Abstract
Objectives
Brief, intermittent cardiopulmonary resuscitation (CPR) training sessions, “Booster Trainings,” improve CPR skill acquisition and short-term retention. The objective of this study was to incorporate arterial blood pressure (ABP) tracings into Booster Trainings to improve CPR skill retention. We hypothesized that ABP-directed CPR “Booster Trainings” would improve intensive care unit (ICU) provider 3 month retention of excellent CPR skills without need for interval retraining.
Methods
A CPR manikin creating a realistic relationship between CC depth and ABP was used for training / testing. 36 ICU providers were randomized to brief, bedside ABP-directed CPR manikin skill re-trainings: 1) Booster Plus (ABP visible during training and testing) vs. 2) Booster Alone (ABP visible only during training, not testing) vs. 3) Control (testing, no intervention). Subjects completed skill tests pre-training (baseline), immediately after training (acquisition), and then retention was assessed at 12 hours, 3 and 6 months. The primary outcome was retention of excellent CPR skills at 3 months. Excellent CPR was defined as systolic blood pressure ≥100 mmHg and compression rate 100-120/min.
Results
Overall, 14 of 24 (58%) participants acquired excellent CPR skills after their initial training (Booster Plus 75% vs. 50% Booster Alone, p=0.21). Adjusted for age, ABP trained providers were 5.2× more likely to perform excellent CPR after the initial training (CI95: 1.3 – 21.2, p=0.02), and to retain these skills at 12 hours (aOR 4.4, CI95 1.3 – 14.9, p=0.018) and 3 months (aOR 4.1, CI95 1.2 – 13.9, p=0.023) when compared to baseline performance.
Conclusions
ABP-directed CPR Booster Trainings improved ICU provider 3-month retention of excellent CPR skills without need for interval retraining.
Keywords: cardiac arrest, cardiopulmonary resuscitation, chest compression
Introduction
Pediatric in-hospital cardiac arrest is not uncommon. Best estimates reveal that between 2-6% of hospitalized children in intensive care units (ICUs) with suffer a cardiac arrest.1-3 This translates to thousands of children each year suffering this potentially devastating event. Survival to discharge following in-hospital cardiac arrests is improving4, but still nearly half will have neurological deficits following the event4-7. As quality of cardiopulmonary resuscitation (CPR) is related to patient survival8-14, novel interventions and educational programs to improve resuscitation quality are potential therapeutic targets.
Real-time CPR feedback systems have been highlighted as one promising technology to improve CPR quality in recent literature15-17. And while this technology has improved CPR skills, they have not demonstrated improved patient outcomes when deployed during actual resuscitations18,19. One possible explanation is that these feedback systems do not allow for differences in patient physiology that may impact the outcome of the resuscitation. In short, a “one-size fits all” approach to CPR may not be optimal.
To that end, animal models of both hypoxic20 and normoxic21 ventricular fibrillation (VF) have demonstrated improved short-term survival when the resuscitation is titrated to arterial blood pressure (ABP). Previous mannequin work has demonstrated improved acquisition of CPR skills with ABP feedback.22 Such an approach is also feasible during actual resuscitations as most pediatric cardiac arrests now occur in intensive care units (ICUs) with invasive monitoring in place at the time of the arrest23. The American Heart Association (AHA) recommends such an approach24, yet our training programs fail to prepare rescuers to use the invasive monitoring available to them.
Prior studies have shown that brief low-dose, high-frequency, bedside CPR skill retraining (booster training) is effective to improve retention of CPR skills of in-hospital providers when providers are retrained every 1 to 3 months using American Heart Association (AHA) absolute rate and depth targets15. At our institution bedside providers (RNs, MDs, RTs) routinely participate in Rolling Refreshers, or bedside CPR skills retraining every 90 days. Using an adolescent sized manikin providers practice AHA standard, depth-guided refreshers until they are able to perform perfect technique CPR for 30 seconds straight. This generally takes no more than 2 minutes at the bedside with any given provider. Building on this previous work, the objective of this study was to evaluate the effectiveness of using arterial blood pressure directed, brief CPR re-trainings (Booster Trainings) to improve skill retention of ICU providers during simulated pediatric cardiac arrest. We hypothesized that when a clinical target such as arterial blood pressure was used to direct CPR Booster Trainings, 3-month retention of excellent CPR skills would be improved, without the need for interval retraining as in our previous studies. 15 As a secondary objective we evaluated whether there was a muscle memory effect of ABP-directed training, by removing the continuous ABP feedback during the testing sessions in one of the treatment groups (details below).
Methods and Materials
This was a prospective, single-blinded, randomized interventional trial, as the ABP recording manikin was “blinded” to the participant group assignment. In order to assess for improvements in CPR quality over time that were attributable to ongoing CPR quality improvement initiatives that were active but separate from our study, a control group was included.” 25 The overall objective was to investigate the effectiveness of ABP-directed, brief CPR re-trainings (Booster Trainings) to improve skill performance of ICU providers during simulated pediatric cardiac arrest. The institutional review board (IRB) at the Children's Hospital of Philadelphia approved the study protocol, including consent procedures. Waiver of written documentation of consent was granted by the IRB, and verbal consent was obtained from all health care providers who participated.
Subjects
All Pediatric Advanced Life Support (PALS) trained in-hospital care providers working in the intensive care unit (nurses, respiratory therapists, and critical care medicine fellow physician trainees) were eligible for inclusion in this study. A convenience sample of providers was approached at the beginning of their normal working hours. To mitigate selection bias all shifts were included (i.e., both daytime and nighttime providers), and all providers on the unit at the time of recruitment were approached.
Novel Manikin with ABP display
The Resusci® Junior manikin (Laerdal Medical, Stavanger, Norway) was modified and specifically engineered to display electrocardiogram, pulse-oximetry, and arterial blood pressure waveforms on a laptop monitor, exactly similar to the patient bedside monitors used at our institution. The manikin uses an internal potentiometer to record chest compression (CC) depth (mm), which was converted into electric voltage differences and subsequently to an arterial blood pressure waveform in LABView (National Instruments, Austin, TX). The relationship between CC depth (mm) delivered and systolic blood pressure (mmHg) displayed was derived from actual patient cardiac arrest data at our institution.26 The “heart rate” displayed in the waveform is generated from the rate of compressions delivered. Data was transferred into MATLAB® (MathWorks, Inc., Natick, MA) and subsequently into Microsoft Excel (Microsoft, Inc., Redmond, WA) for analysis.
Booster Training / Evaluation Sessions
CPR during the training and evaluation sessions was performed on the previously described prototype manikin, which is anatomically similar to an 8 year-old child. Participants were asked to perform 2-rescuer CPR on a simulated intubated patient in cardiac arrest; the participant performed CCs while the investigator provided ventilations. Sessions were performed during the participant's normal working hours, but out of view of other participants. Scripted dialogues were used in the trainings and only one researcher (HW) trained and tested all subjects.
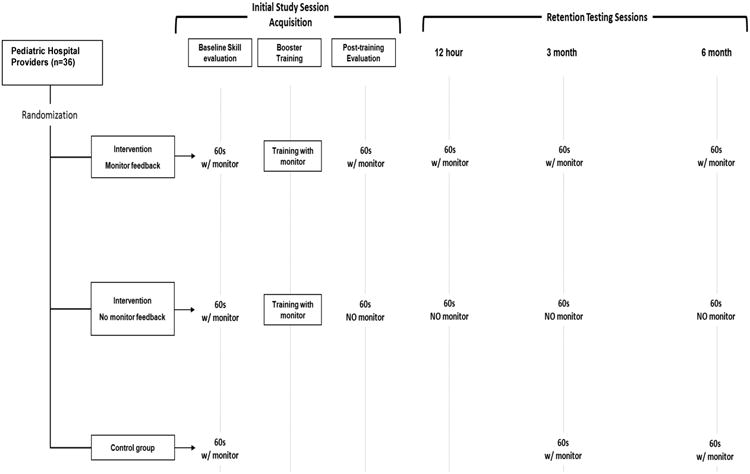
To assess baseline skills, all subjects (interventional arms and control) completed a pretraining evaluation with the ABP tracing visible. The 2 interventional arms in this study were: (1) Booster Alone and (2) Booster Plus. In the Booster Plus group, all evaluation sessions for acquisition and retention were completed with the ABP tracing visible as compared to Booster Alone, which only had ABP visible during training (not during testing). The training sessions were brief (∼120 seconds), ABP-directed Booster Trainings with ABP goals of systolic blood pressure (SBP) of ≥100 mmHg and CC rate of 100-120 CC/min. Subsequent evaluation sessions (60s) in the intervention groups occurred immediately post-training to assess skill acquisition, and then at 12 hours, 3 and 6 months post training to assess retention. In the control arm, the ABP tracing was visible during all sessions, but subjects received no specific training targets and were tested at 3 and 6 months only (Figure 1).
Figure 1. Study Design.
Outcome Variables
The primary outcome variable was a prospectively designated composite variable, excellent CPR, defined as the mean of SBP and compression rate exceeding ≥100 mmHg and 100-120 CC/min respectively, during a given evaluation session. Skill acquisition was tested immediately post-training. Our primary retention time point was 3 months post-training. Univariate analysis with McNemar's test for paired binary data (i.e., excellent CPR) was used to evaluate subjects compared to their baseline performance at each testing session (Table 2). All subjects who completed a 3-month evaluation were considered in the retention analysis even if they did not demonstrate skill acquisition initially (intention to treat). In a prospectively planned secondary analysis, we did investigate if excellent skills were retained to 6 months. Baseline demographic data was collected including sex, age (yrs.), time since last formal CPR education (months), and years of experience in current position.
Table 2.
Percentage of subjects performing excellent CPR (mean systolic blood pressure ≥ 100 mmHg AND mean chest compression rate ≥ 100 CC/min during evaluation session) for A) Booster Plus, B) Booster Alone and C) Control training groups.
| A. Booster Plus: Training and Testing with Monitor | ||
|---|---|---|
|
| ||
| Excellent CPR n (%) | p* | |
| Pre-Training | 2 (17) | NA |
| Post-Training | 9 (75) | 0.02 |
|
| ||
| Retention | ||
| 12 Hours | 8 (67) | 0.03 |
| 3 Month | 7 (58) | 0.06 |
| 6 Month | 3 (33) | 0.38 |
|
| ||
| B. Booster Alone: Training Only with Monitor | ||
|
| ||
| Excellent CPR | p* | |
|
| ||
| Pre-Training | 4 (33) | NA |
| Post-Training | 6 (50) | 0.73 |
|
| ||
| Retention | ||
| 12 Hours | 6 (50) | 0.69 |
| 3 Month | 6 (50) | 0.69 |
| 6 Month | 1 (8) | 0.5 |
| C. Control | ||
|
| ||
| Excellent CPR | p* | |
|
| ||
| Pre-Training | 5 (42) | NA |
|
| ||
| Retention | ||
| 3 Month | 5 (45) | 0.99 |
| 6 Month | 3 (9) | 0.99 |
All comparisons to pre-training via McNemar's test for paired binary data using exact probabilities.
Statistical Analysis
Standard descriptive statistics were calculated as appropriate for the distribution of each variable. For each testing session, categorical variables (excellent CPR) were compared using McNemar's test for paired binary data. In the primary analysis, generalized estimating equations were used to assess differences in the rate of excellent CPR performance over time and between the two training groups. Any candidate demographic variable differing between intervention groups or associated with the primary outcome at a significance cutoff of 0.20 were considered for inclusion in the final model.” As only PICU nurses enrolled in the study, we did not adjust for this in the final model. Based upon our previous work with rolling refreshers, we assumed a baseline excellent performance rate of 15%. Further we assumed that at 3 months, irrespective of training group, that approximately 60% of providers would still perform excellent CPR. With enrollment of 11 providers in each group, we would have 80% power to detect that difference at an alpha level of 0.05. To account for a dropout rate of 10%, we enrolled an additional provider in each interventional group. P-values less than 0.05 were considered statistically significant. Statistical analysis was completed using the Stata-IC statistical package (Version 12.0, StataCorp, College Station, TX).
Results
Between August 24, 2012 and August 31, 2012, thirty-six pediatric intensive care unit (PICU) providers were approached for inclusion. All (100%) met inclusion criteria and provided consent to participate. At study end in March 2013, all but 5 (14%) providers completed all three subsequent retention evaluation sessions (12 hours, 3 months, 6 months). Completion rates by study arm were as follows: 1) Booster Plus: 10 of 12 (83%); 2) Booster Alone: 11 of 12 (92%); and 3) Control (No Structured Training): 10 of 12 (83%). Average time that follow-up sessions were completed was: 3 months: 100 ± 8 days; 6 months: 187 ± 8 days. There was a trend towards differences in age of participant between training groups (Table 1). There were no differences in demographics between the control group and the intervention groups.
Table 1.
Cohort description.
| Booster Plus n = 12 | Booster Alone n = 12 | p | |
|---|---|---|---|
|
| |||
| Age: years median (IQR)* | 24 (23 – 25.5) | 25 (24 – 32.5) | 0.11 |
| Sex: male, n (%)* | 1 (8) | 2 (17) | 0.99 |
| High Degree Obtained, n (%)† | 0.31 | ||
| Associates | 0 (0) | 1 (8) | |
| Bachelors | 12 (100) | 11 (92) | |
| Experience ≥ 1 Year Current Position, n (%) | 10 (83) | 9 (75) | 0.62 |
| Last Formal CPR Instruction, months mean (sd) | 11.3 (7) | 9.4 (4) | 0.44 |
Univariate analysis (Table 2)
Overall, 14 of 24 (58%) of participants acquired excellent CPR skills after their initial training (Booster Plus 75% vs. 50% Booster Alone, p=0.21). In the Booster Plus group, significantly more providers performed excellent CPR immediately after training (75%, p=0.02) and at 12 hours (67%, p=0.03) compared to pre-training (17%). There was a trend towards improved CPR at 3 months (58%, p=0.06). In Booster Alone (no ABP display during testing), improvements were observed, but were not statistically significant.
Multivariate analysis (Table 3)
Table 3.
Multivariable model adjusted for clustering on subject. Odds of subjects performing excellent CPR (systolic blood pressure ≥ 100 mmHg AND chest compression rate ≥ 100 CC/min).
| OR | CI95 | p* | |
|---|---|---|---|
| Acquisition | |||
| Post-training | 5.2 | 1.3-21.2 | 0.02 |
| Retention* | |||
| 12 Hours | 4.4 | 1.3 – 14.9 | 0.018 |
| 3 Months | 4.1 | 1.2 – 13.9 | 0.023 |
| 6 Months | 0.97 | 0.22 – 3.9 | 0.92 |
| Booster Alone† | 0.62 | 0.27 – 1.5 | 0.27 |
Comparison to pre-training evaluation.
Comparison to Booster Plus across all post-training evaluation points.
Adjusted for age of participant, the cohort (all participants in both interventional arms) was 5.2× more likely to perform excellent CPR after the initial training (CI95: 1.3 – 21.2, p=0.02), and to retain these skills at 12 hours (aOR 4.4, CI95 1.3 – 14.9, p=0.018) and at 3 months (aOR 4.1, CI95 1.2 – 13.9, p=0.023) when compared to baseline. At 6 months, excellent CPR performance of the cohort was not different from baseline (OR 0.93, CI95 0.22 – 3.9, p=0.92). In respect to the two intervention arms, compared to Booster Plus, the odds of retention in Booster Alone was not different (OR 0.62, CI95 0.27 – 1.5, p=0.27). Without training (control group) there was no increased likelihood of subjects performing excellent CPR during the next session (OR 0.94, CI95: 0.8 – 1.1, p=0.47).
Discussion
This study demonstrates that a single, brief, bedside CPR Booster Training targeted to arterial blood pressure (ABP) improves ICU provider 3-month retention of excellent CPR skills. Our study was novel in that the blood pressure-to-depth relationship was derived from actual children in cardiac arrest.26 This is also the first study we are aware of that studied skill retention in a model of real-time arterial blood pressure feedback during CPR. We were able to demonstrate improved skill retention at 3 months; however, without re-training skills declined toward baseline at 6 months.
The impetus for this investigation was promising translational data that investigated the effectiveness of titrating resuscitation quality to ABP. In both hypoxic and normoxic20,21 ventricular fibrillation models, such a resuscitative approach improved short-term survival compared to optimal AHA care. Yet as no previous study had used ABP tracings for CPR training, this investigation is an important next step as it begins to fill the gap between the large animal laboratory and the bedside care provider.
We have previously described the effectiveness of Booster trainings to improve skill retention of in-hospital providers15,27,25. However, there is a notable difference between this study and our previous work – subjects of this study did not receive subsequent Booster Trainings at each time point. In this study, a single, less than 2 minute Booster Training resulted in nearly 60% of providers performing excellent CPR at the 3 month assessment – a proportion similar to previous studies when providers had multiple Booster Trainings. Importantly, as skills declined by 6 months in this study, these data provide further evidence that the optimal timing of these Booster Trainings for maintenance of competency may be approximately every 3 months.
The question remains as to why an ABP-directed approach would improve skill retention. In line with concepts of adult learning theory28, CPR education is going to be most effective when targeted to a clinician's setting and role. When training a healthcare provider to a clinical endpoint (i.e., blood pressure), you provide them with a guideline that they are familiar with and can more readily incorporate into a working mental model. Frontline care providers in an intensive care unit are very skilled with titrating vasopressor support to a target ABP, but very few could tell you how deep 5cm is during CPR29. The instructions “push as deep as necessary to obtain a systolic blood pressure of 100 mmHg and push 100-120 times a minute” immediately links the task (CPR) to the patient's response (ABP). We speculate that by using a common clinical endpoint such as ABP, providers are able to retain that information and may explain why we were able to see skill retention out to 3 months without additional Booster Trainings.
This simulation manikin study has notable limitations. First, we only examined CC rate and blood pressure (depth). Other important CPR quality variables such as CC fraction (percentage of time during cardiac arrest that CCs are performed)13,14, incomplete release between CCs30-32, and ventilation rate and quality33-35, were not evaluated. Second, given that most of the study participants were female nurses, there is a theoretical concern that it will be difficult to generalize our findings more broadly to other care providers. However, given that the success of Booster Trainings is most likely attributable to a focus on the needs of the adult learner28, it should be applicable to a broad range of healthcare providers. Next, it is important to note that while we have demonstrated improvements in CPR quality variables in manikins, we do not know if this will translate to higher quality CPR performed during actual resuscitation attempts. This remains an unanswered question. Finally, this educational model was not a mastery-learning model. Not all providers who were trained by these Booster Trainings actually acquired excellent CPR skills, and thus would not be expected to “retain” skills at 3 months. This lack of acquisition of excellent skills may have contributed to the lack of retention of skills, particularly at 6 months. However, it should be noted that the use of the ABP tracings in subsequent evaluation sessions (Booster Plus) is a form of “training.” So as long as the provider recalled the appropriate ABP targets, during each testing session they were “practicing” to achieve those targets and may have led to our improved retention results15. Future studies should consider using a mastery-level training approach to improve retention even further.
Conclusions
As the American Heart Association now recommends monitoring the cardiac arrest victim's physiological response to the resuscitation effort, this investigation represents a vital first link in translating these recommendations to the bedside. In this study, we found that a single, brief, bedside CPR Booster Training targeted to arterial blood pressure (ABP) improved ICU provider 3-month retention of excellent CPR skills. As most cardiac arrests now occur in intensive care units with invasive monitoring in place at the time of the arrest, this new training technique is feasible and holds promise as the resuscitation science community looks for ways to improve CPR educational methods and ultimately patient survival outcomes.
Acknowledgments
We would like to acknowledge the outstanding and dedicated healthcare providers at our institution for their participation in this study and support of the resuscitation research at the Children's Hospital of Philadelphia.
Financial Disclosure: This study was funded by the Laerdal Foundation for Acute Care Medicine and the Endowed Chair of Pediatric Critical Care Medicine at the Children's Hospital of Philadelphia. Dr. Berg is funded by NIH award U10 HD063108. Dr. Nadkarni is funded by NIH award U01 HL107681, U01 HL094345, RO1 HL114484, R01 HL058669, AHRQ R18 HS022469, AHRQ R18 HS022464, and CIHR 2009-09-15. Dr. Sutton is funded through an NIH career development award (K23HD062629).
References
- 1.Suominen P, Olkkola KT, Voipio V, Korpela R, Palo R, Räsänen J. Utstein style reporting of in-hospital paediatric cardiopulmonary resuscitation. Resuscitation. 2000;45(1):17–25. doi: 10.1016/s0300-9572(00)00167-2. [DOI] [PubMed] [Google Scholar]
- 2.Matos RI, Watson RS, Nadkarni VM, et al. Duration of cardiopulmonary resuscitation and illness category impact survival and neurologic outcomes for in-hospital pediatric cardiac arrests. Circulation. 2013;127(4):442–451. doi: 10.1161/CIRCULATIONAHA.112.125625. [DOI] [PubMed] [Google Scholar]
- 3.Slonim AD, Patel KM, Ruttimann UE. Cardiopulmonary resuscitation in pediatric intensive care units. Critical Care Medicine. 1997 doi: 10.1097/00003246-199712000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Ortmann L, Prodhan P, Gossett J, et al. Outcomes After In-Hospital Cardiac Arrest in Children With Cardiac Disease: A Report From Get With the Guidelines-Resuscitation. Circulation. 2011;124(21):2329–2337. doi: 10.1161/CIRCULATIONAHA.110.013466. [DOI] [PubMed] [Google Scholar]
- 5.Tibballs J, Kinney S. A prospective study of outcome of in-patient paediatric cardiopulmonary arrest. Resuscitation. 2006;71(3):310–318. doi: 10.1016/j.resuscitation.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Samson RA, Nadkarni VM, Meaney PA, et al. Outcomes of in-hospital ventricular fibrillation in children. N Engl J Med. 2006;354(22):2328–2339. doi: 10.1056/NEJMoa052917. [DOI] [PubMed] [Google Scholar]
- 7.Zaritsky A, Nadkarni V, Getson P, Kuehl K. CPR in children. Annals of emergency medicine. 1987;16(10):1107–1111. doi: 10.1016/s0196-0644(87)80465-1. [DOI] [PubMed] [Google Scholar]
- 8.Edelson DP, Abella BS, Kramer-Johansen J, et al. Effects of compression depth and pre-shock pauses predict defibrillation failure during cardiac arrest. Resuscitation. 2006;71(2):137–145. doi: 10.1016/j.resuscitation.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Abella BS. Chest Compression Rates During Cardiopulmonary Resuscitation Are Suboptimal: A Prospective Study During In-Hospital Cardiac Arrest. Circulation. 2005;111(4):428–434. doi: 10.1161/01.CIR.0000153811.84257.59. [DOI] [PubMed] [Google Scholar]
- 10.Idris AH, Guffey D, Aufderheide TP, et al. Relationship Between Chest Compression Rates and Outcomes From Cardiac Arrest. Circulation. 2012;125(24):3004–3012. doi: 10.1161/CIRCULATIONAHA.111.059535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheskes S, Schmicker RH, Christenson J, et al. Perishock Pause: An Independent Predictor of Survival From Out-of-Hospital Shockable Cardiac Arrest. Circulation. 2011;124(1):58–66. doi: 10.1161/CIRCULATIONAHA.110.010736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stiell IG, Brown SP, Christenson J, et al. What is the role of chest compression depth during out-of-hospital cardiac arrest resuscitation?*. Critical Care Medicine. 2012;40(4):1192–1198. doi: 10.1097/CCM.0b013e31823bc8bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaillancourt C, Everson-Stewart S, Christenson J, et al. The impact of increased chest compression fraction on return of spontaneous circulation for out-of-hospital cardiac arrest patients not in ventricular fibrillation. Resuscitation. 2011;82(12):1501–1507. doi: 10.1016/j.resuscitation.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christenson J, Andrusiek D, Everson-Stewart S, et al. Chest Compression Fraction Determines Survival in Patients With Out-of-Hospital Ventricular Fibrillation. Circulation. 2009;120(13):1241–1247. doi: 10.1161/CIRCULATIONAHA.109.852202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sutton RM, Niles D, Meaney PA, et al. Low-Dose, High-Frequency CPR Training Improves Skill Retention of In-Hospital Pediatric Providers. Pediatrics. 2011;128(1):e145–e151. doi: 10.1542/peds.2010-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kramer-Johansen J, Myklebust H, Wik L, et al. Quality of out-of-hospital cardiopulmonary resuscitation with real time automated feedback: A prospective interventional study. Resuscitation. 2006;71(3):283–292. doi: 10.1016/j.resuscitation.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Abella BS, Edelson DP, Kim S, et al. CPR quality improvement during in-hospital cardiac arrest using a real-time audiovisual feedback system. Resuscitation. 2007;73(1):54–61. doi: 10.1016/j.resuscitation.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 18.Yeung J, Meeks R, Edelson D, Gao F, Soar J, Perkins GD. The use of CPR feedback/prompt devices during training and CPR performance: A systematic review. Resuscitation. 2009;80(7):743–751. doi: 10.1016/j.resuscitation.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Hostler D, Everson-Stewart S, Rea TD, et al. Effect of real-time feedback during cardiopulmonary resuscitation outside hospital: prospective, cluster-randomised trial. BMJ. 2011;342:d512. doi: 10.1136/bmj.d512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sutton RM, Friess SH, Bhalala U, et al. Hemodynamic directed CPR improves short-term survival from asphyxia-associated cardiac arrest. Resuscitation. 2013;84(5):696–701. doi: 10.1016/j.resuscitation.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friess SH, Sutton RM, Bhalala U, et al. Hemodynamic Directed Cardiopulmonary Resuscitation Improves Short-Term Survival From Ventricular Fibrillation Cardiac Arrest*. Critical Care Medicine. 2013;41(12):2698–2704. doi: 10.1097/ccm.0b013e318298ad6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rieke H, Rieke M, Gado SK, et al. Virtual arterial blood pressure feedback improves chest compression quality during simulated resuscitation. Resuscitation. 2013;84(11):1585–1590. doi: 10.1016/j.resuscitation.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berg RA, Sutton RM, Holubkov R, et al. Ratio of PICU Versus Ward Cardiopulmonary Resuscitation Events Is Increasing*. Critical Care Medicine. 2013;41(10):2292–2297. doi: 10.1097/CCM.0b013e31828cf0c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meaney PA, Bobrow BJ, Mancini ME, et al. Cardiopulmonary Resuscitation Quality: Improving Cardiac Resuscitation Outcomes Both Inside and Outside the Hospital: A Consensus Statement From the American Heart Association. Circulation. 2013;128(4):417–435. doi: 10.1161/CIR.0b013e31829d8654. [DOI] [PubMed] [Google Scholar]
- 25.Niles D, Sutton RM, Donoghue A, et al. “Rolling Refreshers”: A novel approach to maintain CPR psychomotor skill competence. Resuscitation. 2009;80(8):909–912. doi: 10.1016/j.resuscitation.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 26.Sutton RM, French B, Nishisaki A, et al. American Heart Association cardiopulmonary resuscitation quality targets are associated with improved arterial blood pressure during pediatric cardiac arrest. Resuscitation. 2013;84(2):168–172. doi: 10.1016/j.resuscitation.2012.08.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sutton RM, Niles D, Meaney PA, et al. “Booster” training: Evaluation of instructor-led bedside cardiopulmonary resuscitation skill training and automated corrective feedback to improve cardiopulmonary resuscitation compliance of Pediatric Basic Life Support providers during simulated cardiac arrest*. Pediatric Critical Care Medicine. 2011;12(3):e116–e121. doi: 10.1097/PCC.0b013e3181e91271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeWitt TG. The application of social and adult learning theory to training in community pediatrics, social justice, and child advocacy. Pediatrics. 2003;112(3):755–757. [PubMed] [Google Scholar]
- 29.Rodriguez SA, Sutton RM, Berg MD, et al. Simplified dispatcher instructions improve bystander chest compression quality during simulated pediatric resuscitation. Resuscitation. 2014;85(1):119–123. doi: 10.1016/j.resuscitation.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuercher M, Hilwig RW, Ranger-Moore J, et al. Leaning during chest compressions impairs cardiac output and left ventricular myocardial blood flow in piglet cardiac arrest. Critical Care Medicine. 2010;38(4):1141–1146. doi: 10.1097/CCM.0b013e3181ce1fe2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fried DA, Leary M, Smith DA, et al. The prevalence of chest compression leaning during in-hospital cardiopulmonary resuscitation. Resuscitation. 2011;82(8):1019–1024. doi: 10.1016/j.resuscitation.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yannopoulos D, McKnite S, Aufderheide TP, et al. Effects of incomplete chest wall decompression during cardiopulmonary resuscitation on coronary and cerebral perfusion pressures in a porcine model of cardiac arrest. Resuscitation. 2005;64(3):363–372. doi: 10.1016/j.resuscitation.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 33.McInnes AD, Sutton RM, Orioles A, et al. The first quantitative report of ventilation rate during in-hospital resuscitation of older children and adolescents. Resuscitation. 2011;82(8):1025–1029. doi: 10.1016/j.resuscitation.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aufderheide TP, Lurie KG. Death by hyperventilation: A common and life-threatening problem during cardiopulmonary resuscitation. Critical Care Medicine. 2004;32(Supplement):S345–S351. doi: 10.1097/01.CCM.0000134335.46859.09. [DOI] [PubMed] [Google Scholar]
- 35.Aufderheide TP. Hyperventilation-Induced Hypotension During Cardiopulmonary Resuscitation. Circulation. 2004;109(16):1960–1965. doi: 10.1161/01.CIR.0000126594.79136.61. [DOI] [PubMed] [Google Scholar]


