Abstract
Repeated administration of non-competitive N-methyl-d-aspartate (NMDA) receptor antagonists such as phencyclidine (PCP) to rodents causes long-lasting deficits in cognition and memory, and has effects on behaviors that have been suggested to be models of the cognitive impairment associated with schizophrenia (CIAS). Despite this being a widely studied animal model, little is known about the long lasting changes in synapses and circuits that underlie the altered behaviors. Here we examined synaptic transmission ex-vivo in the hippocampus of mice after a subchronic PCP (scPCP) administration regime. We found that after at least one week of drug free washout period when mice have impaired cognitive function, the threshold for long term potentiation (LTP) of CA1 excitatory synapses was elevated. This elevated LTP threshold was directly related to increased inhibitory input to CA1 pyramidal cells through increased activity of GABAergic neurons.
These results suggest repeated PCP administration causes a long-lasting metaplastic change in the inhibitory circuits in the hippocampus that results in impaired LTP, and could contribute to the deficits in hippocampal-dependent memory in PCP-treated mice. Changes in GABA signaling have been described in patients with schizophrenia, therefore our results support using scPCP as a model of CIAS.
Keywords: phencyclidine, hippocampus, long-term potentiation, GABA, inhibitory postsynaptic current
1. Introduction
1.1. Subchronic NMDA receptor antagonism by PCP as a model of cognitive impairment in schizophrenia (CIAS)
Schizophrenia is one of the most common chronic and devastating psychiatric disorders affecting approximately 1% of the population (Sawa and Snyder, 2002). The symptoms of schizophrenia include delusions and hallucinations (positive symptoms), anhedonia, affective flattening, and avolition (negative symptoms), abnormalities in mood, and importantly, because of their impact on outcome, deficits in cognitive functions (Green, 1996; Sawa and Snyder, 2002). The multifaceted clinical syndrome and the complex pathophysiology of schizophrenia including multiple genes, epigenetic and environmental factors are not easily translatable to animals, making the study of the disorder in model organisms difficult (Hall et al., 2014; Jaaro-Peled et al., 2010; Siegel et al., 2013). In particular, attempts to model the cognitive impairment associated with schizophrenia (CIAS) has, of late, been of great interest because the treatment options for this domain are limited (Meltzer et al., 2013; Young et al., 2009). Several lines of evidence have demonstrated that N-methyl-D-aspartate (NMDA) receptor hypofunction may contribute to CIAS, including the observation that non-competitive NMDA receptor antagonists such as phencyclidine (PCP) produce some aspects of CIAS in healthy human subjects and exacerbate symptoms in individuals with schizophrenia (Coyle et al., 2012; Meltzer et al., 2013). While the acute effects of blocking NMDA receptors are noteworthy, repeated administration of NMDA receptor antagonists produce behavioral changes in rodents that persist for many weeks after wash out of the drug (Meltzer et al., 2013; Neill et al., 2010). The post-withdrawal effects include both the positive and negative symptoms of schizophrenia, as well as a pronounced deficit in cognitive function providing the model's face validity (Meltzer et al., 2013; Young et al., 2012). Remodeling of circuits and disruption in glutamatergic and GABAergic signaling are found not only in neonates but also in adolescent (Thomases et al., 2014; Thomases et al., 2013) and adult rodents (Meltzer et al., 2013). Additionally, a variety of atypical antipsychotic drugs are effective in reversing the behavioral alterations in this pharmacologically-induced disease model, including cognitive deficits, demonstrating its predictive validity (Snigdha et al., 2010; Young et al., 2012). Therefore, the chronic administration of NMDA receptor antagonists has become a commonly used paradigm for understanding the basis of cognitive impairment and for preclinical drug discovery for the development of treatments for CIAS and psychosis (Wiescholleck and Manahan-Vaughan, 2013b).
1.2. Involvement of hippocampal function in CIAS
Amongst the neural circuits that are affected in CIAS, there is strong evidence for the involvement of the hippocampus. Consistent with gross morphological changes and functional alterations in the hippocampus (Heckers, 2001; Kraguljac et al., 2014; Rasetti et al., 2014), there are well-established deficits in hippocampal-dependent learning and memory in schizophrenic patients (Perry et al., 2000; Saykin et al., 1991). Similarly, in animal models, chronic administration of NMDA receptor antagonists cause clear deficits in hippocampal-dependent behaviors, such as spatial reference memory tasks as assessed by the Morris Water Maze (Andersen and Pouzet, 2004) and novel object recognition (Horiguchi et al., 2011b; McLean et al., 2009; Snigdha et al., 2010). There has been detailed biochemical, histological (Javitt et al., 2004; Reynolds et al., 2004) and behavioral (Abdul-Monim et al., 2007; Horiguchi et al., 2011a, b; Jenkins et al., 2008) characterization of these pharmacological models of cognitive impairment. However, to date, there have been no published studies that have examined potential functional synaptic alterations in the hippocampus that are correlated with the deficit in hippocampal memory performance.
1.3. Alterations in hippocampal synaptic properties by subchronic PCP (scPCP) relevant to CIAS
Here, we report adaptive changes in the physiology of hippocampal synapses in mice after subchronic PCP (scPCP) injection. After induction of cognitive impairment by repeated administration of PCP to animals and following at least one week of drug washout (Rajagopal et al., 2013), hippocampal sections were made from mice and synaptic plasticity tested in the CA1 region of the hippocampus. We found that long-term potentiation (LTP) at CA3-CA1 synapses was impaired in scPCP treated mice in comparison to vehicle treated controls. We did not observe any alterations in basal excitatory synaptic transmission. However, we found that GABAergic inhibitory synaptic input to the CA1 pyramidal cells was enhanced. The increased GABA transmission was directly responsible for increasing the threshold of LTP induction, because LTP could be induced normally in a disinhibited slice. These results suggest that scPCP causes a long-lasting adaptive enhancement of GABA synapses in the CA1 region of the hippocampus that increases the plasticity threshold of excitatory synapses, and is correlated with alterations in hippocampal-dependent learning. These results suggest a novel and previously unknown elevation in GABA signaling in the hippocampus that could contribute to the hippocampal cognitive dysfunction in rodent models of CIAS and possibly underlies the cognitive disruption in patients with schizophrenia.
2. Methods and Materials
2.1. Animals
All procedures related to the care and treatments of animals were approved by the Northwestern University IAUCUC. Mice on a congenic C57Bl/6 background strain (2 - 3 months old) were purchased from The Jackson Laboratory. Phencyclidine (PCP) (provided by the National Institute on Drug Abuse) was administered (10 mg/kg i.p.) twice daily with a 6-8 hour interval for a total of 7 days. Control animals were handled in exactly the same manner and were injected with vehicle for the same period. After PCP or vehicle treatment, animals underwent greater than one week of washout period before being used for subsequent ex-vivo electrophysiological analysis of hippocampal synaptic transmission.
2.2. Slice preparation and electrophysiology
Horizontal slices containing the ventral hippocampus were prepared using standard techniques. Briefly, animals were deeply anesthetized (xylazine 10mg/kg and ketamine 100mg/kg i.p.) before undergoing cardiac perfusion with an ice-cold sucrose artificial cerebrospinal fluid (ACSF) solution containing (in mM): 85 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 25 glucose, 75 sucrose, 0.5 CaCl2 and 4 MgCl2, equilibrated with 95% O2 and 5% CO2. In a control group of experiments to exclude any potential effects of acute ketamine, animals were anesthetized with isoflurane and directly decapitated. The brain was removed quickly and mounted on the stage of vibratome (Leica Microsystems, Inc). 350μm thick sections were made in the same ice-cold sucrose ACSF. Slices were transferred to a recovery chamber containing the sucrose slicing ACSF solution, which was gradually exchanged for a normal ACSF containing (in mM): 125 NaCl, 2.4 KCl, 1.2 Na2PO4, 25 NaHCO3, 25 glucose, 1 CaCl2 and 2 MgCl2, while the slices were maintained at 30°C. Individual slices were transfer red to a recording chamber and visualized using Dodt contrast optics. For extracellular recordings, slices were perfused with normal ACSF containing (in mM): 125 NaCl, 2.4 KCl, 1.2 Na2PO4, 25 NaHCO3, 25 glucose, 2 CaCl2 and 1 MgCl2. Recording electrodes were manufactured from borosilicate glass pipettes and had resistances of 3-5 M when filled with regular ACSF. Extracellular field postsynaptic potentials (fPSPs) were evoked using a monopolar electrode filled with ACSF placed in the stratum radiatum. LTP was induced by 100Hz tetanic stimulation (1 or 3 trains of 100Hz for 1s with an inter-train interval of 20s). Data were collected and analyzed using pClamp 10 software (Molecular Devices, Sunnyvale, CA). For whole-cell patch clamp experiments, recording electrodes were filled with internal solution containing (in mM) 95 CsF, 25 CsCl, 10 Cs-HEPES, 10 Cs-EGTA, 2 NaCl, 2 Mg-ATP, 10 QX-314, 5 TEA-Cl, 5 4-AP for recording of EPSCs, or 75 CsCH3SO3, 60 CsCl, 1 MgCl2, 0.2 EGTA, 10 HEPES, 2 Mg-ATP 0.3 GTP-Na2, 10 Na2-phosphocreatine,10 TEA, 5 QX-314 for IPSCs. Pyramidal cells were voltage clamped at +40 mV for measurement of NMDAR currents or at -70 mV for measurement of AMPA receptor mediated EPSCs and GABAA mediated IPSCs. EPSCs were isolated by the inclusion of the GABAA antagonist bicuculline (10μM), and IPSCs were isolated by the inclusion of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (10μM) and D-(-)-2-Amino-5-phosphonopentanoic acid (DAPV) (50μM) in the extracellular solution. Miniature IPSCs (mIPSCs) were recorded in the presence of tetrodotoxin (TTX) (1μM) and analyzed using MiniAnalysis (Synaptosoft Inc.).
2.3. Data analysis
Statistical analyses were conducted with Microsoft Excel, Graphpad Prism, and OriginPro9.0 software. Two sample comparisons were made using an unpaired two-tailed Student's t-test and non-parametric data were compared using the Kolmogorov-Smirnov test. For multiple comparisons, repeated two-way analysis of variance (ANOVA) followed by post-hoc Sidak's correction was employed. Differences were considered significant when p < 0.05. Data are shown as mean ± SEM.
3. Results
3.1. Hippocampal LTP is impaired after scPCP treatment
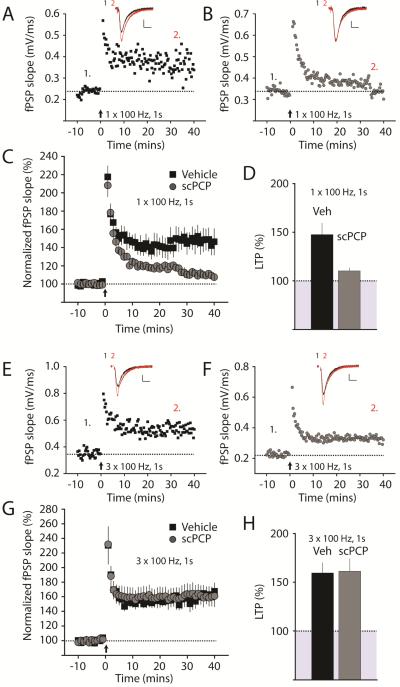
To determine what changes in hippocampal synapses are caused by scPCP treatment, we administered repeated doses of PCP for seven days and then examined mice at least one week post withdrawal (see methods). During this period, animals display elevated locomotor activity and impairments in tests of hippocampal-dependent declarative memory including impaired novel object recognition (Pyndt Jorgensen et al., 2014; Rajagopal et al., 2013). Hippocampal sections were prepared from scPCP-treated mice and vehicle-treated control mice and extracellular field potential recordings were made of postsynaptic potentials (fPSPs). Standard 100Hz tetanic stimulation (single train of 100Hz for 1s) induced robust LTP of Schaffer collateral-CA1 synapses in vehicle-treated mice; however, in interleaved experiments in slices from mice treated with scPCP, the magnitude of LTP was significantly lower. At 30-40 minutes after LTP induction, the slope of the fPSP was 148 ± 12%, n = 9 / 3 mice, in slices from vehicle-treated mice, whereas the potentiation in scPCP-treated mice was 110 ± 3% n = 7 / 3 mice (p < 0.01) (Figure 1A-D). To exclude a potential challenge-induced effect of the ketamine used for anesthesia during the preparation of the slices, we performed a separate set of experiments using slices prepared from mice anesthetized by isoflurane. In these recordings we saw a similar impairment of LTP in the scPCP group (172 ± 12% in vehicle, n = 10 / 3 mice and 117 ± 2% in scPCP, n = 10 / 3 mice) demonstrating that the acute exposure to ketamine was not responsible for the altered plasticity in the scPCP group. In order to determine if the LTP deficit was due to an increase in the threshold for induction in slices from scPCP-treated mice, we employed a stronger stimulation protocol to induce LTP (3 trains at 100Hz for 1s). With this longer induction protocol, there was no difference in the magnitude of LTP between vehicle and scPCP-treated mice. At 30-40 minutes after this longer induction, the fPSP slope was 160 ± 10%, n = 8 / 3 mice, in vehicle-treated mice and 161 ± 13%, n = 7 / 3 mice, (p > 0.05) in scPCP-treated mice (Figure 1E-H). These results suggest scPCP administration results in an increase in the threshold for LTP induction in the hippocampus for periods greater than one week after withdrawal of PCP during a time when there is a severe deficit in recognition memory in the mice (Damgaard et al., 2010).
Figure 1. Impairment of LTP in the hippocampus of scPCP treated mice.
(A) Representative experiment showing timecourse of CA1 LTP for a single recording from vehicle treated mice. LTP was induced at time = 0 with a single trains of 100Hz, 1s tetanic stimulation (arrow). fPSP traces before (1, black) and after (2, red) are shown in the inset above. (B) Timecourse and representative fPSPs recorded in slices from scPCP treated mice. The same induction paradigm failed to produce LTP of a similar magnitude in this recording. Calibration for A & B: 0.2mV, 10ms. (C) Grouped data showing timecourse of LTP from all recordings made from vehicle (black) or scPCP treated (grey) mice (D) Average LTP amplitude measured at 30-40 minutes post-induction. (E & F) LTP induced by longer induction trains (3 x 100Hz, 1s). Representative experiments showing timecourse of CA1 LTP for single recordings from vehicle treated or scPCP treated mice. LTP was induced at time = 0 with three trains of 100Hz, 1s stimulation (arrow). Using this longer stimulation paradigm we did not observe any difference in the magnitude of LTP in the scPCP treated mice. Calibration for E & F: 0.2mV, 10ms. (G & H) Averaged timecourse and magnitude of LTP from all recording from vehicle and scPCP treated mice.
3.2. Basal excitatory synaptic transmission in the hippocampus is not affected in scPCP treated mice
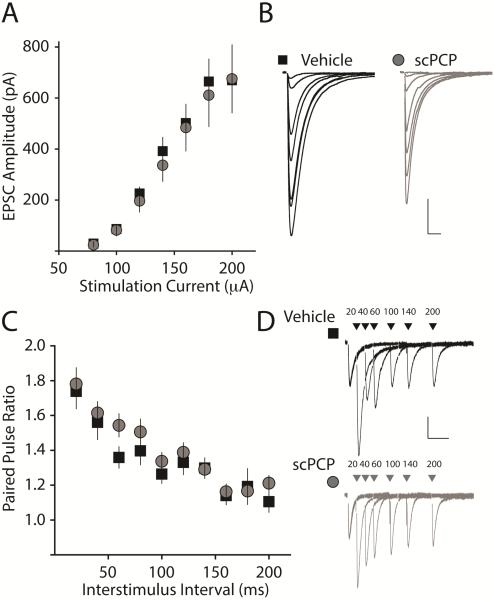
The change in LTP threshold may result from potential alterations in basal synaptic properties of CA1 synapses. To examine whether there were detectable changes in the basal strength of synapses or alterations in short-term plasticity of excitatory synapses on CA1 pyramidal neurons in scPCP-treated mice, we made single-cell recordings in voltage clamp mode. We first compared the input-output (I-O) curve of the excitatory postsynaptic current (EPSC), which is mediated primarily by AMPA receptors at -70mV. Comparisons of EPSC amplitudes at increasing current stimulation intensities demonstrated that there was no significant difference in the size of the EPSC between slices from vehicle and scPCP-treated mice (vehicle: n = 17 / 5 mice: scPCP: n = 15 / 3 mice p > 0.05) (Figure 2 A & B). To determine whether there might be potential changes in release probability or presynaptic short-term plasticity in CA1 synapses after scPCP, we next examined paired-pulse facilitation (PPF) by applying pairs of stimuli separated by inter-stimulus intervals between 20 and 200ms. Comparing PPF in vehicle- and scPCP-treated mice, we found no difference at any of the inter-stimulus intervals tested (vehicle, n = 12 / 3 mice: PCP n = 16 / 3 mice, p > 0.05) (Figure 2 C & D). These results indicate that basal AMPA receptor-mediated excitatory synaptic transmission in CA1 of the hippocampus is not grossly affected after scPCP administration and withdrawal.
Figure 2. AMPAR mediated synaptic currents and short term plasticity is not altered after scPCP treatment.
(A) Input-output (I-O) curve for AMPA receptor mediated EPSCs generated by increasing current used to stimulate Schaffer-collateral inputs to CA1 neurons. No difference was observed in the slope of the curves from vehicle or scPCP treated animals. (B) EPSC traces from single recording made from slices from vehicle treated (black) or scPCP treated (grey) animals. Calibration: 200pA, 20ms. (C) Paired pulse ratio (PPR) of AMPA receptor mediated EPSCs recorded at several interstimulus intervals. There was no difference in the PPR from recordings from vehicle (black squares) or scPCP (grey circles) treated animals. (D) Representative traces from a single experiment showing paired pulse facilitation at all the interstimulus intervals recorded in slices from vehicle or scPCP treated mice. Calibration: 50pA, 100ms.
3.3. NMDAR-mediated synaptic transmission is not altered in the CA1 of PCP injected mice
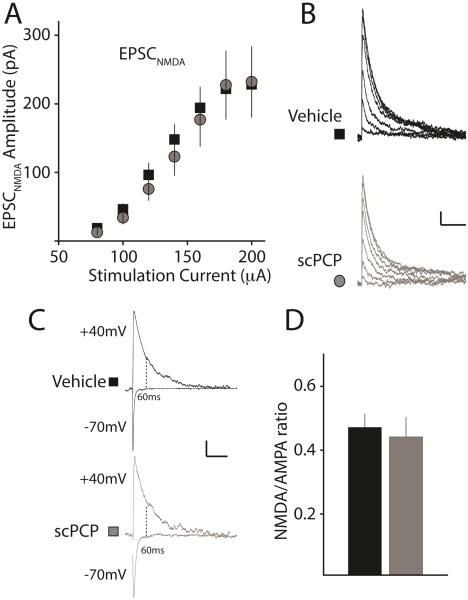
Prior studies have demonstrated that chronic systemic administration of PCP followed by testing the day after withdrawal, results in a significant reduction of the NMDAR-mediated current in pyramidal neurons in the medial prefrontal cortex (mPFC) (Yuen et al., 2012). In our study, we introduced a long delay (greater than one week) between withdrawal and recording to ensure that the drug was not present during the experiments. Nevertheless, it is possible that NMDA receptor function is reduced after washout of PCP due to an adaptive change in the NMDAR-mediated component of excitatory synapses that could contribute to the observed alterations in the ability to induce LTP. To investigate this possibility, we first examined the I-O curve of NMDAR-mediated EPSCs (EPSCNMDA) recorded at +40mV. We did not find any difference in the amplitude of the EPSCNMDA between treatment groups (vehicle: n = 17 / 5 mice; scPCP: n = 15 / 3 mice, p>0.05) (Figure 3 A & B). In addition, comparisons of the NMDA to AMPA (N/A) ratio of EPSCs in CA1 pyramidal neurons demonstrated no differences in slices between vehicle and scPCP-treated animals (Vehicle: 0.47 ± 0.04, n = 18 / 5 mice; scPCP: 0.44 ± 0.06, n = 16 / 3 mice in vehicle and PCP, respectively; p = 0.69) (Figure 3 C & D). These results indicate that scPCP treatment followed by withdrawal does not have an effect on NMDA receptor expression or function at CA1 synapses in hippocampal pyramidal neurons.
Figure 3. NMDAR mediated synaptic current in CA1 is not altered after scPCP treatment.
(A) Input-output (I-O) curve for NMDA receptor mediated EPSCs (EPSCNMDA) recorded at several stimulation intensities isolated in the presence of antagonists of AMPA and GABAA receptors and recorded at +40mV. (B) Representative EPSCNMDA from vehicle (black) or scPCP (grey) treated animals. Calibration: 50pA, 200ms. (C&D) Representative traces and grouped data of NMDA to AMPA ratio recorded from CA1 pyramidal neurons. AMPA receptor mediated currents were measured as the amplitude of the inward current at -70mV and the NMDA receptor current was measured 60ms after the onset of the outward current at +40mV at which point the current is mediated by NMDA receptors (Harlow et al., 2010). Calibration: 50pA, 100ms.
3.4. GABAergic inhibitory transmission is strengthened in the hippocampus of PCP treated mice
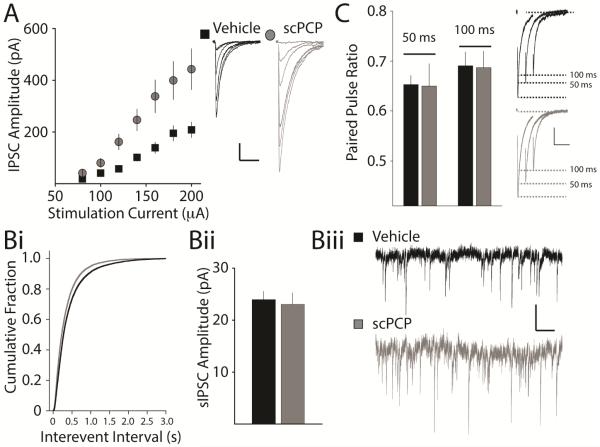
As we did not find evidence that basal glutamatergic signaling was altered after scPCP treatment, we next examined whether inhibitory input to the CA1 neurons was impacted in scPCP-treated mice. We stimulated inputs to CA1 neurons in stratum radiatum and isolated the inhibitory postsynaptic current (IPSC) in the presence of glutamate receptor antagonists. By incrementally changing the current used to stimulate IPSCs, we established I-O curves in slices from vehicle- and scPCP-treated mice. We found that the amplitude of the evoked IPSC was significantly larger across a range of stimulation intensities in scPCP-treated animals (vehicle: n = 18 / 6 mice; scPCP: n = 17 / 4 mice, p < 0.01) (Figure 4A). To further examine inhibitory input to the CA1, we recorded spontaneous IPSCs (sIPSCs) present in pyramidal neurons. In line with the enhancement of inhibitory transmission shown by evoking IPSCs in slices, the frequency of sIPSCs was significantly higher in PCP-treated mice than in vehicle-treated mice (vehicle: 1.95 ± 0.24 Hz, n = 22 / 6 mice; scPCP: 2.97 ± 0.26 Hz; n = 16 / 4 mice, p<0.01) (Figure 4Bi & Biii). Analysis of the amplitudes of sIPSC did not reveal any difference in the size of the sIPSCs between the groups (vehicle: 24.0 ± 1.6 pA, n = 22 / 6 mice; scPCP: 23.1 ± 2.2 pA, n =, 16 / 4 mice, p = 0.74) (Figure 5Bii). As a measure of release probability of inhibitory synapses, we also examined responses to paired stimuli that produce depression of inhibitory transmission. Paired-pulse depression (PPD) of IPSCs at an interstimulus interval of 50ms and 100ms was not different between the two groups (vehicle PPD50: 0.65 ± 0.02, n = 21 / 6 mice; scPCP: PPD50 0.65 ± 0.04, n = 23 / 6 mice, p = 0.93; vehicle PPD100: 0.69 ± 0.03, n = 18 / 6 mice; scPCP PPD100: 0.69 ± 0.03 n = 23 / 6 mice, p=0.92) (Figure 4 C), suggesting release probability of inhibitory synapses is not altered by scPCP administration. We next examined quantal GABA release by measuring miniature IPSCs (mIPSCs) in the presence of tetrodotoxin (TTX, 1μM). The frequency of mIPSC recorded in CA1 neurons was not significantly different in each group (vehicle: 1.94 ± 0.25 Hz, n= 37 / 4 mice; scPCP: 2.40 ± 0.24 Hz n = 32 / 5 mice, p=0.19). However, analysis of the amplitude of mIPSCs uncovered a significantly larger mean amplitude in slices from scPCP-treated mice (vehicle: 15.7 ± 0.6 pA, n = 37 / 4 mice; scPCP: 19.3 ± 0.9 pA, n = 32 / 5 mice, p < 0.01). These results are consistent with an adaptive and persistent increase in inhibitory neurotransmission in the CA1 of the hippocampus after scPCP treatment and withdrawal.
Figure 4. Enhanced inhibitory synaptic transmission onto CA1 neuron in the hippocampus of scPCP treated mice.
(A) I-O curve for IPSCs recorded in CA1 neurons from vehicle and scPCP treated mice. There is a clear difference in the slope of the two curves indicating enhanced inhibitory input in recordings from scPCP treated mice. Right panel shows representative IPSC traces. Calibration: 50pA, 100ms (Bi) Cumulative probability histogram of intervent interval for sIPSCs recorded in CA1 pyramidal neurons. There is a significant increase in frequency after scPCP treatment. (Bii) Average sIPSC amplitude (Biii) Representative traces of sIPSCs from individual recordings from vehicle and scPCP treated mice. Calibration 10pA, 50ms (C) Paired pulse depression of IPSCs recorded at interstimulus intervals of 50ms and 100ms is not altered in scPCP treated mice. Right panel shows representative traces of recordings from vehicle and scPCP treated mice. Calibration: 200pA, 100ms.
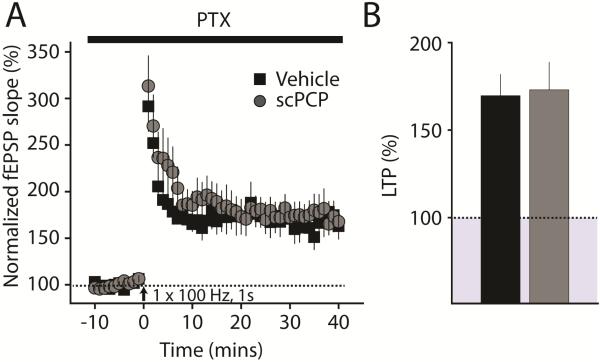
Figure 5. CA1 LTP can be normally induced by shorter trains in a disinhibited slice.
(A) Timecourse of LTP induced by a single 1s train at 100Hz in a disinhibited slice. (B) Average amplitude of LTP in both groups. LTP in both vehicle and scPCP treated animals is of normal amplitude when GABAA receptors are blocked by picrotoxin (50μM PTX).
3.5. Induction of LTP is normalized in a disinhibited slice
The finding that the threshold for LTP induction is elevated and inhibitory input to CA1 neurons is increased, suggests that elevated inhibitory input is causal in modifying the LTP threshold in slices from scPCP-treated mice. In order to test this directly, we blocked all fast GABAergic neurotransmission in our hippocampal slices using the GABAA receptor antagonist picrotoxin (PTX, 50μM) and repeated the LTP experiments in scPCP-treated animals. Using the single train of 100Hz stimulation for 1s in vehicle-treated mice, we obtained large amplitude LTP (in comparison to slices in which inhibition was intact) (170 ± 12%, n = 6 / 4 mice). This was not significantly different from the magnitude of LTP obtained in scPCP-treated mice (173 ± 16%, n = 8 / 5 mice, p = 0.87) (Figure 5). Therefore, the deficit in LTP induction in slices from scPCP-treated mice is completely abolished when inhibitory neurotransmission is blocked, demonstrating that upregulation of inhibitory input to CA1 neurons likely underlies the elevated LTP threshold in the hippocampus.
4. Discussion
Administration of NMDA receptor antagonists such as ketamine, MK-801, and PCP have long-lasting effects on cognitive function and memory in man, non-human primates and rodents. However, there is limited information as to how these altered behaviors are manifest at the synaptic level. Here, we made ex-vivo recordings from adult mice that had undergone repeated PCP administration that results in a severe deficit in recognition memory. We found that the threshold for LTP induction was elevated in hippocampal slices from these mice. The deficits in LTP induction were not a result of alterations in basal glutamatergic synaptic transmission, but were, instead, correlated with an increase in inhibitory GABA input to CA1 pyramidal neurons. LTP could be normally induced in disinhibited slices from scPCP treated mice, strongly suggesting that the increased inhibitory input to CA1 resulted in an elevated threshold for LTP in the hippocampus. PCP administration has been demonstrated to have an effect on cognitive and memory function including long-lasting effects on hippocampal dependent behaviors. However, it has not been clear whether there are long-lasting changes in hippocampal plasticity or synaptic transmission that contribute to the altered behaviors. This study is the first to uncover an adaptive synaptic change in the hippocampus that potentially underlies the long-lasting impairments in hippocampal dependent memory, including recognition memory, that are present after scPCP treatment (Rajagopal et al., 2013).
4.1. LTP of excitatory synapses is altered without changes in basal synaptic properties
We demonstrated that hippocampal LTP is significantly impaired after scPCP treatment. Repeated administration of NMDA receptor antagonists has been used extensively to create animal models of cognitive impairment, because the alterations in behavior produced by these agents are similar to the cognitive dysfunction and psychosis in schizophrenia patients (Wiescholleck and Manahan-Vaughan, 2013a). Prior studies have demonstrated that the NMDA receptor component of excitatory synapses in layer V neurons in the mPFC are reduced after PCP, although in that study recordings were made only one day following PCP withdrawal (Yuen et al., 2012). We did not find a similar reduction in NMDA receptor synaptic currents in hippocampal neurons, which could reflect regional or species differences in the effects of scPCP, or may be due to the different timepoints when measurements were made. Similarly we did not find evidence of a change in AMPA receptor-mediated currents in CA1 synapses. This would suggest that there are no major alterations in excitatory synapses caused by scPCP that could account for a change in the LTP threshold in the hippocampus.
4.2. Enhancement of GABAergic inhibitory synaptic transmission to CA1 pyramidal neurons
Our recordings provide evidence of a robust increase in inhibitory synaptic transmission to the CA1 neurons in the mouse hippocampus following scPCP and washout. This seems to be contrary to the lines of evidence that have demonstrated that parvalbumin immunoreactivity is reduced in the cortex of individuals with schizophrenia and which have been interpreted to indicate a general reduction in inhibitory networks in the cortex of human patients (Reynolds et al., 2004; Zhang and Reynolds, 2002). However, postmortem examination of human tissue allows only measurement of markers of interneurons such as parvalbumin or glutamate decarboxylase (GAD)(Hashimoto et al., 2008) and do not provide a direct measure of alteration in function. In animal models too, including the scPCP model, it has been reported that there is reduced parvalbumin expression in the cortex and the hippocampus (Abdul-Monim et al., 2007; Behrens et al., 2008; Jenkins et al., 2010; McKibben et al., 2010; Wang et al., 2008). More recently, it has been demonstrated that postnatal administration of PCP in rodents causes a reduction in expression of parvalbumin protein without a reduction in the number of interneurons (Kaalund et al., 2013; Powell et al., 2012). Furthermore, an elevated density of cFOS positive interneurons in the hippocampus after repeated NMDA receptor antagonist treatment suggests that there is increased activity of hippocampal interneurons in these models (Bird et al., 1978; Keilhoff et al., 2004; Kjaerby et al., 2013). A recent study using the neonatal MK-801 model found a reduction in the expression of an important K+ channel, and a reduction in spike latency of fast spiking interneurons in the cortex, suggesting that maturation of interneurons is altered after NMDA receptor antagonist treatment (Jones et al., 2014). Likewise exposure to NMDA receptors antagonist during periadolescence has demonstrated sensitivity of GABAergic circuits to altered maturation at times beyond the perinatal period (Thomases et al., 2013). In future work it will be important to directly determine how the intrinsic activity of interneurons in multiple brain areas are affected in these mouse models of CIAS.
Our studies are the first to directly measure functional changes in inhibitory neurotransmission in the hippocampus after scPCP administration. The finding that the frequency of sIPSCs is elevated after scPCP is in line with the interpretation that there are long-lasting increases in interneuron activity or excitability. This is also consistent with the finding that the input-output relationship of evoked IPSCs is increased in slices from scPCP treated mice, since this can be interpreted as the increase of the number of the inhibitory inputs to pyramidal neurons which can be recruited by increasing the intensity of afferent stimulation. There do not appear to be any significant changes in release probability of inhibitory synapses as measured by paired pulse ratio. However, we found that the amplitude of mIPSC was higher suggesting that the strength of individual synapses (i.e. the number of GABAA receptor per synapse) is elevated which is also consistent with the interpretation that inhibitory transmission is enhanced after scPCP. The analysis of mIPSC frequency did not find any differences between the treatment groups supporting the interpretation that the release probability at individual inhibitory synapses is unaltered. Further anatomical analysis will be required to determine directly whether there are changes in the number of inhibitory synapses onto CA1 neurons, as analysis of mIPSCs is not a sensitive or direct measure of this parameter. Taken together the current data are the first to directly demonstrate that there is an adaptive increase in inhibitory transmission to the CA1 region of the hippocampus likely due to an increase in interneuron excitability and / or enhanced inhibitory inputs to pyramidal neurons. The balance between excitation and inhibition is critical to many aspects of brain function. Inhibition not only controls the threshold for induction of synaptic plasticity as we have found, but also regulates synchronous oscillatory activity, which is the basis for cognition in cortical and hippocampal networks, and is hypothesized to be disrupted in individuals with schizophrenia (Gonzalez-Burgos et al., 2010).
4.3. Increase in LTP threshold results from increased inhibition in CA1
The finding most relevant to the known effects of scPCP on hippocampal-dependent memory is that the threshold for LTP is increased in CA1 of the hippocampus. Plasticity of excitatory synapses, particularly LTP and LTD (long term depression), has long been recognized as important to the cellular underpinnings of learning and memory. Therefore, alterations in neural circuits that affect the ability of synapses to undergo LTP will affect the ability of the circuit to respond when memory formation or recall is required. In this case the adaptive changes in inhibition were directly related to the increase in LTP threshold. We were able to demonstrate this by a further set of experiments in which we blocked all inhibition in slices using a GABAA receptor antagonist. In these experiments, we found that the stimulus that had previously been subthreshold in slices from scPCP-treated mice produced normal amplitude LTP, with no difference between the magnitude of LTP in this and the vehicle group. These results strongly suggest that a maladaptive elevation in inhibition after scPCP treatment can act as a metaplastic switch, which can affect cognitive processes and potentially contribute to the aberrant behavior in this animal model of CIAS.
Highlights.
Repeated administration of PCP (scPCP) causes a long lasting increase in the threshold for LTP.
Basal excitatory synaptic transmission is not affected in scPCP treated mice.
Inhibitory synaptic input to CA1 neurons is enhanced in scPCP treated mice.
Enhanced GABAergic input to the CA1 underlies the increased LTP threshold.
Acknowledgements
This work was funded by grants from NIH/NIMH (R01MH099114) and the McKnight Foundation (to AC), and grants from Sumitomo Dainippon Pharma and the Weisman family (to HM). TN was funded by fellowships from The Japan Society for the Promotion of Science and the Nakayama Foundation . PCP was supplied by the National Institute on Drug Abuse. We thank Mr Stephen Kraniotis for technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure:
The authors have no conflicts of interest to declare.
References
- Abdul-Monim Z, Neill JC, Reynolds GP. Sub-chronic psychotomimetic phencyclidine induces deficits in reversal learning and alterations in parvalbumin-immunoreactive expression in the rat. J Psychopharmacol. 2007;21:198–205. doi: 10.1177/0269881107067097. [DOI] [PubMed] [Google Scholar]
- Andersen JD, Pouzet B. Spatial memory deficits induced by perinatal treatment of rats with PCP and reversal effect of D-serine. Neuropsychopharmacology. 2004;29:1080–1090. doi: 10.1038/sj.npp.1300394. [DOI] [PubMed] [Google Scholar]
- Behrens MM, Ali SS, Dugan LL. Interleukin-6 mediates the increase in NADPH-oxidase in the ketamine model of schizophrenia. J Neurosci. 2008;28:13957–13966. doi: 10.1523/JNEUROSCI.4457-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird ED, Spokes EG, Barnes J, Mackay AV, Iversen LL, Shepherd M. Glutamic-acid decarboxylase in schizophrenia. Lancet. 1978;1:156. doi: 10.1016/s0140-6736(78)90455-5. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Basu A, Benneyworth M, Balu D, Konopaske G. Glutamatergic synaptic dysregulation in schizophrenia: therapeutic implications. Handb Exp Pharmacol. 2012:267–295. doi: 10.1007/978-3-642-25758-2_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damgaard T, Larsen DB, Hansen SL, Grayson B, Neill JC, Plath N. Positive modulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors reverses sub-chronic PCP-induced deficits in the novel object recognition task in rats. Behav Brain Res. 2010;207:144–150. doi: 10.1016/j.bbr.2009.09.048. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Hashimoto T, Lewis DA. Alterations of cortical GABA neurons and network oscillations in schizophrenia. Curr Psychiatry Rep. 2010;12:335–344. doi: 10.1007/s11920-010-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Hall J, Trent S, Thomas KL, O'Donovan MC, Owen MJ. Genetic Risk for Schizophrenia: Convergence on Synaptic Pathways Involved in Plasticity. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.07.011. [DOI] [PubMed] [Google Scholar]
- Harlow EG, Till SM, Russell TA, Wijetunge LS, Kind P, Contractor A. Critical period plasticity is disrupted in the barrel cortex of FMR1 knockout mice. Neuron. 2010;65:385–398. doi: 10.1016/j.neuron.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW, Mirnics K, Lewis DA. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus. 2001;11:520–528. doi: 10.1002/hipo.1068. [DOI] [PubMed] [Google Scholar]
- Horiguchi M, Huang M, Meltzer HY. Interaction of mGlu2/3 agonism with clozapine and lurasidone to restore novel object recognition in subchronic phencyclidine-treated rats. Psychopharmacology (Berl) 2011a;217:13–24. doi: 10.1007/s00213-011-2251-2. [DOI] [PubMed] [Google Scholar]
- Horiguchi M, Huang M, Meltzer HY. The role of 5-hydroxytryptamine 7 receptors in the phencyclidine-induced novel object recognition deficit in rats. J Pharmacol Exp Ther. 2011b;338:605–614. doi: 10.1124/jpet.111.180638. [DOI] [PubMed] [Google Scholar]
- Jaaro-Peled H, Ayhan Y, Pletnikov MV, Sawa A. Review of pathological hallmarks of schizophrenia: comparison of genetic models with patients and nongenetic models. Schizophr Bull. 2010;36:301–313. doi: 10.1093/schbul/sbp133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Balla A, Burch S, Suckow R, Xie S, Sershen H. Reversal of phencyclidine-induced dopaminergic dysregulation by N-methyl-D-aspartate receptor/glycine- site agonists. Neuropsychopharmacology. 2004;29:300–307. doi: 10.1038/sj.npp.1300313. [DOI] [PubMed] [Google Scholar]
- Jenkins TA, Harte MK, McKibben CE, Elliott JJ, Reynolds GP. Disturbances in social interaction occur along with pathophysiological deficits following sub-chronic phencyclidine administration in the rat. Behav Brain Res. 2008;194:230–235. doi: 10.1016/j.bbr.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Jenkins TA, Harte MK, Reynolds GP. Effect of subchronic phencyclidine administration on sucrose preference and hippocampal parvalbumin immunoreactivity in the rat. Neurosci Lett. 2010;471:144–147. doi: 10.1016/j.neulet.2010.01.028. [DOI] [PubMed] [Google Scholar]
- Jones KS, Corbin JG, Huntsman MM. Neonatal NMDA Receptor Blockade Disrupts Spike Timing and Glutamatergic Synapses in Fast Spiking Interneurons in a NMDA Receptor Hypofunction Model of Schizophrenia. PLoS One. 2014;9:e109303. doi: 10.1371/journal.pone.0109303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaalund SS, Riise J, Broberg BV, Fabricius K, Karlsen AS, Secher T, Plath N, Pakkenberg B. Differential expression of parvalbumin in neonatal phencyclidine-treated rats and socially isolated rats. J Neurochem. 2013;124:548–557. doi: 10.1111/jnc.12061. [DOI] [PubMed] [Google Scholar]
- Keilhoff G, Becker A, Grecksch G, Wolf G, Bernstein HG. Repeated application of ketamine to rats induces changes in the hippocampal expression of parvalbumin, neuronal nitric oxide synthase and cFOS similar to those found in human schizophrenia. Neuroscience. 2004;126:591–598. doi: 10.1016/j.neuroscience.2004.03.039. [DOI] [PubMed] [Google Scholar]
- Kjaerby C, Broberg BV, Kristiansen U, Dalby NO. Impaired GABAergic Inhibition in the Prefrontal Cortex of Early Postnatal Phencyclidine (PCP)-Treated Rats. Cereb Cortex. 2013 doi: 10.1093/cercor/bht109. [DOI] [PubMed] [Google Scholar]
- Kraguljac NV, White DM, Hadley J, Reid MA, Lahti AC. Hippocampal-parietal dysconnectivity and glutamate abnormalities in unmedicated patients with schizophrenia. Hippocampus. 2014 doi: 10.1002/hipo.22332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKibben CE, Jenkins TA, Adams HN, Harte MK, Reynolds GP. Effect of pretreatment with risperidone on phencyclidine-induced disruptions in object recognition memory and prefrontal cortex parvalbumin immunoreactivity in the rat. Behav Brain Res. 2010;208:132–136. doi: 10.1016/j.bbr.2009.11.018. [DOI] [PubMed] [Google Scholar]
- McLean SL, Idris NF, Woolley ML, Neill JC. D(1)-like receptor activation improves PCP-induced cognitive deficits in animal models: Implications for mechanisms of improved cognitive function in schizophrenia. Eur Neuropsychopharmacol. 2009;19:440–450. doi: 10.1016/j.euroneuro.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Rajagopal L, Huang M, Oyamada Y, Kwon S, Horiguchi M. Translating the N-methyl-D-aspartate receptor antagonist model of schizophrenia to treatments for cognitive impairment in schizophrenia. Int J Neuropsychopharmacol. 2013;16:2181–2194. doi: 10.1017/S1461145713000928. [DOI] [PubMed] [Google Scholar]
- Neill JC, Barnes S, Cook S, Grayson B, Idris NF, McLean SL, Snigdha S, Rajagopal L, Harte MK. Animal models of cognitive dysfunction and negative symptoms of schizophrenia: focus on NMDA receptor antagonism. Pharmacol Ther. 2010;128:419–432. doi: 10.1016/j.pharmthera.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Perry W, Light GA, Davis H, Braff DL. Schizophrenia patients demonstrate a dissociation on declarative and non-declarative memory tests. Schizophr Res. 2000;46:167–174. doi: 10.1016/s0920-9964(99)00229-7. [DOI] [PubMed] [Google Scholar]
- Powell SB, Sejnowski TJ, Behrens MM. Behavioral and neurochemical consequences of cortical oxidative stress on parvalbumin-interneuron maturation in rodent models of schizophrenia. Neuropharmacology. 2012;62:1322–1331. doi: 10.1016/j.neuropharm.2011.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyndt Jorgensen B, Krych L, Pedersen TB, Plath N, Redrobe JP, Hansen AK, Nielsen DS, Pedersen CS, Larsen C, Sorensen DB. Investigating the Long-term Effect of Subchronic Phencyclidine-treatment on Novel Object Recognition and the Association between the Gut Microbiota and Behavior in this Animal Model of Schizophrenia. Physiol Behav. 2014 doi: 10.1016/j.physbeh.2014.12.042. [DOI] [PubMed] [Google Scholar]
- Rajagopal L, Massey BW, Huang M, Oyamada Y, Meltzer HY. The Novel Object Recogniton Test in Rodents in Relation to Cognitive Impairment in Schizophrenia. Curr Pharm Des. 2013 doi: 10.2174/1381612819666131216114240. [DOI] [PubMed] [Google Scholar]
- Rasetti R, Mattay VS, White MG, Sambataro F, Podell JE, Zoltick B, Chen Q, Berman KF, Callicott JH, Weinberger DR. Altered hippocampal-parahippocampal function during stimulus encoding: a potential indicator of genetic liability for schizophrenia. JAMA Psychiatry. 2014;71:236–247. doi: 10.1001/jamapsychiatry.2013.3911. [DOI] [PubMed] [Google Scholar]
- Reynolds GP, Abdul-Monim Z, Neill JC, Zhang ZJ. Calcium binding protein markers of GABA deficits in schizophrenia--postmortem studies and animal models. Neurotox Res. 2004;6:57–61. doi: 10.1007/BF03033297. [DOI] [PubMed] [Google Scholar]
- Sawa A, Snyder SH. Schizophrenia: diverse approaches to a complex disease. Science. 2002;296:692–695. doi: 10.1126/science.1070532. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Gur RC, Gur RE, Mozley PD, Mozley LH, Resnick SM, Kester DB, Stafiniak P. Neuropsychological function in schizophrenia. Selective impairment in memory and learning. Arch Gen Psychiatry. 1991;48:618–624. doi: 10.1001/archpsyc.1991.01810310036007. [DOI] [PubMed] [Google Scholar]
- Siegel SJ, Talpos JC, Geyer MA. Animal models and measures of perceptual processing in schizophrenia. Neurosci Biobehav Rev. 2013;37:2092–2098. doi: 10.1016/j.neubiorev.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snigdha S, Horiguchi M, Huang M, Li Z, Shahid M, Neill JC, Meltzer HY. Attenuation of phencyclidine-induced object recognition deficits by the combination of atypical antipsychotic drugs and pimavanserin (ACP 103), a 5-hydroxytryptamine(2A) receptor inverse agonist. J Pharmacol Exp Ther. 2010;332:622–631. doi: 10.1124/jpet.109.156349. [DOI] [PubMed] [Google Scholar]
- Thomases DR, Cass DK, Meyer JD, Caballero A, Tseng KY. Early adolescent MK-801 exposure impairs the maturation of ventral hippocampal control of basolateral amygdala drive in the adult prefrontal cortex. J Neurosci. 2014;34:9059–9066. doi: 10.1523/JNEUROSCI.1395-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomases DR, Cass DK, Tseng KY. Periadolescent exposure to the NMDA receptor antagonist MK-801 impairs the functional maturation of local GABAergic circuits in the adult prefrontal cortex. J Neurosci. 2013;33:26–34. doi: 10.1523/JNEUROSCI.4147-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, Yang SF, Xia Y, Johnson KM. Postnatal phencyclidine administration selectively reduces adult cortical parvalbumin-containing interneurons. Neuropsychopharmacology. 2008;33:2442–2455. doi: 10.1038/sj.npp.1301647. [DOI] [PubMed] [Google Scholar]
- Wiescholleck V, Manahan-Vaughan D. Long-lasting changes in hippocampal synaptic plasticity and cognition in an animal model of NMDA receptor dysfunction in psychosis. Neuropharmacology. 2013a;74:48–58. doi: 10.1016/j.neuropharm.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Wiescholleck V, Manahan-Vaughan D. Persistent deficits in hippocampal synaptic plasticity accompany losses of hippocampus-dependent memory in a rodent model of psychosis. Front Integr Neurosci. 2013b;7:12. doi: 10.3389/fnint.2013.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Powell SB, Geyer MA. Mouse pharmacological models of cognitive disruption relevant to schizophrenia. Neuropharmacology. 2012;62:1381–1390. doi: 10.1016/j.neuropharm.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Powell SB, Risbrough V, Marston HM, Geyer MA. Using the MATRICS to guide development of a preclinical cognitive test battery for research in schizophrenia. Pharmacol Ther. 2009;122:150–202. doi: 10.1016/j.pharmthera.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Li X, Wei J, Horiguchi M, Meltzer HY, Yan Z. The novel antipsychotic drug lurasidone enhances N-methyl-D-aspartate receptor-mediated synaptic responses. Mol Pharmacol. 2012;81:113–119. doi: 10.1124/mol.111.076141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZJ, Reynolds GP. A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophr Res. 2002;55:1–10. doi: 10.1016/s0920-9964(01)00188-8. [DOI] [PubMed] [Google Scholar]