of force development strategies of spinal manipulation used for thoracic pain. Manual Therapy. 2012;17(3):241–5. doi: 10.1016/j.math.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Cao DY, Khalsa PS, Pickar JG. Dynamic responsiveness of lumbar paraspinal muscle spindles during vertebral movement in the cat. Experimental Brain Research. 2009a;197(4):369–77. doi: 10.1007/s00221-009-1924-0. [DOI] [PubMed] [Google Scholar]
- Cao DY, Pickar JG, Ge W, Ianuzzi A, Khalsa PS. Position sensitivity of feline paraspinal muscle spindles to vertebral movement in the lumbar spine. Journal of Neurophysiology. 2009b;101(4):1722–9. doi: 10.1152/jn.90976.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao DY, Reed WR, Long CR, Kawchuk GN, Pickar JG. Effects of thrust amplitude and duration of high-velocity, low-amplitude spinal manipulation on lumbar muscle spindle responses to vertebral position and movement. Journal of Manipulative and Physiological Therapeutics. 2013;36(2):68–77. doi: 10.1016/j.jmpt.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen MG, Kerkhoff D, Kollasch MW, Cohn L. Job Analysis of Chiropractic. 2005. [Google Scholar]
- Clark BC, Goss DA, Jr, Walkowski S, Hoffman RL, Ross A, Thomas JS. Neurophysiologic effects of spinal manipulation in patients with chronic low back pain. BMC Musculoskelet Disord. 2011;12:170. doi: 10.1186/1471-2474-12-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland JA, Fritz JM, Kulig K, Davenport TE, Eberhart S, Magel J, Childs JD. Comparison of the effectiveness of three manual physical therapy techniques in a subgroup of patients with low back pain who satisfy a clinical prediction rule: a randomized clinical trial. Spine. 2009;34(25):2720–9. doi: 10.1097/BRS.0b013e3181b48809. [DOI] [PubMed] [Google Scholar]
- Conway PJW, Herzog W, Zhang Y, Hasler EM, Ladly K. Forces required to cause cavitation during spinal manipulation of the thoracic spine. Clinical Biomechanics. 1993;8:210–4. doi: 10.1016/0268-0033(93)90016-B. [DOI] [PubMed] [Google Scholar]
- Cook C, Learman K, Showalter C, Kabbaz V, O’Halloran B. Early use of thrust manipulation versus non-thrust manipulation: a randomized clinical trial. Manual Therapy. 2013;18(3):191–8. doi: 10.1016/j.math.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Cramer GD, Cambron J, Cantu JA, Dexheimer JM, Pocius JD, Gregerson D, Fergus M, McKinnis R, Grieve TJ. Magnetic resonance imaging zygapophyseal joint space changes (gapping) in low back pain patients following spinal manipulation and side-posture positioning: a randomized controlled mechanisms trial with blinding. Journal of Manipulative and Physiological Therapeutics. 2013;36(4):203–17. doi: 10.1016/j.jmpt.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descarreaux M, Nougarou F, Dugas C. Standardization of spinal manipulation therapy in humans: development of a novel device designed to measure dose-response. Journal of Manipulative and Physiological Therapeutics. 2013;36(2):78–83. doi: 10.1016/j.jmpt.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Edgecombe TL, Kawchuk GN, Long CR, Pickar JG. The effect of application site of spinal manipulative therapy (SMT) on spinal stiffness. The Spine Journal. 2013 doi: 10.1016/j.spinee.2013.07.480. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, Van Rompay M, Kessler RC. Trends in alternative medicine use in the United States, 1990–1997: Results of a follow-up national survery. Journal of the American Medical Association. 1998;280(18):1569–75. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- Francois N, Claude D, Loranger M, Page I, Descarreaux M. The role of preload forces in spinal manipulation: experimental investigation of kinematic and electromyographic responses in healthy adults. Journal of Manipulative and Physiological Therapeutics. 2014;37(5):287–93. doi: 10.1016/j.jmpt.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Ge W, Pickar JG. The decreased responsiveness of lumbar muscle spindles to a prior history of spinal muscle lengthening is graded with the magnitude of change in vertebral position. Journal of Electromyography and Kinesiology. 2012;22(6):814–20. doi: 10.1016/j.jelekin.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette RG. A speculative argument for the coactivation of diverse somatic receptor populations by forceful chiropractic adjustments. Manual Medicine. 1987;3:1–14. [Google Scholar]
- Greenman PE. Principles of Manual Medicine. 1989. [Google Scholar]
- Haldeman S. Spinal manipulative therapy; a status report. Clinical Orthopaedics and Related Research. 1983;179:62–70. [PubMed] [Google Scholar]
- Harvey RJ, Matthews PB. The response of de-efferented muscle spindle endings in the cat’s soleus to slow extension of the muscle. Journal of Physiology. 1961;157:370–92. doi: 10.1113/jphysiol.1961.sp006729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawk C, Long CR, Boulanger KT. Prevalence of nonmusculoskeletal complaints in chiropractic practice: report from a practice-based research program. Journal of Manipulative and Physiological Therapeutics. 2001;24(3):157–69. [PubMed] [Google Scholar]
- Henderson CNR. Three neurophysiologic theories on the chiropractic subluxation. In: Gatterman MI, editor. Foundations of chiropractic: Subluxation. St. Louis: Elsevier Mosby; 2005. pp. 296–303. [Google Scholar]
- Herzog W, Conway PJ, Kawchuk GN, Zhang Y, Hasler EM. Forces exerted during spinal manipulative therapy. Spine. 1993;18(9):1206–12. doi: 10.1097/00007632-199307000-00014. [DOI] [PubMed] [Google Scholar]
- Herzog W, Kats M, Symons B. The effective forces transmitted by high-speed, low-amplitude thoracic manipulation. Spine. 2001;26(19):2105–10. doi: 10.1097/00007632-200110010-00012. [DOI] [PubMed] [Google Scholar]
- Hessell BW, Herzog W, Conway PJW, McEwen MC. Experimental measurement of the force exerted during spinal manipulation using the Thompson technique. Journal of Manipulative and Physiological Therapeutics. 1990;13(8):448–53. [PubMed] [Google Scholar]
- Hooper PD. Evolution and basic principles of the chiropractic adjustment and manipulation. In: Haldeman S, Dagenais S, Budgell B, Grunnet-Nilsson N, Hooper PD, Meeker WC, Triano J, editors. Principles and Practice of Chiropractic. New York: McGraw-Hill; 2005. pp. 745–54. [Google Scholar]
- Hunt CC, Ottoson D. Initial burst of primary endings of isolated mammalian muscle spindles. Journal of Neurophysiology. 1976;39(2):324–30. doi: 10.1152/jn.1976.39.2.324. [DOI] [PubMed] [Google Scholar]
- Ianuzzi A, Khalsa PS. Comparison of human lumbar facet joint capsule strains during simulated high-velocity, low-amplitude spinal manipulation versus physiological motions. The Spine Journal. 2005a;5(3):277–90. doi: 10.1016/j.spinee.2004.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianuzzi A, Khalsa PS. High loading rate during spinal manipulation produces unique facet joint capsule strain patterns compared with axial rotations. Journal of Manipulative and Physiological Therapeutics. 2005b;28(9):673–87. doi: 10.1016/j.jmpt.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Korr IM. Proprioceptors and somatic dysfunction. Journal of the American Osteopathic Association. 1975;74:638–50. [PubMed] [Google Scholar]
- Korr IM. The Neurobiologic Mechanisms in Manipulative Therapy. 1978. [Google Scholar]
- Leach RA. The Chiropractic Theories. 2004. p. 4. [Google Scholar]
- Matthews PBC. Mammalian muscle receptors and their central actions. 1972. [Google Scholar]
- Matthews PBC, Stein RB. The sensitivity of muscle spindle afferents to small sinusoidal changes of length. Journal of Physiology. 1969;200:723–43. doi: 10.1113/jphysiol.1969.sp008719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan M, Keller TS. Measurement and analysis of the in vivo posteroanterior impulse response of the human thoracolumbar spine: a feasibility study. Journal of Manipulative and Physiological Therapeutics. 1994;17(7):431–41. [PubMed] [Google Scholar]
- Nougarou F, Dugas C, Deslauriers C, Page I, Descarreaux M. Physiological responses to spinal manipulation therapy: investigation of the relationship between electromyographic responses and peak force. Journal of Manipulative and Physiological Therapeutics. 2013;36(9):557–63. doi: 10.1016/j.jmpt.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Page I, Nougarou F, Dugas C, Descarreaux M. The effect of spinal manipulation impulse duration on spine neuromechanical responses. Journal of the Canadian Chiropractic Association. 2014;58(2):141–8. [PMC free article] [PubMed] [Google Scholar]
- Perle SM, Kawchuk GN. Pressures generated during spinal manipulation and their association with hand anatomy. Journal of Manipulative and Physiological Therapeutics. 2005;28(4):e1–e7. doi: 10.1016/j.jmpt.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Peterson DH, Bergmann TF. The spine: anatomy, biomechanics, assessment, and adjustive techniques. In: Bergmann TF, Peterson DH, Lawrence DJ, editors. Chiropractic Technique. New York: Churchill Livingstone; 1993. pp. 197–522. [Google Scholar]
- Pickar JG. An in vivo preparation for investigating neural responses to controlled loading of a lumbar vertebra in the anesthetized cat. Journal of Neuroscience Methods. 1999;89:87–96. doi: 10.1016/s0165-0270(99)00060-6. [DOI] [PubMed] [Google Scholar]
- Pickar JG. Neurophysiological effects of spinal manipulation. The Spine Journal. 2002;2:357–71. doi: 10.1016/s1529-9430(02)00400-x. [DOI] [PubMed] [Google Scholar]
- Pickar JG, Bolton PS. Spinal manipulative therapy and somatosensory activation. Journal of Electromyography and Kinesiology. 2012 doi: 10.1016/j.jelekin.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickar JG, Kang YM. Paraspinal muscle spindle responses to the duration of a spinal manipulation under force control. Journal of Manipulative and Physiological Therapeutics. 2006;29(1):22–31. doi: 10.1016/j.jmpt.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Pickar JG, Sung PS, Kang YM, Ge W. Response of lumbar paraspinal muscle spindles is greater to spinal manipulative loading compared with slower loading under length control. The Spine Journal. 2007;7(5):583–95. doi: 10.1016/j.spinee.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickar JG, Wheeler JD. Response of muscle proprioceptors to spinal manipulative-like loads in the anesthetized cat. Journal of Manipulative and Physiological Therapeutics. 2001;24(1):2–11. doi: 10.1067/mmt.2001.112017. [DOI] [PubMed] [Google Scholar]
- Reed WR, Cao DY, Long CR, Kawchuk GN, Pickar JG. Relationship between biomechanical characteristics of spinal manipulation and neural responses in an animal model: effect of linear control of thrust displacement versus force, thrust amplitude, thrust duration, and thrust rate. Evidence-based Complementary and Alternative Medicine: eCAM. 2013;2013:492039. doi: 10.1155/2013/492039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed WR, Long CR, Kawchuk GN, Pickar JG. Neural responses to the mechanical parameters of a high-velocity, low-amplitude spinal manipulation: effect of preload parameters. Journal of Manipulative and Physiological Therapeutics. 2014;37(2):68–78. doi: 10.1016/j.jmpt.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein SM, Terwee CB, Assendelft WJ, de Boer MR, van Tulder MW. Spinal manipulative therapy for acute low back pain: an update of the cochrane review. Spine (Phila Pa 1976) 2013;38(3):E158–E177. doi: 10.1097/BRS.0b013e31827dd89d. [DOI] [PubMed] [Google Scholar]
- Shekelle PG, Adams AH, Chassin MR, Hurwitz EL, Brook RH. Spinal manipulation for low-back pain. Annals of Internal Medicine. 1992;117(7):590–8. doi: 10.7326/0003-4819-117-7-590. [DOI] [PubMed] [Google Scholar]
- Sorensen LP, Stochkendahl MJ, Hartvigsen J, Nilsson NG. Chiropractic patients in Denmark 2002: an expanded description and comparison with 1999 survey. Journal of Manipulative and Physiological Therapeutics. 2006;29(6):419–24. doi: 10.1016/j.jmpt.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Sung PS, Kang YM, Pickar JG. Effect of spinal manipulation duration on low threshold mechanoreceptors in lumbar paraspinal muscles: a preliminary report. Spine. 2005;30(1):115–22. doi: 10.1097/01.brs.0000147800.88242.48. [DOI] [PubMed] [Google Scholar]
- Suter E, Herzog W, Conway PJ, Zhang YT. Reflex response associated with manipulative treatment of the thoracic spine. Journal of the Neuromusculoskeletal System. 1994;2(3):124–30. [Google Scholar]
- Triano J. The mechanics of spinal manipulation. In: Herzog W, editor. Clinical Biomechanics of Spinal Manipulation. New York: Churchill Livingstone; 2000. pp. 92–190. [Google Scholar]
- Triano J, Schultz AB. Loads transmitted during lumbosacral spinal manipulative therapy. Spine. 1997;22(17):1955–64. doi: 10.1097/00007632-199709010-00003. [DOI] [PubMed] [Google Scholar]
- Triano JJ. Biomechanics of spinal manipulative therapy. The Spine Journal. 2001;1(2):121–30. doi: 10.1016/s1529-9430(01)00007-9. [DOI] [PubMed] [Google Scholar]
- Vaillant M, Edgecombe T, Long CR, Pickar JG, Kawchuk GN. The effect of duration and amplitude of spinal manipulative therapy on the spinal stiffness. Manual Therapy. 2012;17(6):577–83. doi: 10.1016/j.math.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillant M, Pickar JG, Kawchuk GN. Performance and reliability of a variable rate, force/displacement application system. Journal of Manipulative and Physiological Therapeutics. 2010;33(8):585–93. doi: 10.1016/j.jmpt.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]




