Abstract
Objectives
Patients with febrile neutropenia are at high risk of morbidity and mortality from infectious causes. Decreasing time to antibiotic (TTA) administration is associated with improved patient outcomes. We sought to reduce TTA for children presenting to the Emergency Department (ED) with fever and neutropenia.
Methods
In a prospective cohort study with historical comparison, TTA administration was evaluated in patients with neutropenia presenting to the Children’s of Alabama ED. A protocol was established to reduce delays in antibiotic administration and increase the percentage of patients who receive treatment within 60 minutes of presentation. One hundred pre-protocol patient visits between August 2010 and December 2011 were evaluated and 153 post-protocol visits were evaluated between August 2012 and September 2013. We reviewed individual cases to determine barriers to rapid antibiotic administration.
Results
Antibiotics were administered in 96.9 ± 57.8 minutes in the pre-protocol patient group and only 35% of patients received antibiotics within 60 minutes of presentation and 70% received antibiotics within 120 minutes. After implementation of the protocol, TTA for neutropenic patients was decreased to 64.3 ± 28.4 minutes (p < 0.0001) with 51.4% receiving antibiotics within 60 minutes and 93.2% within 120 minutes.
Conclusion
Implementing a standard approach to patients at risk for neutropenia decreased TTA. There are numerous challenges in providing timely antibiotics to children with febrile neutropenia. Identified delays included venous access (time to effect of topical anesthetics, and difficulty obtaining access), physicians waiting on laboratory results, and antibiotic availability.
Keywords: febrile neutropenia, quality improvement, time to antibiotics
Introduction
Pediatric oncology patients undergoing chemotherapy are at high risk of morbidity and mortality from infectious causes due to impairment of innate and adaptive immunity. Fever may be the only indication of a severe infection due to the blunting of classical signs and symptoms of inflammation.1 There is a high incidence of fever and neutropenia in pediatric oncology patients, as over 80% of individuals with a hematologic malignancy will develop fever during at least one chemotherapy cycle.1 While there have been attempts to classify patients with neutropenic fever into a high and low risk category, no universally accepted consensus has been established.1-4 Children who develop fever while undergoing myelosuppressive chemotherapy, or have a history of benign hematologic immunosuppression, require swift treatment with broad-spectrum antibiotics. Delays in treatment can lead to rapid progression of infection and sepsis without overt signs of disease.5 The importance of timely antibiotics in septic patients was demonstrated in adult patients as delay in initiation of antimicrobial therapy after onset of hypotension resulted in a 7.6% decreased rate of survival per hour over the first six hours.6
Time to antibiotic (TTA) administration in febrile neutropenic patients has recently become an important quality of care measurement. Rapid antimicrobial administration is becoming a universal goal in these patients due to the evidence that rapid antibiotic administration can improve outcomes.7, 8 While the importance of timely administration has been recognized, there has not been a consensus on the time window in which antimicrobials should be given to febrile neutropenic patients. Various institutions have established timeline goals from patient presentation at the emergency department (ED) to administration of antibiotics.5, 7, 9-13 Published guidelines for TTA have ranged from 30 to 120 minutes. Meeting this time window has led to decreased adverse effects during the subsequent hospitalization in some studies.13, 14 Some reports, however, do not demonstrate a correlation between time to antibiotics and patient outcomes.10, 15 One study found that administration of antibiotics from 60-120 minutes after presentation resulted in worse outcomes than antibiotic administration within the first 60 minutes of presentation.14
In an evaluation of 337 cases of febrile neutropenia admitted to St. Jude Children’s Research Hospital, a pathogen was confirmed through laboratory tests in 25% of the episodes, with bacterial isolates identified in 63% of these patients. In addition, 22% of visits were classified as probable infections based on clinical findings without a pathogen identified.16 Because of the high incidence of infections in patients with febrile neutropenia, swift administration of antibiotics has the potential to improve patient outcomes and reduce subsequent inpatient complications.5 Thus, the benefit of rapid antibiotic administration to high risk patients likely outweighs any potential harm of administering a single dose of antibiotic prior to laboratory confirmation of neutropenia in patients on active chemotherapy or with a history of congenital or acquired neutropenia.
Children’s of Alabama (COA) is a tertiary care center in Birmingham, Alabama, and the medical home for approximately 300 pediatric oncology patients annually. Children with a history of receiving chemotherapy or benign hematologic neutropenia frequently present to the ED with fever. While they are triaged into an urgent category, utilizing the five level Emergency Severity Index triage system, there was no previous standardized care plan for these patients. We developed a protocol to improve TTA in febrile patients at risk for neutropenia for use in the ED. We sought to reduce delays in initial antibiotic administration with a standardized care protocol for this group of patients. Our management goal was to provide the first dose of antibiotic within 60 minutes from patient triage. The time period of 60 minutes for antibiotic administration was a consensus decision by the Divisions of Pediatric Hematology/Oncology and Emergency Medicine. In this review, we studied time to antibiotics before and after protocol initiation.
Materials and Methods
We performed a prospective cohort study with a historical comparison evaluating the effect of a standardized care protocol for patient visits to the COA ED. The aim of the study was to evaluate the effect of protocol implementation on TTA for pediatric patients at risk for neutropenia. The pre-protocol patient group (historical control) included a chart review of ED patient visits between August 2010 and December 2011. Based on these findings, a protocol was developed to reduce TTA. After protocol development in July 2012, we prospectively evaluated patients presenting to the ED during a 14 month period which included a 2 month period for staff training and implementation of the protocol (August 1, 2012 through September 30, 2012) and the subsequent 12 months (October 1, 2012 through September 30, 2013). Approval for this research was obtained from the local institutional review board.
Within the pre-protocol group, ED patient visits triaged as “fever-immunosuppressed” and those who had a designation of “protective isolation” were queried between August 2010 and December 2011. Individual patient visits were reviewed within the electronic medical record system and those containing patients on chemotherapy were selected for further review.The charts for these visits were evaluated to determine if the patients met the inclusion criteria of severe neutropenia (absolute neutrophil count [ANC] < 500) and fever (any single temperature of ≥38.3° C or two measured temperatures 38-38.2° C twice within a 12 hour period). In a review of this historical group, it was determined that waiting for the result of a complete blood count (CBC) to determine whether a patient was actually neutropenic resulted in significant delays in antibiotic administration. Therefore, a protocol was developed to apply to all febrile hematology and oncology patients known to be at risk for neutropenia (oncology patients on active chemotherapy or within one month of the end of therapy, and hematology patients with benign neutropenia including congenital neutropenia). After protocol implementation, visits of febrile patients at risk for neutropenia were evaluated from August 1, 2012 through September 30, 2013. The protocol was created in July 2012. August 1, 2012 toSeptember 30, 2012 were used as a two month lead in time for training and for the ED staff to familiarize themselves with use of the protocol. After the two month training period, a 12 month period (October 1, 2012 to September 30, 2013) was analyzed by division into four quarters to assess potential variation in protocol adherence over a one year period. Nursing and physician notes were reviewed in detail for patient visits where TTA was beyond 60 minutes with the goal to identify potential points of delay.Subset analysis of this post-protocol group also included evaluation of those patients found to have severe neutropenia.
We reviewed and collected data from the electronic medical record system for each patient visit, including both pre and post-protocol visits. The following were obtained from every patient visit: hematologic or oncologic diagnosis, ANC, time in minutes to antibiotic order (defined as time from triage check-in to time of order in the electronic medical record system), time in minutes to antibiotic administration (defined as time from triage check-in to infusion of initial antibiotic dose). In collecting the data, physician and nursing documentation was reviewed to identify potential causes of antibiotic administration delay.
Interventions
A protocol for the rapid administration of antibiotics in febrile patients at risk for neutropenia was formulated by the Divisions of Pediatric Hematology/Oncology and Emergency Medicine.(Figure 1) Points of delay identified in the historical group were addressed in the formulation and implementation of the protocol.(Table 1) Common barriers identified included delays in obtaining central access (achieving adequate topical analgesia, parents requesting specific nurses to obtain access, time and number of attempts necessary to gain access), delay in obtaining the ordered medication (time required to obtain the medicine from pharmacy), and delay in ordering antibiotics (often due to the physician waiting on CBC results). Patient education (including a letter sent to all patients on active treatment) explained the new protocol and reminded families to place a topical anesthetic agent over the point of access en route to the ED. This allows the ED staff to begin obtaining central venous access upon arrival to the ED rather than waiting for topical anesthesia. The ED nurses received increased training regarding use of the subcutaneous venous access device (SVAD or port) in order to decrease required attempts to achieve central venous access. Families were also asked to allow ED staff to obtain central venous access rather than asking for specific nurses who work in departments outside of the ED. For this group of patients, cefepime was placed in a Pyxis Medstation® system to reduce wait times required to obtain the medication from the inpatient pharmacy. ED nursing and triage staff were also educated on the importance of rapid triage to decrease patient wait times after triage check-in.
Figure 1.
Protocol for febrile patients at risk for neutropenia presenting to the emergency department
Table 1.
Identified points of delay in the historical group and responses/interventions that were addressed in the formulation and implementation of the protocol.
| Barriers Identified | Response/Intervention |
|---|---|
| Delays in obtaining central access |
|
| Delay in antibiotic order |
|
| Delay in obtaining ordered antibiotics from pharmacy |
|
In order to quickly administer cefepime, the medication must be readily available to ED nursing staff. Previous studies have demonstrated that by placing broad-spectrum antibiotics in the ED in a readily available emergency cart with standard dosing, TTA was reduced.10, 12 This eliminates the transit time that is necessary to deliver the medication from the inpatient pharmacy. As part of our protocol implementation, cefepime was made immediately available in a Pyxis Medstation® system. Once ED staff ensures that there is no history of beta-lactam allergy and a physician places an order, the medication may be quickly retrieved and administered. While this removes the delay of retrieving the medication from the pharmacy, it places increased responsibility on the ED physicians and nursing staff to ensure proper dosing of the medication, as there is no longer a pharmacist double-checking the ordered dose. To ensure accuracy, the electronic order system in the ED has built in standardized dosing (50 mg/kg) within a unique order set for patients triaged with immunosuppressed fever.
Waiting for laboratory results, which demonstrated whether or not a patient was neutropenic, caused significant delays in administration of antibiotics. Therefore, the protocol required that all patients at risk for neutropenia (oncology patients on active chemotherapy or patients with a known benign neutropenic condition) be initially treated as potentially neutropenic and receive rapid antibiotics without waiting for a CBC result. Further management of the patient including admission for ongoing antibiotic treatment could be determined after laboratory studies resulted.
After initial evaluation including vital signs and triage level, labs are to be obtained including CBC with differential, blood cultures (aerobic and anaerobic from each lumen of central line or peripheral blood cultures when central line is not present), and other cultures as clinically appropriate. If clinical signs of sepsis are present (hypotension, tachycardia out of proportion to fever, delayed capillary refill) cefepime (50 mg/kg IV with a maximum dose of 2 grams) is administered with additional supportive care and antimicrobials as clinically indicated. In a well appearing child at risk for neutropenia who has not had a CBC in the past 24 hours, antibiotics are to be administered. ED physicians may wait for the CBC result to determine if antibiotics are necessary only if there has been a recently documented ANC greater than 1,000 in the past 12 hours or ANC greater than 1,500 in the past 24 hours. In this case, following result of the new CBC, an ANC less than 500 would require cefepime. After administration of cefepime, complete evaluation follows including full history and physical, chest x-ray, and other labs and work up if needed. ED nursing staff was educated on the protocol prior to initiation and received continuing yearly re-education. Quarterly results of the median time to antibiotics for this patient population were shared at faculty and staff meetings to encourage improvement and adherence to the protocol. When a patient is triaged and determined to be at risk for neutropenia, an ED attending physician is notified to ensure proper and timely use of the protocol. Thus activation of the protocol is not dependent on medical trainees (such as resident physicians) who rotate through the ED and may have less familiarity with the protocol.
Primary Data Analysis
For analysis we evaluated time in minutes to initial antibiotic dose in the pre and post-protocol groups. Within each group the proportion of patients receiving antibiotics within the first 60 and 120 minutes of presentation was determined. Within the post-protocol group, data from the one-year period October 1, 2012 to September 30, 2013 was further divided into four quarters in order to determine the trend in ED personnel use of the protocol. The four quarters were defined as quarter 1: October 1-December 31, 2012, quarter 2: January 1-March 31, 2013, quarter 3: April 1-June 30, 2013, and quarter 4: July 1-September 30, 2013. The first two months of the protocol implementation were not included in this breakdown as this time was considered a lead-in period to educate families and hospital staff. Quarterly reports were made available to ED staff.
Patient characteristics were summarized using descriptive statistics and compared between groups using a chi-squared (or Fisher’s exact) test and Wilcoxon rank-sum test. In neutropenic patients, TTA administration was assessed between pre and post-protocol groups using a Student’s t-test. The proportions of those receiving antibiotics within 60 and 120 minutes before and after protocol implementation were examined using the chi-squared test. In a subset of post-protocol group, the same method was used to compare TTA administration between the truly neutropenic and the at risk groups. TTA administration, time from ED triage check-in to order, and time from order to administration over four consecutive quarters were evaluated via an analysis of variance (ANOVA), followed by post-hoc tests using Tukey-Kramer method. The proportions of neutropenic patients receiving antibiotics within 60 and 120 minutes were also examined between the four quarters by the chi-squared test or Fisher’s exact test. For variables that deviated from the assumptions of statistical tests, appropriate transformation or non-parametric procedure was applied. A p-value of < 0.05 was considered statistically significant in two-tailed statistical tests. All analyses were conducted using SAS 9.3 (SAS Institute, Cary NC) software. The graphical displays were created by GraphPad Prism 6 (GraphPad, San Diego, CA) software.
Results
The pre-protocol group included 100 neutropenic patients treated in the ED during a 17-month period from August 2010 through December 2011.The post-protocol group comprised 153 patients including 74 neutropenic patients treated in the ED during a 14-month period from August 1, 2012 through September 30, 2013. In comparison of the pre-protocol and post-protocol groups, there was no significant difference in median age, rates of ICU admission or death. (Table 2) As described in the Methods section above, the pre-protocol group included only oncology patients with severe neutropenia while the post-protocol group included all patients at risk for neutropenia and only a subset (48.4%) had severe neutropenia. Hence the groups differed significantly in their median ANC. However in order to control for ANC as a confounder, a sub-group analysis is presented below comparing only the 74 patients with severe neutropenia in the post-protocol group to the 100 patients with severe neutropenia in the pre-protocol group. The groups also differed in underlying diagnosis with the post-protocol group having a significantly greater proportion of patients with leukemia and lymphoma as an oncologic diagnosis. (Table 2)
Table 2.
Comparison of clinical characteristics and laboratory findings between the pre-protocol and post-protocol groups
| Pre Protocol (n=100) | Post Protocol (n=153) | p-value | |
|---|---|---|---|
| Age in years, median (range) | 8 (0-23) | 6 (0-22) | 0.22 |
| ANC (cells/μL), median (range) | 34.5 (0-485) | 559 (0-14,020) | <.0001* |
| Severe Neutropenia (ANC <500/μL), n (%) | 100 (100)1 | 74 (48.4) | <.0001* |
| Leukemia/Lymphoma, n (%) | 51 (51) | 102 (66.7) | 0.019* |
| Other Oncologic Diagnoses, n (%) | 48 (48) | 45 (29.4) | 0.003* |
| Benign Hematology, n (%) | 0 (0) | 6 (3.9) | 0.08 |
| ICU Admission, n (%) | 5 (5) | 2 (1.3) | 0.12 |
| Death, n (%) | 2 (2) | 0 (0) | 0.16 |
By design, all patients in the pre-protocol group had severe neutropenia with ANC <500/μL (see methods)
Denotes statistical significance at p < 0.05
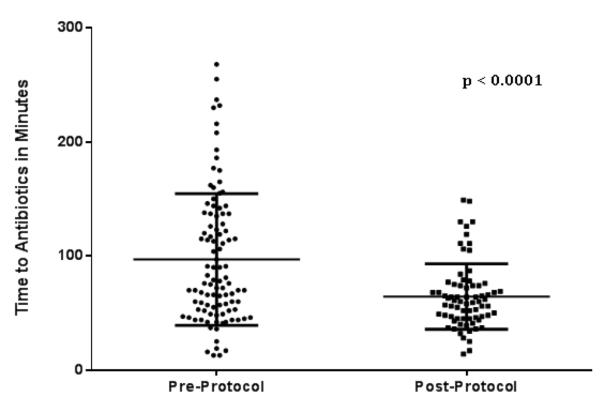
Comparing only patients with severe neutropenia in the pre and post-protocol groups, the mean TTA improved significantly after implementation of the protocol to 64.3 ± 28.4 minutes, compared to the pre-protocol group, 96.9 ± 57.8 minutes (p < 0.0001).(Figure 2) The percentage of neutropenic patients receiving antibiotics within 60 minutes improved from 35% to 51.4% (p=0.031) after protocol implementation. Administration within 120 minutes improved from 70% to 93.2% (p=0.0002).(Figure 3)
Figure 2.
Mean time to antibiotic administration was significantly decreased following implementation of a standardized care protocol in the emergency department.
Figure 3.
Percentage of patients receiving antibiotics within 60 and 120 minutes improved following implementation of standardized care protocol.
Analysis of the historical control group determined that waiting for CBC results had caused a significant delay in TTA. Therefore, the protocol was designed to apply to all patients at significant risk for neutropenia. During the 14 month post-protocol evaluation period, a total of 153 patients met the protocol inclusion criteria. Among these patients, 48.4% (74/153) were truly neutropenic. The mean TTA for the 153 patients at risk for neutropenia was 69.5 ± 35 minutes. In this group, the mean TTA was not significantly different (p=0.076) between patients who were truly neutropenic and merely at risk (neutropenic: n=74, 64.3 ± 28.4 minutes; only at-risk: n=79, 74.3 ± 40 minutes).
Next we examined whether there was a change in effect of the protocol over time. Since the above analysis revealed no difference in TTA for those with or without severe neutropenia, all patients at risk for neutropenia where included in this analysis. However, patients seen during the first two months of the protocol implementation (August 1-September 30, 2012) were excluded since this was a period of training. The remaining patients (N=144) treated during the 12 month period from October 1, 2012 to September 30, 2013 were evaluated by quarter of presentation (four cohorts divided into 3 month periods of presentation). This quarterly analysis was done to follow the trend of protocol use and effectiveness over the first year of use. The means for TTA in quarters 1 to 4 were 82.8 ± 45.2 minutes (n=38), 60.1 ± 19.5 minutes (n=29), 68.2 ± 32.8 minutes (n=33), and 59.3 ± 27.4 minutes (n=44) respectively (Figure 4a). Overall, there were significant differences in TTA between quarters (p=0.0078), specifically between quarter 1 and 4 (p=0.0087), with a shorter TTA in quarter 4. There was a 22.3% and 11.2% improvement in antibiotics administration within 60 and 120 minutes respectively (p=0.044 and 0.136) when comparing quarter 4 to quarter 1 (Figure 4b). The total number of patient visits in the ED during these four quarters were 18,847 in quarter 1 (October-December 2012), 16,631 in quarter 2 (January-March 2013), 15,693 in quarter 3 (April-June 2013), and 15,717 in quarter 4 (July-September 2013). During quarter 1 TTA was longest and ED volume was highest while during quarter 4 TTA was shortest and ED volume was second lowest although we had no direct measurement to determine whether ED volume affected the TTA.
Figure 4.
Time to antibiotics (TTA) by quarter following implementation of protocol. Mean TTA decreased significantly from quarter 1 to 4 (a), and percentage of cases with TTA less than 60 and 120 minutes increased significantly from quarter 1 to 4 (b).
Patient charts were reviewed to determine any points of delay in TTA. Time from ED triage check-in to antibiotic order by an ED physician was evaluated along with time from antibiotic order to administration. Significant differences in time to order were seen between quarters (p=0.0014), there was an improvement for at risk patients between quarter 1 and 4 from 46.4 ± 41.8 minutes to 27.1 ± 22.8 minutes respectively (p=0.042). However, no apparent improvement was noted in time from order to medication between quarter 1 and 4 (p=0.867).
Discussion
Implementation of standardized guidelines was successful in reducing time from ED triage to antibiotic administration in patients at risk for neutropenia. A 33.6% reduction in wait time for neutropenic patients was demonstrated from the pre-protocol to post-protocol groups. Moreover, as the ED staff became more familiar and comfortable with the guidelines waiting times were significantly reduced for both time to order and administration of antibiotics between the first and last quarter of the initial post-protocol year. Certain delays were commonly noted when developing the protocol and reviewing patient charts. These include achieving topical anesthesia prior to central venous access, obtaining central access, waiting on laboratory results, and waiting on antibiotics from the pharmacy.
Obtaining central venous access is a crucial first step of treatment in this patient group as most children undergoing chemotherapy at our center have a subcutaneous venous access device (SVAD or port). Topical anesthesia of the soft tissue overlying the SVAD with a mixture of lidocaine-prilocaine may take up to 60 minutes to achieve a desired effect.17 By educating families to apply a topical anesthetic en route to the ED, central venous access may be achieved with adequate analgesia upon presentation. Nursing access of the SVAD has also caused delays. Due to frequent inpatient hospitalizations during the course of chemotherapy patient families often become comfortable with the nursing providers on the hematology/oncology inpatient unit. This provides a challenge in the ED, however, when requests are made by families for a particular oncology nurse to access their SVAD rather than the ED nursing staff. In an attempt to reduce such requests, patient families are being educated on the importance of timely central access in the event of fever and potential neutropenia. The ED nursing staff also received additional training to improve SVAD access skills. Pre-approved order sets were implemented that allowed nursing staff to begin obtaining central access and blood work without the requirement of a physician order. Following the order for an antibiotic, there may be further delays in delivery from the pharmacy. By making the antibiotic readily available in the ED (through use of a medication storage and dispensing system) this delay can be averted. It is possible that this practice could result in medication errors as there is not a pharmacist checking the dose. However, this risk can be mitigated by use of computerized order entry that employs an indication specific standard dosing and automated dose calculation. Using this approach at our center there have been no reported medication errors or adverse effects of antibiotic toxicity after placing the antibiotic supply in the ED rather than dispensing from the hospital pharmacy.
Emergency department physicians may contribute to the delay by waiting for the results of the CBC to determine if a patient is neutropenic. In addition to waiting for the CBC, one study demonstrated that another delay was the time between obtaining laboratory results and administering the initial dose of antibiotic.9 The described protocol has eliminated this delay by requiring cefepime administration prior to CBC results unless there has been a very recent CBC demonstrating that the patient is not neutropenic. Differing from pre-protocol practices where initial antibiotic treatment decisions were dictated by ANC, the current protocol ensures prompt initial treatment to each patient in this high-risk group. Further decision making regarding the need for inpatient hospitalization and ongoing antibiotic treatment are made based on clinical judgment along with laboratory studies. This practice increases the number of patients at risk for neutropenia presenting to the ED with fever who will receive a single dose of antibiotic because both neutropenic and non-neutropenic patients receive a dose of antibiotic, whereas in the past well-appearing non-neutropenic children may have been discharged without antibiotic treatment. However, among patients treated as at risk for neutropenia 48.4% were found to be truly neutropenic.
Reduction in TTA took place between triage check-in and antibiotic order, as there was a 41.1% reduction in time between quarter 1 and 4 of the post-protocol group. This is likely a result of an improved triage system by the ED staff and physicians ordering cefepime upon patient arrival rather than waiting for laboratory results. The sizeable reduction in time to order between the first and fourth quarter of the post-protocol year demonstrates that significant improvements can be made within this time window by encouraging rapid response by the ED staff upon patient arrival. No significant difference was seen between the two groups from antibiotic order to antibiotic administration. This lack of improvement may imply that the events within this time period (topical anesthesia, central venous access, and antibiotic retrieval) need to be further addressed.
A limitation of this study is that the historical control group included a retrospective chart review of patient visits queried from the ED. Certain patient visits that should have been included in the review may have been missed due to inconsistent ED triage. Another potential limitation is that our institution is a training hospital in which the ED staff includes rotating residents and medical students whose knowledge and understanding of the protocol may have varied from month-to-month. This is a challenge to ongoing adherence and education regarding the protocol. However, we have attempted to address this by encouraging attending physicians to be responsible for initiating the antibiotic order within this protocol. Due to seasonal variability within a pediatric ED it is possible that the varying patient volume during each quarter of the post-protocol group may have impacted TTA outcomes. Quarter 1 contained the highest number of patients of the four quarters and also had the longest TTA. How ED volume may have directly impacted TTA in patients at risk for neutropenia is not known. As a single institution study, future evaluation at other institutions may help to understand if the protocol and practices used are universally applicable.
In summary, the development and implementation of a standardized protocol for febrile patients at risk for neutropenia resulted in significantly decreased time to initial antibiotic dose. In addition, the proportion of patients receiving the initial dose of antibiotic within the first 60 minutes of presentation increased over a year long period suggesting increased compliance as ED staff became more familiar with the protocol rather than showing a pattern of initial enthusiasm and subsequent waning of compliance. Ongoing efforts are underway to identify and address specific barriers in order to further decrease the time to antibiotic administration in this patient population. While it is unknown if these changes are sustainable over the long-term, continued evaluation of TTA with the protocol will determine long-term benefit.
Acknowledgments
Financial Support: Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR00165.
Footnotes
Meetings: Preliminary data was presented at the Southern Regional Meeting in New Orleans, Louisiana on February 23, 2013
Conflicts of Interest: The authors in this publication have not conflicts of interest to disclose.
Author Contributions: Kathy Monroe, and Matthew Kutny conceived the study, and developed the described protocol. Amber King and Clay Cohen participated in data extraction. Clay Cohen performed the chart reviews with the oversight of Matthew Kutny. Chee Paul Lin assisted in data analysis. Clay Cohen drafted the manuscript with contributions from Chee Paul Lin, Gregory K. Friedman, Kathy Monroe, and Matthew Kutny.
References
- 1.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011 Feb 15;52(4):e56–93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez-Barca E, Fernandez-Sevilla A, Carratala J, et al. Prognostic factors influencing mortality in cancer patients with neutropenia and bacteremia. Eur J Clin Microbiol Infect Dis. 1999 Aug;18(8):539–544. doi: 10.1007/s100960050345. [DOI] [PubMed] [Google Scholar]
- 3.Elting LS, Rubenstein EB, Rolston KV, Bodey GP. Outcomes of bacteremia in patients with cancer and neutropenia: observations from two decades of epidemiological and clinical trials. Clin Infect Dis. 1997 Aug;25(2):247–259. doi: 10.1086/514550. [DOI] [PubMed] [Google Scholar]
- 4.Santolaya ME, Alvarez AM, Aviles CL, et al. Admission clinical and laboratory factors associated with death in children with cancer during a febrile neutropenic episode. Pediatr Infect Dis J. 2007 Sep;26(9):794–798. doi: 10.1097/INF.0b013e318124aa44. [DOI] [PubMed] [Google Scholar]
- 5.Lin MY, Weinstein RA, Hota B. Delay of active antimicrobial therapy and mortality among patients with bacteremia: impact of severe neutropenia. Antimicrob Agents Chemother. 2008 Sep;52(9):3188–3194. doi: 10.1128/AAC.01553-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006 Jun;34(6):1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 7.McCavit TL, Winick N. Time-to-antibiotic administration as a quality of care measure in children with febrile neutropenia: a survey of pediatric oncology centers. Pediatr Blood Cancer. 2012 Feb;58(2):303–305. doi: 10.1002/pbc.23148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ammann RA, Bodmer N, Hirt A, et al. Predicting adverse events in children with fever and chemotherapy-induced neutropenia: the prospective multicenter SPOG 2003 FN study. J Clin Oncol. 2010 Apr 20;28(12):2008–2014. doi: 10.1200/JCO.2009.25.8988. [DOI] [PubMed] [Google Scholar]
- 9.Burry E, Punnett A, Mehta A, Thull-Freedman J, Robinson L, Gupta S. Identification of educational and infrastructural barriers to prompt antibiotic delivery in febrile neutropenia: a quality improvement initiative. Pediatr Blood Cancer. 2012 Sep;59(3):431–435. doi: 10.1002/pbc.23418. [DOI] [PubMed] [Google Scholar]
- 10.Corey AL, Snyder S. Antibiotics in 30 minutes or less for febrile neutropenic patients: a quality control measure in a new hospital. J Pediatr Oncol Nurs. 2008 Jul-Aug;25(4):208–212. doi: 10.1177/1043454208319971. [DOI] [PubMed] [Google Scholar]
- 11.Volpe D, Harrison S, Damian F, et al. Improving timeliness of antibiotic delivery for patients with fever and suspected neutropenia in a pediatric emergency department. Pediatrics. 2012 Jul;130(1):e201–210. doi: 10.1542/peds.2012-0153. [DOI] [PubMed] [Google Scholar]
- 12.Amado VM, Vilela GP, Queiroz A, Jr., Amaral AC. Effect of a quality improvement intervention to decrease delays in antibiotic delivery in pediatric febrile neutropenia: a pilot study. J Crit Care. 2011 Feb;26(1):103, e109–112. doi: 10.1016/j.jcrc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 13.Pakakasama S, Surayuthpreecha K, Pandee U, et al. Clinical practice guidelines for children with cancer presenting with fever to the emergency room. Pediatr Int. 2011 Dec;53(6):902–905. doi: 10.1111/j.1442-200X.2011.03363.x. [DOI] [PubMed] [Google Scholar]
- 14.Fletcher M, Hodgkiss H, Zhang S, et al. Prompt administration of antibiotics is associated with improved outcomes in febrile neutropenia in children with cancer. Pediatr Blood Cancer. 2013 Aug;60(8):1299–1306. doi: 10.1002/pbc.24485. [DOI] [PubMed] [Google Scholar]
- 15.Szwajcer D, Czaykowski P, Turner D. Assessment and management of febrile neutropenia in emergency departments within a regional health authority-a benchmark analysis. Curr Oncol. 2011 Dec;18(6):280–284. doi: 10.3747/co.v18i6.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hakim H, Flynn PM, Knapp KM, Srivastava DK, Gaur AH. Etiology and clinical course of febrile neutropenia in children with cancer. J Pediatr Hematol Oncol. 2009 Sep;31(9):623–629. doi: 10.1097/MPH.0b013e3181b1edc6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lullmann B, Leonhardt J, Metzelder M, et al. Pain reduction in children during port-a-cath catheter puncture using local anaesthesia with EMLA. Eur J Pediatr. 2010 Dec;169(12):1465–1469. doi: 10.1007/s00431-010-1244-1. [DOI] [PubMed] [Google Scholar]