Abstract
Endometrial cancer incidence is increasing, due in part to a strong association with obesity. Mutations in the phosphatidylinositol 3‐kinase (PI3K) pathway, the central relay pathway of insulin signals, occur in the majority of endometrioid adenocarcinomas, the most common form of endometrial cancer. We sought to determine the impact of PI3K pathway alterations on progression free survival in a cohort of endometrioid endometrial cancers. Prognostic utility of PIK3CA, PIK3R1, and PTEN mutations, as well as PTEN protein loss by immunohistochemistry, was explored in the context of patient body mass index. Reverse‐phase protein arrays were utilized to assess protein expression based on PTEN status. Among 187 endometrioid endometrial cancers, there were no statistically significant associations between PFS and PIK3CA, PIK3R1, PTEN mutation or loss. When stratified by body mass index, PTEN loss was associated with improved progression free survival (P < 0.006) in obese (body mass index ≥ 30) patients. PTEN loss resulted in distinct protein changes: Canonical PI3K pathway activation was observed only in the non‐obese population while decreased expression of β‐CATENIN and phosphorylated FOXO3A was observed in obese patients. These data suggest the impact of PTEN loss on tumor biology and clinical outcomes must be interpreted in the context of body mass index, and provide a potential explanation for discrepant reports on the effect of PTEN status and obesity on prognosis in endometrial cancer. This reveals a clinically important interaction between metabolic state and tumor genetics that may unveil the biologic underpinning of obesity‐related cancers and impact ongoing clinical trials with PI3K pathway inhibitors.
Keywords: Endometrial cancer, PTEN loss, Obesity, Survival, PI3K/AKT pathway
Highlights
PTEN loss is associated with improved PFS in obese endometrial cancer patients.
Impact of PTEN loss may be considered in the context of BMI in endometrial cancer.
Distinct protein changes are found in EC based on patient BMI and PTEN status.
Abbreviations
- phosphatidylinositol 3-kinase (PI3K)
BMI
- body mass index
PFS
- progression free survival
MDACC
- MD Anderson Cancer Center
FFPE
- Formalin-fixed
paraffin embedded
- DSS
disease specific survival
- IHC
immunohistochemical
- TMA
tissue microarray
- RPPA
reverse phase protein array
- PR
progesterone receptor
- AMPK
AMP-activated protein kinase
1. Introduction
Endometrial cancer is the most common gynecologic malignancy in the developed world and its incidence and mortality rate are increasing due, in part, to the obesity epidemic (Siegel et al., 2012). Obesity is an established risk factor for developing endometrial cancer with a 3‐fold increase in risk (Reeves et al., 2007). The biology underlying the associations between obesity, endometrial cancer risk and outcomes remains incompletely understood. However, chronic over‐exposure to hormones elevated in the obese state, such as insulin‐like growth factors and estrogens, likely contribute (Schmandt et al., 2011). Reports on the impact of obesity on endometrial cancer outcomes are conflicting. Two large population based studies report increased cancer‐specific mortality from endometrial cancer in patients with increased body mass index (BMI). In fact, this association was strongest in endometrial cancer (Calle et al., 2003; Reeves et al., 2007). In other studies of endometrial cancer, however, obesity was associated with less aggressive pathologic features (Bokhman, 1983; Munstedt et al., 2008) and similar or improved outcomes (Anderson et al., 1996; Bokhman, 1983; Crosbie et al., 2012; Mauland et al., 2011; Munstedt et al., 2008; von Gruenigen et al., 2006). This may be due to the inclusion of both serous and endometrioid subtypes, which have different genomic underpinnings (Getz et al., 2013).
Endometrial cancers have the highest rates of PI3K pathway alterations reported to date (Cheung et al., 2011; Ruderman et al., 1990; Salvesen et al., 2009). The most common PI3K pathway alteration in endometrial cancer is PTEN loss, which occurs via mutation, methylation, or chromosomal loss, and is reported in over 50% of cases (Kong et al., 1997; Risinger et al., 1997; Tashiro et al., 1997). PTEN protein can also be lost through effects of microRNAs and protein degradation (Fata et al., 2012). Mutations in PIK3CA, the gene that encodes the catalytic subunit of PI3K (p110α), and mutations in PIK3R1, the gene that encode the regulatory subunit of PI3K (p85α) occur in 50% and 30% of endometrial cancers, respectively (Cheung et al., 2011; Getz et al., 2013; Rudd et al., 2011; Urick et al., 2011).
Thus far, studies evaluating the correlation between PI3K aberrations and clinical outcome have reported discrepant results. Loss of PTEN protein expression has been reported to portend a more favorable (Dellas et al., 2009; Mackay et al., 2010) or less favorable prognosis (Athanassiadou et al., 2007; Kanamori et al., 2002; Salvesen et al., 2002; Terakawa et al., 2003) in endometrial cancer. PTEN mutation has been associated with favorable prognosis, early stage, and endometrioid histology (Garcia‐Dios et al., 2013, 2001, 1997, 1998, 2004). Mutations in the kinase domain of PIK3CA are associated with higher stage, higher grade, and poor prognosis (Catasus et al., 2008; Garcia‐Dios et al., 2013) or a better prognosis among tumors that are estrogen receptor negative (Dong et al., 2012). Other studies have failed to detect any significant association for any PI3K aberration with patient outcome (Salvesen et al., 2004). Improved outcomes in obese patients with concurrent p27Kip and PTEN loss have been reported, suggesting a link between metabolism and the consequence of tumor suppressor loss (Dellas et al., 2009).
We sought to determine the impact of PI3K pathway alterations on progression free survival (PFS) in a cohort of clinically and genetically annotated endometrioid endometrial cancers. Based on links of metabolism with the PI3K pathway (Engelman et al., 2006), we explored potential interactions between obesity and PI3K pathway alteration status on patient outcome. Strikingly, PTEN loss in obese patients was associated with an improved outcome while PTEN loss in non‐obese patients was associated with a worse outcome. Thus, obesity appears to modulate the clinical impact of PTEN loss on survival. This observation may explain prior discrepant studies of PTEN, obesity and prognosis. Proteomic analyses suggest avenues of investigation to further understand the underlying biology of this clinical observation.
2. Materials and methods
2.1. Clinical data
Patients treated for endometrial cancer with adequate tissue for molecular testing between 2000‐2009 were identified through the University of Texas MD Anderson Cancer Center (MDACC). Gynecologic Oncology Tumor Bank under an institutional review board‐approved protocol. All patients were treated with hysterectomy and bilateral salpingo‐oophorectomy. The performance of staging lymphadenectomy was undertaken in patients in accordance with Mayo Clinic criteria (Mariani et al., 2000). Adjuvant therapy was based on final pathologic findings and FIGO stage in accordance with National Comprehensive Cancer Network (NCCN) guidelines. Histologic diagnosis was confirmed by a gynecologic pathologist (RB). Patients with non‐endometrioid histology were excluded.
Collected clinical data included baseline demographic and clinical characteristics at the time of diagnosis, as well as treatment course and survival. BMI was used to categorize non‐obese (BMI < 30 kg/m2) and obese (BMI ≥ 30 kg/m2). Patients were followed for outcomes per NCCN surveillance guidelines and institutional practice. PFS was calculated from the date of surgery to the date of recurrence or death from any cause.
2.2. Validation cohort
A prospectively studied population‐based cohort of patients with endometrioid endometrial cancer from Norway was identified for validation. Formalin‐fixed, paraffin‐embedded (FFPE) tissues were prospectively collected from patients diagnosed with endometrial carcinoma in Hordaland County from 2001 to 2009. Clinico‐pathological parameters were registered as previously reported. Disease‐specific survival (DSS) was calculated using surgery date as the entry date. Patients who died from other causes were censored at the date of death (Trovik et al., 2011). The study was approved by the Norwegian Data Inspectorate (961478‐2), Norwegian Social Science Data Services (15501), and local Institutional Review Board (REKIII nr. 052.01).
2.3. Mutational analysis
Whole exome sequencing was performed on DNA extracted from FFPE tumors using a 454‐well platform; hot‐spot mutations were confirmed with a Sequenom panel as previously described (Cheung et al., 2011; Liang et al., 2012).
2.4. Immunohistochemical (IHC) analysis and scoring
FFPE sections of endometrial carcinoma were analyzed by IHC; PTEN expression was graded as positive, negative, or heterogeneous, as reported previously (Djordjevic et al., 2012). PTEN negative categorization required retained positive expression of adjacent stromal cells, to verify assay function. PTEN heterogenous tumors had separate foci that were PTEN positive and PTEN negative. For survival and multivariate analyses, tumors that were PTEN negative were classified as PTEN loss. PTEN heterogeneous and PTEN positive tumors were classified as PTEN retained.
For the Norwegian cohort, tissue microarrays (TMAs) were generated as previously described and validated (Stefansson et al., 2004). IHC evaluation was carried out blinded for patient characteristics and outcome and recorded as previously described (Krakstad et al., 2012; Salvesen et al., 2000). Briefly, the staining index was calculated as a product of staining intensity (0–3) and area of positive tumor cells (1=<10%, 2 = 10–50%, 3=>50%). Staining index was categorized in tertiles for statistical analyses, considering the frequency of marker distribution, the subgroups size and event number in each category.
2.5. Reverse phase protein array (RPPA)
RPPA was performed on tumor specimens as described previously (Cheung et al., 2011; Hennessy et al., 2010; Hu et al., 2007; Liang et al., 2012). Briefly, proteins were extracted from frozen endometrioid endometrial tumor tissue from the MDACC cohort and probed with 135 antibodies. The signal intensities on the RPPA arrays were quantitated using MicroVigene software (VigeneTech, Inc., Carlisle, MA), and processed using the R package SuperCurve (version‐1.01, available at http://bioinformatics.mdanderson.org/OOMPA).
2.6. Statistical analysis
Chi‐squared test was used to analyze the association between obesity status and PTEN status. Two‐sample Student's t‐test was used to compare protein expression levels between groups. A multivariate Cox proportional hazard model was used to study the association between PFS and stage, grade, PTEN loss, and obesity. Based on findings from the Kaplan–Meier analysis, an interaction term for PTEN loss and obesity was included in this model. Robust markers were selected using a pairwise algorithm for the Cox proportional hazard model described by Simon et al (Simon et al., 2011). The lasso penalty was used (alpha = 1) and a 10‐fold cross validation was run over these paths to find the optimal predictors for survival with the smallest error rate.
For analysis of the Norwegian cohort, patients with BMI≥30 (n = 120) were investigated for the association between PTEN expression and survival. Univariate survival analyses were performed using the Kaplan–Meier method. Differences in survival between groups were estimated using the log‐rank trend test.
For all analyses, a two‐sided P < 0.05 was considered statistically significant, with no adjustment for multiple tests. All computations were done using R version 2.14.0 (http://www.r‐project.org/).
3. Results
3.1. Patient characteristics
Two hundred forty two patients with endometrial carcinoma had adequate tissue for analysis; 187 (77%) were of endometrioid histology and included for more detailed analysis. Clinical and demographic features are summarized (Table 1.) Thirty five percent of patients (n = 65) were non‐obese (BMI < 30) and 65% (n = 120) were classified as obese (BMI ≥ 30). The majority of patients had early stage disease and grade 2 endometrioid tumors.
Table 1.
Patient characteristics of 187 women with endometrioid endometrial cancer.
| N | PFS | PTEN protein loss | PTEN mutation | PIK3CA mutation | |
|---|---|---|---|---|---|
| ALL | 187 | 28.3 | 103 | 101 | 76 |
| FIGO Stage | |||||
| I | 115 | 32.5 | 66 | 58 | 43 |
| II | 24 | 36.5 | 11 | 13 | 10 |
| III | 32 | 18.2 | 19 | 21 | 17 |
| IV | 16 | 17.0 | 7 | 9 | 6 |
| Grade* | |||||
| 1 | 25 | 34.3 | 13 | 16 | 11 |
| 2 | 122 | 29.9 | 65 | 59 | 47 |
| 3 | 39 | 26.1 | 24 | 25 | 17 |
| BMI** | |||||
| <30 | 65 | 29.2 | 30 | 35 | 30 |
| ≥30 | 120 | 28.0 | 71 | 65 | 45 |
N: number, PFS: Median progression free survival, FIGO: International Federation of Gynecology and Obstetrics, BMI: Body mass index.* Data missing from one patient; **Data missing from two patients.
3.2. PFS, pathologic factors and molecular aberrations
At a median follow up of 28.3 months, median PFS was significantly shorter with increasing histologic grade of tumor (P=<0.0001; Supplementary Figure S1A) and lower stage at diagnosis was associated with improved PFS (P < 0.0001; Supplementary Figure S1B). These data are consistent with previous data for endometrial carcinoma.
We tested if there were univariate relationships between molecular aberrations in the PI3K pathway and outcomes. Among the MD Anderson endometrioid cohort, PIK3CA and PIK3R1 mutations had no significant effect on PFS (Figure 1A, 1B). Recent studies suggest that PTEN loss by IHC is a better indication of PTEN functional loss than PTEN mutation and is highly reproducible (Djordjevic et al., 2012; Garg et al., 2012). In the MDACC endometrioid cohort, there was no association between PFS and PTEN mutation (Figure 1C), PTEN loss by IHC (Figure 2A), or grouped PTEN loss and/or PTEN mutation (Figure S2).
Figure 1.
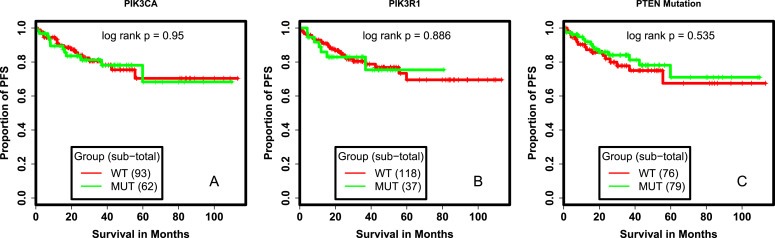
Survival analysis of patients with PI3K and PTEN mutant tumors. (A.) Kaplan Meier Survival curves comparing PFS of patients with PIK3CA mutated and PIK3CA wildtype tumors (A.), with PIK3R1 mutated and PIK3R1 wildtype tumors (B.), with PTEN mutated and PTEN wildtype tumors (C.) WT: wildtype, MUT: mutant, PFS: progression free survival.
Figure 2.
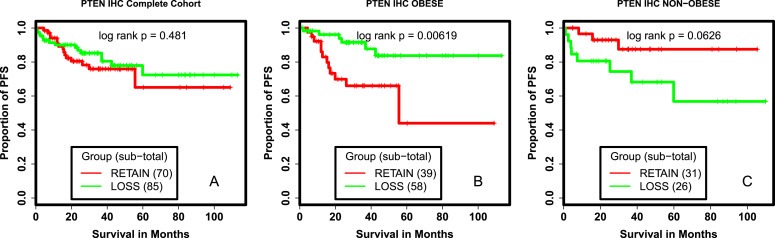
Survival analysis of patients with PTEN LOSS and PTEN retained tumors. Kaplan Meier survival curves comparing PFS of patients with PTEN LOSS and PTEN RETAINED tumors (A.), obese patients with PTEN LOSS and PTEN RETAINED tumors (B.), non‐obese patients with PTEN LOSS and PTEN RETAINED tumors (C.). IHC: Immuno‐histochemistry, RETAIN: PTEN RETAINED tumors as measured by IHC, LOSS:PTEN LOSS tumors as measured by IHC, PFS: progression free survival.
There is a strong association between obesity and risk of developing endometrial cancer. Further, PI3K signals are important in the regulation of metabolism (Engelman et al., 2006), and key downstream regulators of insulin and other growth factors. We hypothesized mutations in the PI3K pathway might have different roles in cancer biology in the presence of excess nutrients associated with the obese state. We tested for an interaction between BMI and PI3K pathway alterations. There was no association between PIK3CA, PIK3R1 or PTEN mutations and PFS in either the obese or non‐obese population (data not shown). However, PTEN loss was associated with improved PFS as compared to PTEN retention among obese patients (P = 0.006; Figure 2B). In contrast, PTEN loss was associated with a trend toward decreased PFS in non‐obese patients (P = 0.06; Figure 2C). We evaluated the potential impact of obesity on patients whose tumors had either lost or retained PTEN. BMI had no association with PFS (P = 0.975) in the collective population (Figure 3A). In patients whose tumors had lost PTEN, obesity was associated with improved PFS (P = 0.03; Figure 3B). In contrast, in patients whose tumors retained PTEN expression, obesity was associated with decreased PFS (P = 0.02; Figure 3C).
Figure 3.
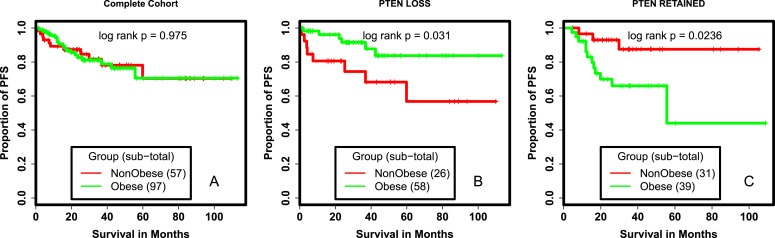
Survival analysis of obesity. Kaplan Meier survival curves comparing PFS of obese and non‐obese patients (A.), obese and non‐obese patients whose tumors had PTEN LOSS (B.), obese and non‐obese patients whose tumors had PTEN RETAINED (C.). RETAIN: PTEN retained tumors as measured by IHC, LOSS:PTEN loss tumors as measured by IHC, PFS: progression free survival.
We explored another clinically‐annotated cohort of endometrial cancer patients from Norway to validate these findings. Categorized as low, medium, or high PTEN expression, obese patients with low PTEN expression trended toward more favorable survival (P = 0.059; Supplementary Figure S3). No prognostic impact of PTEN expression was seen for non‐obese patients (data not shown.)
PTEN protein loss has been reported to be associated with lower tumor grade and stage (Athanassiadou et al., 2007; Salvesen et al., 2004). A multivariate analysis was performed controlling for these factors and their interaction. As shown in Table 2, the interaction between PTEN status and obesity is the most significant predictor of improved PFS (HR 0.15; 95% CI 0.02, 0.89). Obesity and advanced stage also predicted worse PFS.
Table 2.
Multivariate Cox model proportional hazard for PFS controlling for key clinical and pathologic factors including the interaction between PTEN loss and obesity.
| Variable | Hazard Ratio | Standard error | Lower limit CI (95%) | Higher limit CI (95%) | P ‐value |
|---|---|---|---|---|---|
| Grade 2 | 1.46 | 1.1 | 0.17 | 12.67 | 0.73 |
| Grade 3 | 2.78 | 1.13 | 0.3 | 25.61 | 0.37 |
| Stage II | 3.69 | 0.67 | 1 | 13.61 | 0.05 |
| Stage III | 6.51 | 0.6 | 2.01 | 21.06 | 0.0018 |
| Stage IV | 11.74 | 0.59 | 3.7 | 37.31 | <0.0001 |
| PTEN Status (Loss) | 2.04 | 0.73 | 0.48 | 8.61 | 0.33 |
| Obesity | 3.71 | 0.66 | 1 | 13.76 | 0.05 |
| PTEN Status (Loss):Obesity | 0.15 | 0.9 | 0.03 | 0.9 | 0.038 |
CI: Confidence interval, PTENSTATUS (LOSS):OBESITY: interaction term.
Significance at <0.05.
3.3. Effect of PTEN loss on cell signaling
Using RPPA, we examined the total protein and phospho‐protein expression changes associated with PTEN loss in obese and non‐obese patients separately. The proteins with statistically significant changes of the largest magnitude are shown in Figure 4 (See Supplementary Tables S1–4 for complete data set). In the non‐obese population, the most significant alterations in protein expression associated with PTEN loss were in PTEN, phosphorylated AKT, phosphorylated 4EBP1, and phosphorylated AMP‐activated protein kinase (AMPK; shown in Figure 4A, left panel). In the obese population, decreased PTEN protein expression was significantly associated with PTEN loss. The largest magnitude of expression changes in the obese population were seen in β‐CATENIN, phosphorylated FOXO3A, and phosphorylated YAP (Figure 4A, right panel). The absolute median expression of phosphorylated AKT was increased in obese patients with PTEN loss as compared to those with PTEN retained, but was not significantly different (Supplementary Figure S4). In contrast to changes in the non‐obese population, among the obese patients, phosphorylated 4EBP1 was decreased with PTEN loss as compared to those with PTEN retained, and was marginally significantly different (Supplementary Figure S4). We next compared the impact of obesity on protein expression changes separately in tumors lacking or retaining PTEN (Figure 4B). The largest changes were seen in phosphorylated AMPK and IGFBP‐2 in the PTEN loss tumors. Progesterone receptor (PR) levels were markedly increased in obese patients independent of PTEN status.
Figure 4.
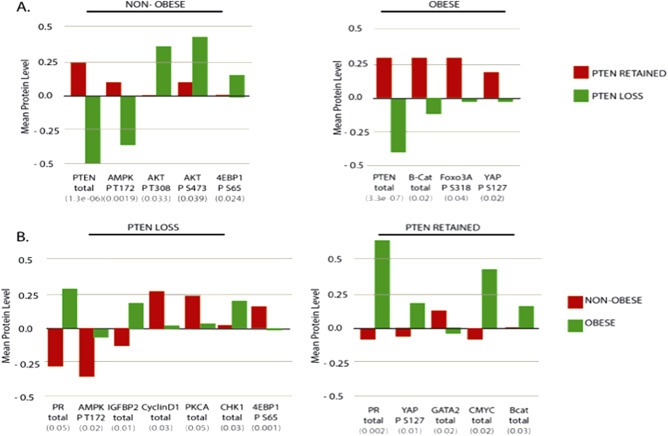
Comparisons of protein expression in tumors. (A.) Proteins with the largest magnitudes of change between PTEN LOSS and PTEN RETAINED tumors in non‐obese (left panel) and obese (right panel) populations. (B.) Proteins with the largest magnitudes of change between tumors from obese and non‐obese patients in PTEN LOSS (left panel) and PTEN RETAINED (right panel) tumors. p‐value of Student's t‐test shown in grey below the protein name. PTEN: Phosphatase and Tensin Homolog, AMPK P T172: Adenosine Monophosphate‐Activated Protein Kinase Phosphorylated at T172, AKT P T308: AKT phosphorylated at T308, AKT P S473: AKT phosphorylated at S473, 4EBP1 P S65: Eukaryotic Translation Initiation Factor 4E binding protein 1 phosphorylated at S65, B‐cat: Beta‐Catenin, Foxo3a P S318: Forkhead Box O 3A phosphorylated at S318, YAP P S127: Yes‐associated protein phosphorylated at S127, PR: Progesterone Receptor, IGFBP2: Insulin‐like Growth Factor Binding Protein2 Cyclin D1: Cyclin D1, PKCA: Protien Kinase C alpha, CHK1: Serine/threonine‐protein kinase Chk1, GATA 2: GATA binding protein 2, MYC: Myc protein.
4. Discussion
Endometrial cancers have the highest rates of PI3K pathway alteration (Cheung et al., 2011; Ruderman et al., 1990) and the strongest association with obesity of all studied cancers (Calle et al., 2003; Cheung et al., 2011; Schmandt et al., 2011). Given the fundamental role of the PI3K pathway in regulating metabolism, we explored the potential clinical interaction of obesity and tumor‐specific PI3K pathway alterations in endometrioid endometrial tumors. We report distinct clinical outcomes for obese patients with PTEN loss as compared to non‐obese patients with PTEN loss tumors in two independent cohorts of patients with endometrial cancer. This observed genetic‐environmental interaction may explain the discrepant reports of both PTEN expression and BMI with clinical outcomes. These data describe a clinically important interaction between metabolic state and tumor genetics that could potentially unveil the biologic underpinning of obesity‐related cancers and may be relevant to biomarker evaluation for clinical trials with PI3K pathway inhibitors.
Numerous studies have investigated the association of PTEN loss and endometrial cancer prognosis with discrepant results (Athanassiadou et al., 2007, 2009, 2001, 2002, 2010). These differences are likely due to differences in study methodologies including the study population, PTEN antibodies applied for IHC, use of IHC versus sequencing, and use of full length versus hot spot sequencing. Our study population excluded all non‐endometrioid tumors, given the known low rates of PTEN loss and overall worse outcomes compared to endometrioid tumors (Hecht and Mutter, 2006). The association of molecular aberrations with histology type has likely contributed to findings of PTEN loss and improved outcomes in earlier studies. In addition, our data suggest that differences in BMI likely contribute to these discrepant results, particularly in studies that have evaluated endometrioid endometrial cancers only. Studies in which PTEN loss was associated with worse outcome were completed in Japanese populations (Kanamori et al., 2001, 2002). Though BMI was not reported in these studies, presumably this population had a significantly lower BMI than studies performed in Western populations. One study reported the loss of both PTEN and p27 protein expression was associated with improved outcomes in obese patients, though non‐endometrioid tumors were included and only 29% of the patients were obese (Dellas et al., 2009). The high proportion of patients with a BMI over 30 in our cohort, as well as a separate cohort from Norway, may have provided increased power to detect the association between PTEN loss and improved survival in obese patients.
The impact of obesity on endometrial cancer survival has also been controversial. Higher BMI was associated with worse all cause and cancer‐related mortality in two large population‐based studies that did not consider any pathologic factors (Calle et al., 2003; Reeves et al., 2007). In contrast, the majority of retrospective and unplanned subset analyses of prospective trials have reported improved or stable outcomes among obese patients compared to those with a BMI <30. Based on our findings, it appears that the impact of obesity may relate to the molecular profile of the tumor, specifically PTEN loss, which may provide insight into the prior contradictory findings. It would seem that neither factor should be considered individually, but rather, in the context of each other.
Our results have potential clinical implications. PI3K pathway inhibitors are being explored as therapeutic agents in endometrial cancer (www.clinicaltrials.gov). Several of these studies have embedded translational studies to evaluate molecular markers that may predict response to these agents. It has been hypothesized that PTEN loss or mutation may predict response to PI3K pathway inhibition. In fact, aberrations in the PI3K pathway, such as PIK3CA mutation, have been associated with higher response to PI3K‐directed therapies in tissue‐independent early phase trials (Janku et al., 2011). However, in endometrial cancer, retrospective molecular analyses have failed to demonstrate any association between presence of a PI3K pathway aberration and response to mTOR inhibitors/rapalogs. Specifically, assessment of PTEN loss by IHC has been evaluated and failed to predict response to rapalogs (Mackay et al., 2012; Meyer et al., 2011; Oza et al., 2011). Our RPPA analyses suggest that PTEN loss in non‐obese patients may be a better marker for canonical PI3K pathway activation than in obese patients. Thus, revisiting these analyses to incorporate BMI and including BMI as a factor in prospective biomarker studies is warranted.
Our results have implications for endometrial cancer classification. In this era of targeted therapy, there is a strong drive to prospectively molecularly categorize tumors to guide treatment decisions. Our data suggest that the context in which the molecular alterations developed in endometrial cancers has biological and clinical importance. The categorization of breast cancers by histologic type, hormone receptor expression, and HER2 amplification confer essential prognostic and predictive information. Similar categorizations have been attempted in endometrial cancer based on molecular aberrations with little success (Mackay et al., 2012; Meyer et al., 2011). This study, however, emphasizes that further clinical categorization of endometrioid endometrial cancer is of potential clinical utility.
One advantage of our dataset is the potential to interrogate protein signaling changes underlying differences in observed clinical outcome. RPPA analyses from this study suggest signaling pathways that should be further explored for their roles in endometrial cancer biology. The RPPA data further suggest that there are distinct consequences of PTEN loss in the obese or non‐obese setting. These are associative studies, thus, we cannot explain the mechanisms of this difference. However, there are many intriguing findings to explore.
In obese patients, the proteins with the largest, most significant changes between PTEN loss and retained tumors were β‐CATENIN and phosphorylated FOXO3A, which were both increased in PTEN retained tumors. Phosphorylated FOXO3A, a target of AKT, would traditionally be expected to increase in PTEN loss tumors. However, a recent study performed in colon cancer cell lines has demonstrated an important interaction of the PI3K and WNT/β‐CATENIN pathways. In these studies, inhibition of PI3K results in the hyper‐activation of β‐catenin, measured by phosphorylated FOX03A, and is associated with increased survival (Tenbaum et al., 2012). It is thus possible that aberrations in β‐catenin signaling associated with PTEN retention could be a dominant regulator of FOXO3A phosphorylation and may be important in endometrial cancers.
Changes in YAP phosphorylation were observed; obese patients with PTEN retained had higher YAPS127 phosphorylation as compared to those with PTEN loss. YAP is a member of the HIPPO tumor suppressor pathway, which can regulate PTEN levels by transcriptional activation of mir29, a microRNA that targets PTEN (Tumaneng et al., 2012). The association of decreased YAP phosphorylation with PTEN loss suggests this regulation should be evaluated in tumors as well. Finally, increased AMPK phosphorylation and IGFBP2 expression were observed in obese patients with PTEN loss. These findings are markers of nutrient deprivation, which is unexpected in the context of obesity. The changes in these proteins suggest continued evaluation of the impact of cellular metabolism and nutrient bioavailability on endometrial tumor biology.
In summary, we detected that PTEN loss associates with a better prognosis in obese, but not non‐obese patients. We also provide data for distinct protein changes related to PTEN loss supporting PI3K pathway activation as being more dominant in the non‐obese than obese patient population. Thus, the data presented describe an intriguing interaction between measurable molecular features of a tumor (PTEN status) and tumor microenvironment (obesity) that characterizes a clinical group with improved cancer outcomes. Measured changes in protein expression in these distinct clinical groups reveal signaling pathways for future studies in endometrial cancer biology. These molecular and clinical variables should be evaluated in ongoing and planned therapeutic studies for endometrial cancer.
Financial support
NIH K12 Calabresi Scholar Award (K12 CA088084) to SNW, NCI SPORE in Uterine Cancer (2P50 CA098258‐06) to SNW, RRB, KHL, GBM. NCI Cancer Center Support Grant (P30 CA016672) to MD Anderson Cancer Center Stand Up to Cancer Dream Team Translational Research Grant, a Program of the Entertainment Industry Foundation (SU2C‐AACR‐DT0209) to SNW, RRB, RLC, KHL, LCC, GBM, APM, Helse Vest, Research Council of Norway and The Norwegian Cancer Society, Harald Andersens legat to HBS.
Supporting information
The following are the supplementary data related to this article:
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data 1.
1.1.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molonc.2015.04.014.
Westin Shannon N., Ju Zhenlin, Broaddus Russell R., Krakstad Camilla, Li Jane, Pal Navdeep, Lu Karen H., Coleman Robert L., Hennessy Bryan T., Klempner Samuel J., Werner Henrica M.J., Salvesen Helga B., Cantley Lewis C., Mills Gordon B., Myers Andrea P., (2015), PTEN loss is a context-dependent outcome determinant in obese and non-obese endometrioid endometrial cancer patients, Molecular Oncology, 9, doi: 10.1016/j.molonc.2015.04.014.
References
- Anderson, B. , Connor, J.P. , Andrews, J.I. , Davis, C.S. , Buller, R.E. , Sorosky, J.I. , Benda, J.A. , 1996. Obesity and prognosis in endometrial cancer. 1171-1178; discussion Am. J. Obstetrics Gynecol. 174, 1178–1179. [DOI] [PubMed] [Google Scholar]
- Athanassiadou, P. , Athanassiades, P. , Grapsa, D. , Gonidi, M. , Athanassiadou, A.M. , Stamati, P.N. , Patsouris, E. , 2007. The prognostic value of PTEN, p53, and beta-catenin in endometrial carcinoma: a prospective immunocytochemical study. Int. J. Gynecol. Cancer. 17, 697–704. [DOI] [PubMed] [Google Scholar]
- Bokhman, J.V. , 1983. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 15, 10–17. [DOI] [PubMed] [Google Scholar]
- Calle, E.E. , Rodriguez, C. , Walker-Thurmond, K. , Thun, M.J. , 2003. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 348, 1625–1638. [DOI] [PubMed] [Google Scholar]
- Catasus, L. , Gallardo, A. , Cuatrecasas, M. , Prat, J. , 2008. PIK3CA mutations in the kinase domain (exon 20) of uterine endometrial adenocarcinomas are associated with adverse prognostic parameters. Mod. Pathol. 21, 131–139. [DOI] [PubMed] [Google Scholar]
- Cheung, L.W. , Hennessy, B.T. , Li, J. , Yu, S. , Myers, A.P. , Djordjevic, B. , Lu, Y. , Stemke-Hale, K. , Zhang, F. , Ju, Z. , Cantley, L.C. , Scherer, S.E. , Liang, H. , Lu, K.H. , Broaddus, R.R. , Mills, G.B. , 2011. High frequency of PIK3R1 and PIK3R2 mutations in endometrial cancer elucidates a novel mechanism for regulation of PTEN protein stability. Cancer Discov. 1, 170–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosbie, E.J. , Roberts, C. , Qian, W. , Swart, A.M. , Kitchener, H.C. , Renehan, A.G. , 2012. Body mass index does not influence post-treatment survival in early stage endometrial cancer: results from the MRC ASTEC trial. Eur. J. Cancer. 48, 853–864. [DOI] [PubMed] [Google Scholar]
- Dellas, A. , Jundt, G. , Sartorius, G. , Schneider, M. , Moch, H. , 2009. Combined PTEN and p27kip1 protein expression patterns are associated with obesity and prognosis in endometrial carcinomas. Clin. Cancer Res. 15, 2456–2462. [DOI] [PubMed] [Google Scholar]
- Djordjevic, B. , Hennessy, B.T. , Li, J. , Barkoh, B.A. , Luthra, R. , Mills, G.B. , Broaddus, R.R. , 2012. Clinical assessment of PTEN loss in endometrial carcinoma: immunohistochemistry outperforms gene sequencing. Mod. Pathol. 25, 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, Y. , Yang, X. , Wong, O. , Zhang, X. , Liang, Y. , Zhang, Y. , Wong, W. , Nong, L. , Liao, Q. , Li, T. , 2012. PIK3CA mutations in endometrial carcinomas in Chinese women: phosphatidylinositol 3'-kinase pathway alterations might be associated with favorable prognosis. Hum. Pathol. 43, 1197–1205. [DOI] [PubMed] [Google Scholar]
- Engelman, J.A. , Luo, J. , Cantley, L.C. , 2006. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 7, 606–619. [DOI] [PubMed] [Google Scholar]
- Fata, J.E. , Debnath, S. , Jenkins, E.C. , Fournier, M.V. , 2012. Nongenomic mechanisms of PTEN regulation. Int. J. Cel. Biol. 2012, 379685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Dios, D.A. , Lambrechts, D. , Coenegrachts, L. , Vandenput, I. , Capoen, A. , Webb, P.M. , Ferguson, K. , Akslen, L.A. , Claes, B. , Vergote, I. , Moerman, P. , Van Robays, J. , Marcickiewicz, J. , Salvesen, H.B. , Spurdle, A.B. , Amant, F. , 2013. High-throughput interrogation of PIK3CA, PTEN, KRAS, FBXW7 and TP53 mutations in primary endometrial carcinoma. Gynecol. Oncol. 128, 327–334. [DOI] [PubMed] [Google Scholar]
- Garg, K. , Broaddus, R.R. , Soslow, R.A. , Urbauer, D.L. , Levine, D.A. , Djordjevic, B. , 2012. Pathologic scoring of PTEN immunohistochemistry in endometrial carcinoma is highly reproducible. Int. J. Gynecol. Pathol. 31, 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz, G. , Gabriel, S.B. , Cibulskis, K. , Lander, E. , Sivachenko, A. , Sougnez, C. , Lawrence, M. , Kandoth, C. , Dooling, D. , Fulton, R. , Fulton, L. , Kalicki-Veizer, J. , McLellan, M.D. , O'Laughlin, M. , Schmidt, H. , Wilson, R.K. , Ye, K. , Ding, L. , Mardis, E.R. , Ally, A. , Balasundaram, M. , Birol, I. , Butterfield, Y.S. , Carlsen, R. , Carter, C. , Chu, A. , Chuah, E. , Chun, H.J. , Dhalla, N. , Guin, R. , Hirst, C. , Holt, R.A. , Jones, S.J. , Lee, D. , Li, H.I. , Marra, M.A. , Mayo, M. , Moore, R.A. , Mungall, A.J. , Plettner, P. , Schein, J.E. , Sipahimalani, P. , Tam, A. , Varhol, R.J. , Gordon Robertson, A. , Cherniack, A.D. , Pashtan, I. , Saksena, G. , Onofrio, R.C. , Schumacher, S.E. , Tabak, B. , Carter, S.L. , Hernandez, B. , Gentry, J. , Salvesen, H.B. , Ardlie, K. , Winckler, W. , Beroukhim, R. , Meyerson, M. , Hadjipanayis, A. , Lee, S. , Mahadeshwar, H.S. , Park, P. , Protopopov, A. , Ren, X. , Seth, S. , Song, X. , Tang, J. , Xi, R. , Yang, L. , Zeng, D. , Kucherlapati, R. , Chin, L. , Zhang, J. , Todd Auman, J. , Balu, S. , Bodenheimer, T. , Buda, E. , Neil Hayes, D. , Hoyle, A.P. , Jefferys, S.R. , Jones, C.D. , Meng, S. , Mieczkowski, P.A. , Mose, L.E. , Parker, J.S. , Perou, C.M. , Roach, J. , Shi, Y. , Simons, J.V. , Soloway, M.G. , Tan, D. , Topal, M.D. , Waring, S. , Wu, J. , Hoadley, K.A. , Baylin, S.B. , Bootwalla, M.S. , Lai, P.H. , Triche, T.J. , Van Den Berg, D.J. , Weisenberger, D.J. , Laird, P.W. , Shen, H. , Cho, J. , Dicara, D. , Frazer, S. , Heiman, D. , Jing, R. , Lin, P. , Mallard, W. , Stojanov, P. , Voet, D. , Zhang, H. , Zou, L. , Noble, M. , Reynolds, S.M. , Shmulevich, I. , Arman Aksoy, B. , Antipin, Y. , Ciriello, G. , Dresdner, G. , Gao, J. , Gross, B. , Jacobsen, A. , Ladanyi, M. , Reva, B. , Sander, C. , Sinha, R. , Onur Sumer, S. , Taylor, B.S. , Cerami, E. , Weinhold, N. , Schultz, N. , Shen, R. , Benz, S. , Goldstein, T. , Haussler, D. , Ng, S. , Szeto, C. , Stuart, J. , Benz, C.C. , Yau, C. , Zhang, W. , Annala, M. , Broom, B.M. , Casasent, T.D. , Ju, Z. , Liang, H. , Liu, G. , Lu, Y. , Unruh, A.K. , Wakefield, C. , Weinstein, J.N. , Zhang, N. , Liu, Y. , Broaddus, R. , Akbani, R. , Mills, G.B. , Adams, C. , Barr, T. , Black, A.D. , Bowen, J. , Deardurff, J. , Frick, J. , Gastier-Foster, J.M. , Grossman, T. , Harper, H.A. , Hart-Kothari, M. , Helsel, C. , Hobensack, A. , Kuck, H. , Kneile, K. , Leraas, K.M. , Lichtenberg, T.M. , McAllister, C. , Pyatt, R.E. , Ramirez, N.C. , Tabler, T.R. , Vanhoose, N. , White, P. , Wise, L. , Zmuda, E. , Barnabas, N. , Berry-Green, C. , Blanc, V. , Boice, L. , Button, M. , Farkas, A. , Green, A. , Mackenzie, J. , Nicholson, D. , Kalloger, S.E. , Blake Gilks, C. , Karlan, B.Y. , Lester, J. , Orsulic, S. , Borowsky, M. , Cadungog, M. , Czerwinski, C. , Huelsenbeck-Dill, L. , Iacocca, M. , Petrelli, N. , Rabeno, B. , Witkin, G. , Nemirovich-Danchenko, E. , Potapova, O. , Rotin, D. , Berchuck, A. , Birrer, M. , Disaia, P. , Monovich, L. , Curley, E. , Gardner, J. , Mallery, D. , Penny, R. , Dowdy, S.C. , Winterhoff, B. , Dao, L. , Gostout, B. , Meuter, A. , Teoman, A. , Dao, F. , Olvera, N. , Bogomolniy, F. , Garg, K. , Soslow, R.A. , Levine, D.A. , Abramov, M. , Bartlett, J.M. , Kodeeswaran, S. , Parfitt, J. , Moiseenko, F. , Clarke, B.A. , Goodman, M.T. , Carney, M.E. , Matsuno, R.K. , Fisher, J. , Huang, M. , Rathmell, W.K. , Thorne, L. , Van Le, L. , Dhir, R. , Edwards, R. , Elishaev, E. , Zorn, K. , Goodfellow, P.J. , Mutch, D. , Kahn, A.B. , Bell, D.W. , Pollock, P.M. , Wang, C.D.A.W. , Shinbrot, E. , Ayala, B. , Chu, A.L. , Jensen, M.A. , Kothiyal, P. , Pihl, T.D. , Pontius, J. , Pot, D.A. , Snyder, E.E. , Srinivasan, D. , Mills Shaw, K.R. , Sheth, M. , Davidsen, T. , Eley Martin, L.F.G. , Demchok, J.A. , Guyer, M.S. , Ozenberger, B.A. , Sofia, H.J. , 2013. Integrated genomic characterization of endometrial carcinoma. Nature. 497, 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht, J.L. , Mutter, G.L. , 2006. Molecular and pathologic aspects of endometrial carcinogenesis. J. Clin. Oncol. 24, 4783–4791. [DOI] [PubMed] [Google Scholar]
- Hennessy, B.T. , Lu, Y. , Gonzalez-Angulo, A.M. , Carey, M.S. , Myhre, S. , Ju, Z. , Davies, M.A. , Liu, W. , Coombes, K. , Meric-Bernstam, F. , Bedrosian, I. , McGahren, M. , Agarwal, R. , Zhang, F. , Overgaard, J. , Alsner, J. , Neve, R.M. , Kuo, W.L. , Gray, J.W. , Borresen-Dale, A.L. , Mills, G.B. , 2010. A technical assessment of the utility of reverse phase protein arrays for the study of the functional proteome in non-microdissected human breast cancers. Clin. Proteomics. 6, 129–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, J. , He, X. , Baggerly, K.A. , Coombes, K.R. , Hennessy, B.T. , Mills, G.B. , 2007. Non-parametric quantification of protein lysate arrays. Bioinformatics. 23, 1986–1994. [DOI] [PubMed] [Google Scholar]
- Janku, F. , Tsimberidou, A.M. , Garrido-Laguna, I. , Wang, X. , Luthra, R. , Hong, D.S. , Naing, A. , Falchook, G.S. , Moroney, J.W. , Piha-Paul, S.A. , Wheler, J.J. , Moulder, S.L. , Fu, S. , Kurzrock, R. , 2011. PIK3CA mutations in patients with advanced cancers treated with PI3K/AKT/mTOR axis inhibitors. Mol. Cancer Ther. 10, 558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori, Y. , Kigawa, J. , Itamochi, H. , Shimada, M. , Takahashi, M. , Kamazawa, S. , Sato, S. , Akeshima, R. , Terakawa, N. , 2001. Correlation between loss of PTEN expression and Akt phosphorylation in endometrial carcinoma. Clin. Cancer Res. 7, 892–895. [PubMed] [Google Scholar]
- Kanamori, Y. , Kigawa, J. , Itamochi, H. , Sultana, H. , Suzuki, M. , Ohwada, M. , Kamura, T. , Sugiyama, T. , Kikuchi, Y. , Kita, T. , Fujiwara, K. , Terakawa, N. , 2002. PTEN expression is associated with prognosis for patients with advanced endometrial carcinoma undergoing postoperative chemotherapy. Int. J. Cancer. 100, 686–689. [DOI] [PubMed] [Google Scholar]
- Kong, D. , Suzuki, A. , Zou, T.T. , Sakurada, A. , Kemp, L.W. , Wakatsuki, S. , Yokoyama, T. , Yamakawa, H. , Furukawa, T. , Sato, M. , Ohuchi, N. , Sato, S. , Yin, J. , Wang, S. , Abraham, J.M. , Souza, R.F. , Smolinski, K.N. , Meltzer, S.J. , Horii, A. , 1997. PTEN1 is frequently mutated in primary endometrial carcinomas. Nat. Genet. 17, 143–144. [DOI] [PubMed] [Google Scholar]
- Krakstad, C. , Trovik, J. , Wik, E. , Engelsen, I.B. , Werner, H.M.J. , Birkeland, E.E. , Ræder, M.B. , Øyan, A.M. , Stefansson, I.M. , Kalland, K.-H. , Akslen, L.A. , Salvesen, H.B. , 2012. Loss of GPER identifies new targets for therapy among a subgroup of ER alpha-positive endometrial cancer patients with poor outcome. Br. J. Cancer. 106, 1682–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, H. , Cheung, L.W. , Li, J. , Ju, Z. , Yu, S. , Stemke-Hale, K. , Dogruluk, T. , Lu, Y. , Liu, X. , Gu, C. , Guo, W. , Scherer, S.E. , Carter, H. , Westin, S.N. , Dyer, M.D. , Verhaak, R.G. , Zhang, F. , Karchin, R. , Liu, C.G. , Lu, K.H. , Broaddus, R.R. , Scott, K.L. , Hennessy, B.T. , Mills, G.B. , 2012. Whole-exome sequencing combined with functional genomics reveals novel candidate driver cancer genes in endometrial cancer. Genome Res. 22, 2120–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay, H. , Eisenhauer, E. , Kamel-Reid, S. , Clarke, B. , Walsh, W. , Karakasis, K. , Salvesen, H.B. , Oza, A. , 2012. Molecular determinants of outcome with mTOR inhibition in endometrial cancer (EC). J. Clin. Oncol. 30, (suppl; abstr 5010) [Google Scholar]
- Mackay, H.J. , Gallinger, S. , Tsao, M.S. , McLachlin, C.M. , Tu, D. , Keiser, K. , Eisenhauer, E.A. , Oza, A.M. , 2010. Prognostic value of microsatellite instability (MSI) and PTEN expression in women with endometrial cancer: results from studies of the NCIC Clinical Trials Group (NCIC CTG). Eur. J. Cancer. 46, 1365–1373. [DOI] [PubMed] [Google Scholar]
- Mariani, A. , Webb, M.J. , Keeney, G.L. , Haddock, M.G. , Calori, G. , Podratz, K.C. , 2000. Low-risk corpus cancer: is lymphadenectomy or radiotherapy necessary?. Am. J. obstetrics Gynecol. 182, 1506–1519. [DOI] [PubMed] [Google Scholar]
- Mauland, K.K. , Trovik, J. , Wik, E. , Raeder, M.B. , Njolstad, T.S. , Stefansson, I.M. , Oyan, A.M. , Kalland, K.H. , Bjorge, T. , Akslen, L.A. , Salvesen, H.B. , 2011. High BMI is significantly associated with positive progesterone receptor status and clinico-pathological markers for non-aggressive disease in endometrial cancer. Br. J. Cancer. 104, 921–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, L.A. , Slomovitz, B.M. , Djordjevic, B. , Galbincea, J.M. , Johnston, T.A. , Munsell, M. , Burzawa, J.K. , Huang, M. , Broaddus, R. , Iglesias, D.A. , Coleman, R.L. , Gershenson, D.M. , Burke, T.W. , Wolf, J. , Lu, K.H. , 2011. The search continues: looking for predictive biomarkers for response mTOR inhibition in endometrial cancer. J. Clin. Oncol. 29, (Suppl; abstr 5016) [Google Scholar]
- Minaguchi, T. , Yoshikawa, H. , Oda, K. , Ishino, T. , Yasugi, T. , Onda, T. , Nakagawa, S. , Matsumoto, K. , Kawana, K. , Taketani, Y. , 2001. PTEN mutation located only outside exons 5, 6, and 7 is an independent predictor of favorable survival in endometrial carcinomas. Clin. Cancer Res. 7, 2636–2642. [PubMed] [Google Scholar]
- Munstedt, K. , Wagner, M. , Kullmer, U. , Hackethal, A. , Franke, F.E. , 2008. Influence of body mass index on prognosis in gynecological malignancies. Cancer Causes Control. 19, 909–916. [DOI] [PubMed] [Google Scholar]
- Oza, A.M. , Elit, L. , Tsao, M.S. , Kamel-Reid, S. , Biagi, J. , Provencher, D.M. , Gotlieb, W.H. , Hoskins, P.J. , Ghatage, P. , Tonkin, K.S. , Mackay, H.J. , Mazurka, J. , Sederias, J. , Ivy, P. , Dancey, J.E. , Eisenhauer, E.A. , 2011. Phase II study of temsirolimus in women with recurrent or metastatic endometrial cancer: a trial of the NCIC Clinical Trials Group. J. Clin. Oncol. 29, 3278–3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves, G.K. , Pirie, K. , Beral, V. , Green, J. , Spencer, E. , Bull, D. , 2007. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 335, 1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risinger, J.I. , Hayes, A.K. , Berchuck, A. , Barrett, J.C. , 1997. PTEN/MMAC1 mutations in endometrial cancers. Cancer Res. 57, 4736–4738. [PubMed] [Google Scholar]
- Risinger, J.I. , Hayes, K. , Maxwell, G.L. , Carney, M.E. , Dodge, R.K. , Barrett, J.C. , Berchuck, A. , 1998. PTEN mutation in endometrial cancers is associated with favorable clinical and pathologic characteristics. Clin. Cancer Res. 4, 3005–3010. [PubMed] [Google Scholar]
- Rudd, M.L. , Price, J.C. , Fogoros, S. , Godwin, A.K. , Sgroi, D.C. , Merino, M.J. , Bell, D.W. , 2011. A unique spectrum of somatic PIK3CA (p110alpha) mutations within primary endometrial carcinomas. Clin. Cancer Res. 17, 1331–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman, N.B. , Kapeller, R. , White, M.F. , Cantley, L.C. , 1990. Activation of phosphatidylinositol 3-kinase by insulin. Proc. Natl. Acad. Sci. U. S. A. 87, 1411–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvesen, H.B. , Carter, S.L. , Mannelqvist, M. , Dutt, A. , Getz, G. , Stefansson, I.M. , Raeder, M.B. , Sos, M.L. , Engelsen, I.B. , Trovik, J. , Wik, E. , Greulich, H. , Bo, T.H. , Jonassen, I. , Thomas, R.K. , Zander, T. , Garraway, L.A. , Oyan, A.M. , Sellers, W.R. , Kalland, K.H. , Meyerson, M. , Akslen, L.A. , Beroukhim, R. , 2009. Integrated genomic profiling of endometrial carcinoma associates aggressive tumors with indicators of PI3 kinase activation. Proc. Natl. Acad. Sci. U. S. A. 106, 4834–4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvesen, H.B. , Das, S. , Akslen, L.A. , 2000. Loss of nuclear p16 protein expression is not associated with promoter methylation but defines a subgroup of aggressive endometrial carcinomas with poor prognosis. Clin. Cancer Res. 6, 153–159. [PubMed] [Google Scholar]
- Salvesen, H.B. , Stefansson, I. , Kalvenes, M.B. , Das, S. , Akslen, L.A. , 2002. Loss of PTEN expression is associated with metastatic disease in patients with endometrial carcinoma. Cancer. 94, 2185–2191. [DOI] [PubMed] [Google Scholar]
- Salvesen, H.B. , Stefansson, I. , Kretzschmar, E.I. , Gruber, P. , MacDonald, N.D. , Ryan, A. , Jacobs, I.J. , Akslen, L.A. , Das, S. , 2004. Significance of PTEN alterations in endometrial carcinoma: a population-based study of mutations, promoter methylation and PTEN protein expression. Int. J. Oncol. 25, 1615–1623. [PubMed] [Google Scholar]
- Schmandt, R.E. , Iglesias, D.A. , Co, N.N. , Lu, K.H. , 2011. Understanding obesity and endometrial cancer risk: opportunities for prevention. Am. J. Obstetrics Gynecol. 205, 518–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel, R. , Naishadham, D. , Jemal, A. , 2012. Cancer statistics, 2012. CA Cancer J. Clin. 62, 10–29. [DOI] [PubMed] [Google Scholar]
- Simon, N. , Friedman, J. , Hastie, T. , Tibshirani, R. , 2011. Regularization paths for Cox's proportional hazards model via coordinate descent. J. Stat. Softw. 39, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson, I.M. , Salvesen, H.B. , Akslen, L.A. , 2004. Prognostic impact of alterations in P-cadherin expression and related cell adhesion markers in endometrial cancer. J. Clin. Oncol. 22, 1242–1252. [DOI] [PubMed] [Google Scholar]
- Tashiro, H. , Blazes, M.S. , Wu, R. , Cho, K.R. , Bose, S. , Wang, S.I. , Li, J. , Parsons, R. , Ellenson, L.H. , 1997. Mutations in PTEN are frequent in endometrial carcinoma but rare in other common gynecological malignancies. Cancer Res. 57, 3935–3940. [PubMed] [Google Scholar]
- Tenbaum, S.P. , Ordonez-Moran, P. , Puig, I. , Chicote, I. , Arques, O. , Landolfi, S. , Fernandez, Y. , Herance, J.R. , Gispert, J.D. , Mendizabal, L. , Aguilar, S. , Ramon y Cajal, S. , Schwartz, S. , Vivancos, A. , Espin, E. , Rojas, S. , Baselga, J. , Tabernero, J. , Munoz, A. , Palmer, H.G. , 2012. beta-catenin confers resistance to PI3K and AKT inhibitors and subverts FOXO3a to promote metastasis in colon cancer. Nat. Med. 18, 892–901. [DOI] [PubMed] [Google Scholar]
- Terakawa, N. , Kanamori, Y. , Yoshida, S. , 2003. Loss of PTEN expression followed by Akt phosphorylation is a poor prognostic factor for patients with endometrial cancer. Endocr. Relat. Cancer. 10, 203–208. [DOI] [PubMed] [Google Scholar]
- Trovik, J. , Wik, E. , Stefansson, I.M. , Marcickiewicz, J. , Tingulstad, S. , Staff, A.C. , Njolstad, T.S. , Vandenput, I. , Amant, F. , Akslen, L.A. , Salvesen, H. , 2011 May 15. Stathmin overexpression identifies high risk patients and lymph node metastasis in endometrial cancer. Clin. Cancer Res. 17, (10) 3368–3377. [DOI] [PubMed] [Google Scholar]
- Tumaneng, K. , Schlegelmilch, K. , Russell, R.C. , Yimlamai, D. , Basnet, H. , Mahadevan, N. , Fitamant, J. , Bardeesy, N. , Camargo, F.D. , Guan, K.L. , 2012. YAP mediates crosstalk between the Hippo and PI(3)K-TOR pathways by suppressing PTEN via miR-29. Nat. Cell Biol. 14, 1322–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urick, M.E. , Rudd, M.L. , Godwin, A.K. , Sgroi, D.C. , Merino, M. , Bell, D.W. , 2011 Jun 15. PIK3R1 (p85-alpha/p85{alpha}) is somatically mutated at high frequency in primary endometrial cancer. Cancer Res. 71, (12) 4061–4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gruenigen, V.E. , Tian, C. , Frasure, H. , Waggoner, S. , Keys, H. , Barakat, R.R. , 2006. Treatment effects, disease recurrence, and survival in obese women with early endometrial carcinoma : a Gynecologic Oncology Group study. Cancer. 107, 2786–2791. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following are the supplementary data related to this article:
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data


