Abstract
OBJECTIVE
Renal cell carcinoma with sarcomatoid dedifferentiation(sRCC) is associated with higher stage of presentation and worse survival. The objective of this study was to examine the clinicopathological characteristics associated with overall survival(OS), specifically examining the percentage of sarcomatoid component(PSC).
METHODS
We reviewed clinicopathologic data for all nephrectomized patients with confirmed sRCC. Histologic slides were re-reviewed by dedicated GU pathologists to ascertain PSC. Patient characteristics were tabulated overall and by disease stage. Cutpoints in the PSC providing a meaningful difference in OS were identified by recursive partitioning analysis(RPA). Factors selected included age group, gender, race, clinical stage, tumor histology, presurgical systemic therapy, lymphovascular invasion, and tumor size. Kaplan-Meier method and log-rank test were used to assess differences in OS.
RESULTS
Among 186 patients with sRCC, 64(34%) had localized, and 122(66%) had metastatic disease at presentation. Patients had primarily clear cell histology(73%). Median follow-up was 12.1 months(range 0.1–242.2 months). Median OS was 12.6 months (95%CI 10.7–14.9 months). Univariate RPA identified a PSC cutpoint of 10% as prognostically significant. Patients with PSC>10% were at higher risk of death compared to patients with ≤10%(45% vs. 61% 1-year OS;P=0.04). Multivariate RPA revealed that tumor size, presence of metastatic disease, and PSC were significantly associated with OS. Among 4 identified groups, patients with localized disease and tumor size ≤10cm were most likely to be alive at 1 year(89%), and patients with metastatic disease and PSC>40% were least likely to be alive at 1 year(28%;p<0.001).
CONCLUSION
PSC appears to be a prognostic factor in patients with sRCC, with larger percentage of involvement portending a worse survival, especially in patients with metastatic disease.
Keywords: Sarcomatoid, Renal Cell Carcinoma, Percentage, Nephrectomy, Recursive Partitioning Analysis
1. INTRODUCTION
Renal cell carcinoma with sarcomatoid dedifferentiation (sRCC) is characterized by malignant spindle cells, similar to those present in sarcomas, within a background of epithelioid cells of renal cell carcinoma. First described by Farrow and colleagues in 1968, [1] it was initially thought to represent a distinct entity of renal neoplasms. However, current research hypothesizes a common pathway through which sarcomatoid dedifferentiation occurs.[2–4] Up to 10% of renal cell carcinomas are estimated to contain sarcomatoid features and clinically, the presence of sarcomatoid elements is associated with tumor aggressiveness.[5]
As the biology of sRCC is being actively elucidated in the laboratory, the clinical implications are still being investigated. Specifically, the presence of sarcomatoid elements is associated with higher stage at presentation, aggressive disease course, and decreased patient survival, both in the localized and metastatic settings.[6–9] Although there have been multiple reports of various chemotherapeutic regimens in the literature, the response rates have been modest at best.[10, 11] Recently, several studies have reported the use of systemic targeted therapy in sRCC patients, however, response rates varied between 0% to 15.8% with no statistically significant differences between targeted agents and chemotherapy, indicating a need for better risk stratification.[12, 13] Despite these data, a correlation of pathological characteristics with prognosis has been performed in only a limited number of studies. Among these pathologic characteristics, the PSC could potentially be an important prognostic indicator for patients both in the localized and metastatic setting. However, there has been no statistically-established threshold in the literature indicating what PSC cutpoint may portend worse outcomes. In addition, reports have indicated that the PSC may in turn directly determine the responsiveness to certain anti-angiogenic, immunotherapeutic, or chemotherapeutic targets.[11, 12]
The objective of this study was to specifically examine the effect of PSC on overall survival in a large cohort of sRCC patients.
2. PATIENTS AND METHODS
2.1 Patient Selection & Clinical Review
We retrospectively reviewed clinicopathologic data for all nephrectomized patients with pathologically confirmed sRCC from 1987–2011 with institutional board review approval. Our database contained information on 273 patients who were identified as having sRCC. Patients who were lost to follow-up or are currently participating in an unreported clinical trial were excluded. Complete clinical and pathologic data were available for 230 patients who underwent nephrectomy and had sRCC in their primary nephrectomy specimen. Among 230 patients, 186 patients with full histologic slides available for re-review by dedicated GU pathologists were identified and included in the current study. Patient characteristics, including age, gender, and ethnicity were collected. TNM stage was assigned according to the 2009 AJCC classification.[14] Tumor size was defined as the greatest tumor diameter based on evaluation of the pathological specimen. In cases of multifocal disease the largest tumor size was used for statistical analysis. Patients were treated with systemic therapy (presurgical or salvage) at the discretion of the treating GU medical oncologist (included targeted therapy, cytotoxic chemotherapy, immunotherapy, or combinations thereof, since sRCC has no standard therapy at present).
2.2 Pathological Evaluation
All available H&E stained slides of tumor samples from the resected specimens were reviewed by dedicated genitourinary pathologists who were blinded to patient outcomes. Pathologic characteristics including stage, histology, presence of lymphovascular invasion, necrosis, tumor size and PSC were collected. Classification of RCC subtypes and presence of sarcomatoid components were based on the ISUP grading system for RCC.[15] The percentages of epithelioid and sarcomatoid histologic components were determined based on evaluation of morphologic features of the tumor cells on H&E stained slides. The epithelioid component was comprised of cohesive tumor cells of variable nuclear grade with a rounded shape and an architecture and cytology compatible with the underlying histological subtype of renal carcinoma. The sarcomatoid component showed tumor cells that were spindled in shape, of high nuclear grade, less cohesive and with architectural and cytological features reminiscent of sarcoma. The PSC represented the proportion of tumor with sarcomatoid histology divided over the total tumor (sarcomatoid+epithelioid). Assessment of PSC was done in 5% increments. There was adequate representative sampling of all kidney tumors, including grossly different tumor areas, with at least 1 section taken per centimeter of tumor diameter.
2.3 Statistical Analysis
Patient characteristics were tabulated overall and by clinical disease status at time of nephrectomy (metastatic vs. localized). Overall survival(OS) is defined as the time interval between the date of nephrectomy and the date of death. Patients who were alive at the last follow-up date were censored at that time. Cutpoints in the PSC providing a meaningful difference in OS were identified by recursive partitioning analysis(RPA), both as univariate, and multivariate models[16] In a univariate model, that variable is examined for the best split by looking at two-sided p-values from log-rank tests of OS for all possible splits, and selecting the most significant split meeting specified sample size restrictions. In the multivariate model, all variables are examined for the best split in combination, using similar methods. Even though not all variables appear in the diagram (regression tree), the p-values used for splitting are calculated using all variables in the model.[16, 17] Potential factors selected for consideration included age group, gender, race, tumor stage, clinical stage, tumor histology, presurgical systemic therapy, lymphovascular invasion, necrosis, and tumor size. For RPA, the minimum number of patients in any terminal subgroup was set to 20. The log-rank test[18] was applied in each split and splitting was stopped when the p-value for any split was greater than 0.05. Terminal subgroups were compared using log-rank tests, and subgroups with p-values greater than 0.05 were combined. Any missing values were handled using surrogate splits, which utilizes the information of other predictors to impute the missing value.[17] Kaplan-Meier plots[19] were used to present the differences in survival between the groups. RPA was performed in R[The R Foundation for Statistical Computing], the Kaplan-Meier curves were created in Stata 13.1[Stata Corp, College Station, TX], and all other analyses were carried out in SAS 9.3[SAS Institute Inc., Cary, NC].
3. RESULTS
A total of 186 patients with sRCC who underwent nephrectomy between 1987 and 2011 and had the numeric PSC available were identified and included in the current study. Table 1 presents the patient characteristics at the time of diagnosis for all patients and by disease status.
Table 1.
Patient Characteristics
| All Patients | Metastatic | Localized | |
|---|---|---|---|
| Patient Characteristics | N (%) | N (%) | N (%) |
| All | |||
| 186 (100%) | 122 (100%) | 64 (100%) | |
| Age | |||
| <50 | 46 (25%) | 32 (26%) | 14 (22%) |
| 50–69 | 115 (62%) | 81 (66%) | 34 (53%) |
| ≥70 | 25 (13%) | 9 (8%) | 16 (25%) |
| Gender | |||
| Female | 68 (37%) | 45 (37%) | 23 (36%) |
| Male | 118 (63%) | 77 (63%) | 41 (64%) |
| Race/Ethnicity | |||
| Black | 9 (5%) | 5 (4%) | 4 (6%) |
| Hispanic | 28 (15%) | 15 (12%) | 13 (20%) |
| White | 142 (76%) | 97 (80%) | 45 (71%) |
| Other | 7 (4%) | 5 (4%) | 2 (3%) |
| Pathologic Tumor Stage | |||
| T1 and T2 | 35 (19%) | 23 (19%) | 12 (19%) |
| T3 | 119 (63%) | 84 (69%) | 35 (55%) |
| T4 | 29 (16%) | 14 (11%) | 15 (23%) |
| Unknown | 3 (2%) | 1 (1%) | 2 (3%) |
| Clinical N and M Stage | |||
| N0M0 | 64 (34%) | 0 (0%) | 64 (100%) |
| N0M1 | 93 (50%) | 93 (76%) | 0 (0%) |
| N1M0 | 16 (9%) | 16 (13%) | 0 (0%) |
| N1M1 | 13 (7%) | 13 (11%) | 0 (0%) |
| Histology | |||
| Clear Cell | 136 (73%) | 87 (71%) | 49 (77%) |
| Other | 50 (27%) | 35 (29%) | 15 (23%) |
| Lymphovascular Invasion | |||
| Yes | 42 (23%) | 29 (24%) | 13 (20%) |
| No | 142 (76%) | 92 (75%) | 50 (78%) |
| Unknown | 2 (1%) | 1 (1%) | 1 (2%) |
| Necrosis | |||
| Yes | 86 (46%) | 62 (51%) | 24 (38%) |
| No Unknown |
97 (52%) 3 (2%) |
58 (47%) 2 (2%) |
39 (61%) 1 (1%) |
| Presurgical Systemic Therapy | |||
| Yes No |
24 (13%) 162 (87%) |
24 (20%) 98 (80%) |
0 64 (100%) |
| Percentage sarcomatoid – median (IQR) | |||
| N=186 | 25 (5, 60) | 30 (10, 60) | 17.5 (5, 50) |
| Tumor size in cm – median (IQR) | |||
| N=168 | 10 (8, 13.5) | 10 (8.25, 13.55) | 10 (8, 12.5) |
IQR = InterQuartile Range
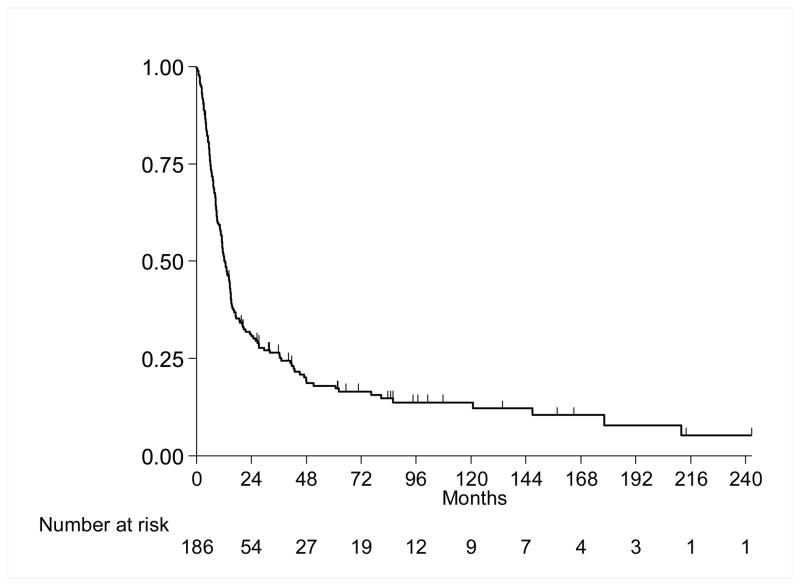
Median follow-up for the entire cohort was 12.1 months (range 0.1–242.2 months). 155(83%) patients died and 31(17%) were alive at last follow-up. Median follow-up for survivors was 58.4 months (range 4–242.2 mo). Figure 1 presents the OS experience for all patients. Median survival was 12.6 months (95% CI 10.7, 14.9 months), and the 1-year OS was 51%(SE=4%).
Figure 1.
Overall Survival
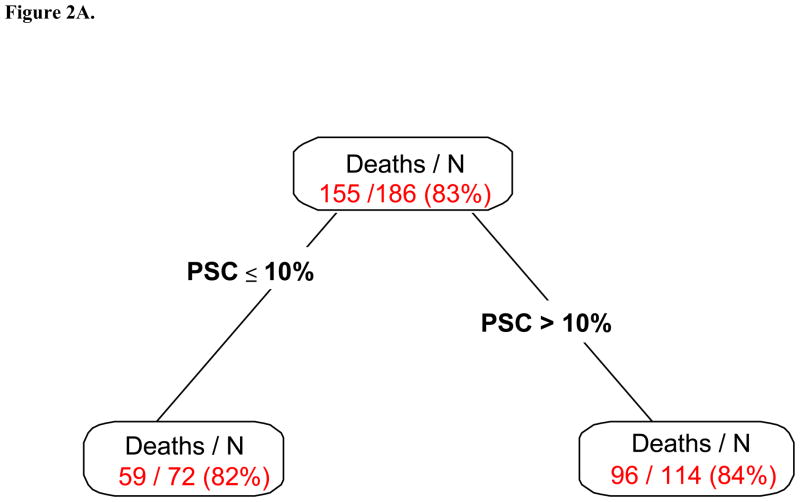
Figure 2A presents the RPA partitions considering PSC only. Two subgroups were identified with a cutpoint of 10% for PSC. The OS comparison between subgroups is summarized in Table 2 and presented in Figure 2B. Patients with PSC >10% were at higher risk of death (45% alive at 1 year) compared to patients with PSC≤10% (61%; p=0.04)
Figure 2.
Figure 2A. Univariate Recursive Partitioning Analysis for Overall Survival. The PSC was split at 10% to distinguish patients with better and worse survival. Patients with higher sarcomatoid percentage were more likely to die. PSC= Percent sarcomatoid component
Figure 2B. Overall Survival by Percent Sarcomatoid; PSC= Percent sarcomatoid component
Table 2.
Overall Survival Estimates by the Risk Groups Identified by Univariate RPA
| Group No. | Category | Deaths/N | Median OS (95% CI) | 1-Year OS (SE) | P-value |
|---|---|---|---|---|---|
| 1 (Low risk) | PSC ≤ 10% | 59/72 | 15.1 (12.2, 36.5) | 61% (6%) | 0.04 |
| 2 (High risk) | PSC > 10% | 96/114 | 11.1 (8.5, 14.2) | 45% (5%) |
RPA=Recursive Partitioning Analysis; PSC=Percent sarcomatoid component; OS=Overall survival
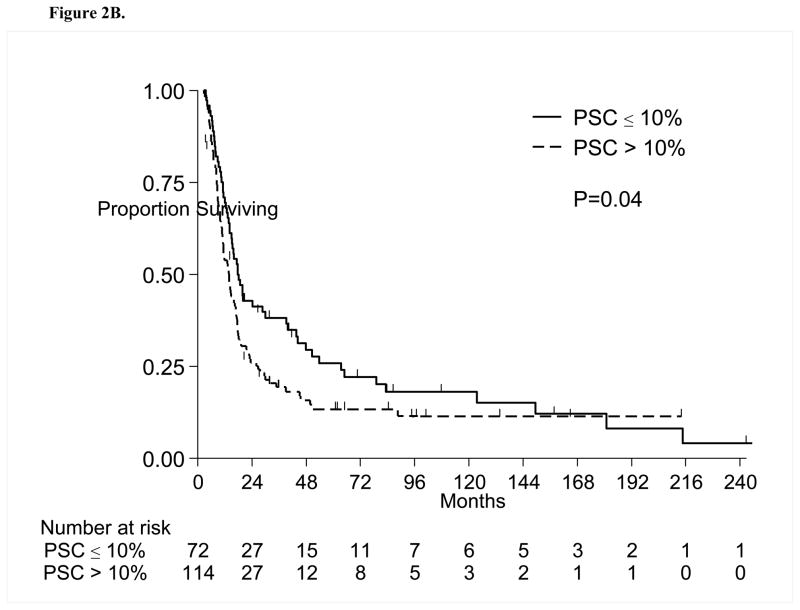
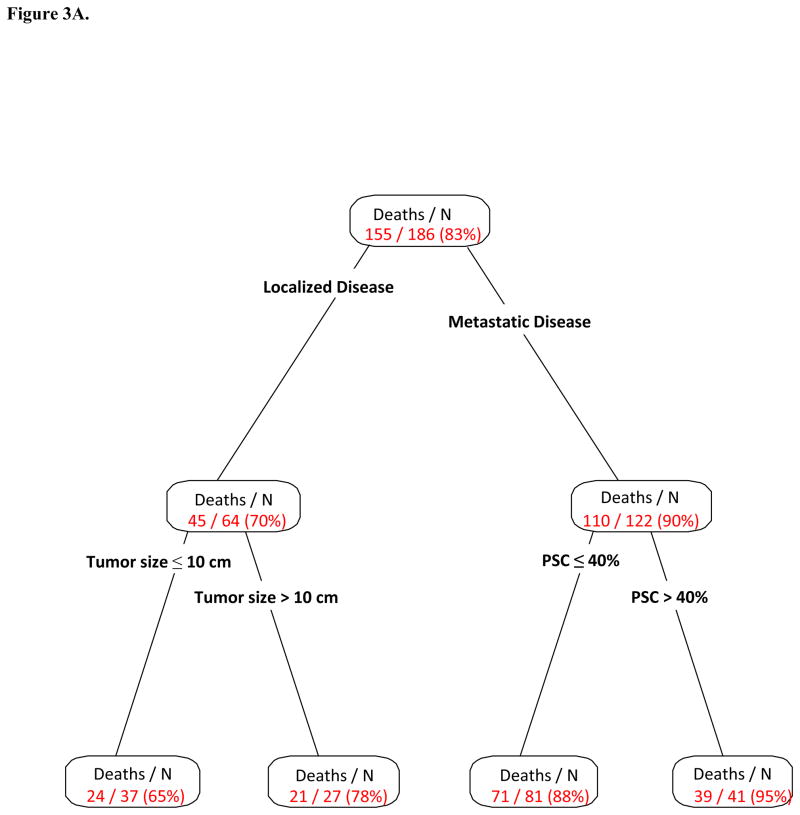
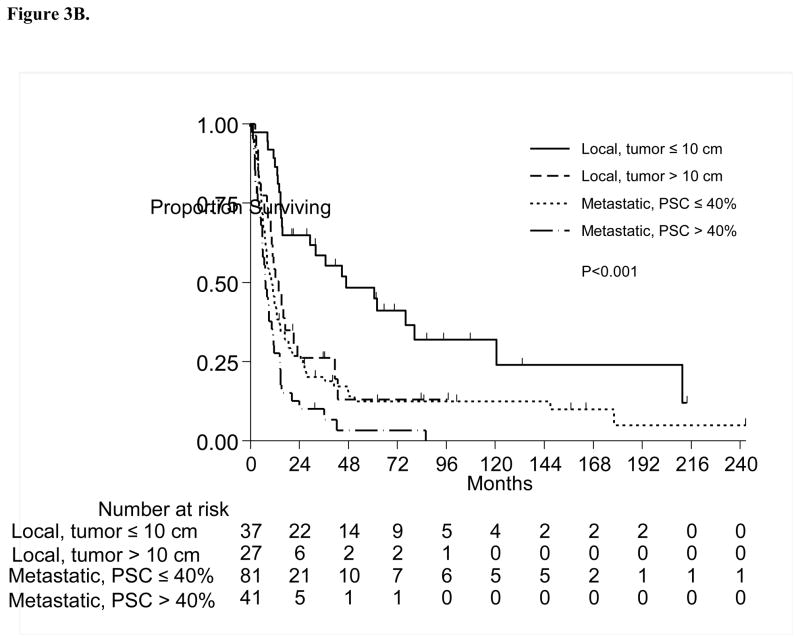
Figure 3A presents the multivariate RPA partitions, including PSC as well as other relevant patient characteristics previously stated. Partitions for clinical stage, tumor size and percent sarcomatoid were associated with OS. From the 186 patients, a group of 64 patients with localized disease was separated from the rest in the first split, then the second split occurred at tumor size ≤10 cm versus >10 cm. For the remaining 122 patients with metastatic disease, the second and last split occurred for those with PSC ≤40% versus all others. Table 3 presents the OS comparison among these subgroups. Patients with localized disease and tumor size ≤10 cm or >10 cm were most likely to be alive at 1 year(89% and 54%, respectively), while patients with metastatic disease and PSC ≤40% or >40% had 1-year OS of 44% vs. 28%, respectively(P<0.001; Figure 3B).
Figure 3.
Figure 3A. Multivariate Recursive Partitioning Analysis for Overall Survival. The primary factor associated with survival was clinical stage (localized versus metastatic). Among patients with localized disease, tumor size was the strongest factor associated with survival. Among patients with metastatic disease, the percentage sarcomatoid was the strongest factor associated with survival. In this setting, having a sarcomatoid percentage more than 40% is associated with the worst survival. PSC= Percent sarcomatoid component
Figure 3B. Overall Survival by Multivariate Recursive Partitioning Analysis Groups; PSC= Percent sarcomatoid component
Table 3.
Overall Survival Estimates by the Risk Groups Identified by Multivariate RPA
| Group | Category | Deaths/N | Median OS (95% CI) | 1-Year OS (SE) | P-value |
|---|---|---|---|---|---|
| 1 (Low risk) | Localized Disease; Tumor size ≤ 10 cm | 24/37 | 47.1 (29.6, NR) | 89% (5%) | <0.001 |
| 2 | Localized Disease; Tumor size >10 cm | 21/27 | 14.2 (10.2, 41.6) | 54% (10%) | |
| 3 | Metastatic Disease; PSC ≤ 40% | 71/81 | 10.6 (8.1, 14.6) | 44% (6%) | |
| 4 (High risk) | Metastatic Disease; PSC > 40% | 39/41 | 7.3 (5.9, 11.2) | 28% (7%) |
RPA= Recursive Partitioning Analysis; OS=Overall Survival; PSC= Percent sarcomatoid component; NR=Not reached
4. DISCUSSION
In this study of 186 patients with localized and metastatic sRCC, we have identified four independent distinct prognostic groups using readily available preoperative and postoperative clinical variables that stratify this cohort of patients from lowest risk to highest risk groups. We have also identified a distinct cutpoint value for the PSC that best distinguishes the difference in OS, and have incorporated this into our overall model for risk stratification. On one hand, patients with localized disease and tumor size ≤10 cm are categorized as being on the lower end of the risk spectrum and have the longest median OS in our cohort. On the other hand, patients with metastatic disease and PSC >40% have the poorest median OS and are considered to be in the highest risk of mortality. Interestingly, patients with localized disease and tumor size >10 cm had similar survival to those with metastatic disease and PSC≤40%. In addition, a cutpoint value of 10% was significant in the entire group of patients when considering PSC alone, with higher PSC in the final pathologic specimen resulting in a significantly worse OS.
To our knowledge, this is the first report to find a distinct cutpoint value for PSC that can risk stratify patients with sRCC. There have been multiple studies that showed that the mere presence of a sarcomatoid component, regardless of the percentage involvement, results in adverse outcomes. A prior study of 108 patients with sRCC from our institution with only 25 patients presenting with localized disease reported a median OS of 9 months for the entire cohort.[20] In addition, stratification of patients based on focal versus extensive PSC(<25% vs. ≥25%) showed slightly longer median survival(9 vs. 12 months) in the focal group, however, this difference did not reach statistical significance.[20] To assess prognostic significance of the PSC, a series by de Peralta and colleagues evaluated 101 sRCC patients with a median follow up of 40 months and divided them into four subsets(<10%, 11–25%, 26–50%, >50%).[21] The only significant subset difference was in the categories of greater than or less than 50% for all pathologic stages. However, only TNM staging remained significant on multivariate analysis. In a similar report by Cheville and colleagues, 102 patients stratified by PSC from 5–10%(27 patients), 10–50%(36 patients), and >50%(39 patients) failed to show a statistically significant difference in both univariate and multivariate analysis with regards to PSC’s ability to independently determine cancer-specific mortality.[5] More recently, Shuch and colleagues demonstrated that PSC was an important predictor of survival on univariate, but not multivariate analysis. Even after assessing PSC by categorization into quartiles or as a continuous variable, neither method could demonstrate a significant association on multivariate analysis, despite approaching statistical significance as a continuous variable(p=0.08).[22] More recently, Kim and colleagues showed that increasing PSC has a significant association with disease specific mortality, although this series only reported on 59 patients with sRCC.[23] All of the above-mentioned series demonstrate that the presence of sarcomatoid component portends a poor outcome, and a few show worsening outcomes to be associated with increased PSC, mostly in univariate but not multivariate analysis. In addition, none of these series identified a distinct independent prognostic threshold level for PSC, with each report either assigning PSC cutpoint by quartiles[22], median[24], or arbitrarily[5, 20, 21]. Zhang and colleagues recently reported the only study to date that showed the amount of sarcomatoid dedifferentiation as an independent prognostic indicator for patients with sRCC. In their study, 204 patients with sRCC were compared with 207 patients with grade 4 RCC with no sarcomatoid features.[24] However, the authors simply chose the median(30%) to assign the PSC cutpoint[24].
Consistent with this and other reports, we were able to identify stage, tumor size, and presence of sarcomatoid component as the most important discriminating clinical factors for determining overall survival in our cohort of patients with localized and metastatic sRCC. However, in contradistinction to previous reports, we then proceeded to identify a potential diagnostic threshold that might best stratify various risk groups with the use of RPA. Also known as Classification and Regression Tree method, RPA is a multivariate analysis model which partitions all variables into homogeneous groups by searching through all possible variables entered to divide groups with the most prognostic differences first, and then repeats this algorithm for each partition(node) until all data have been divided into meaningful subgroups based on the most prognostic variables.[17] The advantage of such an analysis is that it generates a clinically meaningful “RPA tree” that can be used algorithmically for risk stratification of patients with sRCC. A diagnostic threshold of 10% was identified for PSC among the entire cohort during univariate RPA. Also, in our multivariate model, the most powerful discriminative factor was indeed localized versus metastatic disease, which is clinically intuitive, and consistent with previous studies that demonstrate significant differences in these two patient groups[5, 20]. Among patients with localized disease, tumor diameter was recognized as the next most prognostic indicator in this group with a cutpoint of 10 cm. No other variables reached statistically significant thresholds to further stratify this group. Among patients with metastatic disease, a PSC of 40% was found to be the next most discriminative factor, with a difference in OS median of 3.3 months. Our analysis distinguishes the lower risk group of patients with localized sRCC with smaller tumors from the highest risk group, namely patients with metastatic disease and PSC>40%.
Clinical evidence to date points to the fact that the presence of the sarcomatoid component portends a worse outcome in the subset of patients with sRCC, however, characterizing the sarcomatoid component in and of itself as a distinct, biologically aggressive entity has been difficult. It is unclear if the epithelioid component of the RCC histology also contributes to the biologically aggressive nature of the disease, as high grade and low grade components have been identified in both primary and metastatic specimens, in addition to all the various histologic subtypes.[25] Despite the unclear biological nature of the sarcomatoid elements, the PSC has been shown to be a potentially useful clinical indicator for predicting the nature of distant metastases and predicting response to chemotherapeutic or targeted therapy agents. Shuch and colleagues evaluated 32 patients with sRCC and resected metastases.[25] Among the 52 metastatic sites, 58% contained pure sarcomatoid pattern and 38% contained pure epithelioid histology, with only 4% showing mixed pattern. In addition, patients with greater than 30% PSC in the primary tumor were more likely to predict sarcomatoid histology in the metastasis site.[25] On the predictive aspect of PSC, the results of ECOG 8802 demonstrated that higher response rates to doxorubicin-gemcitabine in patients with locally advanced or metastatic sRCC seemed to correlate with higher PSC, [11] while Golshayan and colleagues demonstrated that partial responses in patients with metastatic sRCC treated with targeted therapy were limited to those with less than 20% PSC and clear cell histology.[12] Our study has now added to the body of literature that seems to indicate that PSC plays an important role in sRCC, not only for prediction of response to therapy, but overall outcome from disease.
The limitations of our study include its retrospective nature, its long span of patient inclusion, and the inherent potential for bias in choosing the appropriate surgical candidate for definitive or cytoreductive nephrectomy. Although the calculation of PSC can be inherently subjective, we limited the variability by re-review of all cases by dedicated GU pathologists in a blinded fashion. Additionally, we did not have a sufficient sample size to run the RPA on a training and confirmation set, so confirmation in an independent sample will be the next step in establishing the importance of PSC as more pathologists consistently assign a numeric score. In addition, it would be valuable to externally validate our findings in a larger multi-institutional series.
5. CONCLUSIONS
The PSC appears to be a prognostic factor for OS of patients with sRCC. Patients with localized disease and tumor size less than 10 cm had significantly improved survival compared with patients with metastatic disease and PSC greater than or equal to 40%. Consistent with international guidelines, any sarcomatoid elements present in the nephrectomy specimen should be reported, without the use of any PSC cutpoint to make the diagnosis of sRCC. Given that sRCC is a rare disease entity with significant morbidity and mortality, multi-institutional efforts are needed to better understand the biology and behavior of this aggressive disease, and ultimately find effective therapies and cures.
Table 4.
Summary of the studies investigating the association between PSC cutpoint and outcome of sRCC patients.
| Studies | Number of Patients | Time of Study | Median Follow-up (months) | PSC cutpoint used (Method of PSC cutpoint determination) | Median OS (months) | Remarks |
|---|---|---|---|---|---|---|
| Current study | 186 Localized 64 Metastatic 122 |
1987–2011 | 12.1 (overall) 58.4 (survivors) |
Univariate – 10% Multivariate – 40% for metastatic patients (RPA) |
12.6 | Univariate-PSC 10% cutpoint; Multivariate – Localized vs Metastatic, tumor size, PSC 40% cutpoint |
| De Peralta et al., 2001 [21] | 101 Localized 13 Metastatic 88 |
Not specified | 40 | <10%, 11–25%, 26–50%, >50% (Not specified) | 19 | PSC only significant at 50% cutpoint, only TNM significant on multivariate analysis |
| Mian et al., 2002 [20] | 108 Localized 25 Metastatic 83 |
1987–1998 | 52 (survivors only) | 25% (Not specified) | 9 | |
| Cheville et. al., 2004 [5] | 102 Localized 46 Metastatic 56 |
1970–2000 | 17 (overall) 64.8 (survivors) |
5–10%, 10–50%, >50% (Not specified) | Not specified | PSC not associated with cancer-specific survival |
| Shuch et al., 2012 [22] | 104 Localized 32 Metastatic 72 |
1989–2009 | 12.1 (survivors only) | <25%, 26–50%, 51–75%, >75% (quartiles; continuous) | 5.9 | PSC predictor only on univariate, but not multivariate analysis |
| Kim et al., 2014 [23] | 59 Localized 26 Metastatic 33 |
1999–2012 | 21.5 | ≤25%, >25% (Not specified) | 8.7 | PSC significantly associated with OS |
| Zhang et al., 2014 [24] | 204 Localized 111 Metastatic 93 |
1970–2009 | 20.4 | <30%, ≥30% (median; continuous) | Not specified | PSC significantly associated with cancer-specific survival |
Acknowledgments
Supported by the NIH/NCI under award number P30CA016672 (for the Biostatistics Resource Group).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Farrow GM, Harrison EG, Jr, Utz DC, ReMine WH. Sarcomas and sarcomatoid and mixed malignant tumors of the kidney in adults. I. Cancer. 1968;22:545–50. doi: 10.1002/1097-0142(196809)22:3<545::aid-cncr2820220308>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 2.He H, Magi-Galluzzi C. Epithelial-to-mesenchymal transition in renal neoplasms. Advances in anatomic pathology. 2014;21:174–80. doi: 10.1097/PAP.0000000000000018. [DOI] [PubMed] [Google Scholar]
- 3.Ficarra V, Brunelli M, Cheng L, Kirkali Z, Lopez-Beltran A, Martignoni G, et al. Prognostic and therapeutic impact of the histopathologic definition of parenchymal epithelial renal tumors. European urology. 2010;58:655–68. doi: 10.1016/j.eururo.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Delahunt B. Sarcomatoid renal carcinoma: the final common dedifferentiation pathway of renal epithelial malignancies. Pathology. 1999;31:185–90. doi: 10.1080/003130299104945. [DOI] [PubMed] [Google Scholar]
- 5.Cheville JC, Lohse CM, Zincke H, Weaver AL, Leibovich BC, Frank I, et al. Sarcomatoid renal cell carcinoma: an examination of underlying histologic subtype and an analysis of associations with patient outcome. The American journal of surgical pathology. 2004;28:435–41. doi: 10.1097/00000478-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Brookman-May S, May M, Shariat SF, Zigeuner R, Chromecki T, Cindolo L, et al. Prognostic effect of sarcomatoid dedifferentiation in patients with surgically treated renal cell carcinoma: a matched-pair analysis. Clinical genitourinary cancer. 2013;11:465–70. doi: 10.1016/j.clgc.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 7.Beuselinck B, Lerut E, Wolter P, Dumez H, Berkers J, Van Poppel H, et al. Sarcomatoid Dedifferentiation in Metastatic Clear Cell Renal Cell Carcinoma and Outcome on Treatment With Anti-Vascular Endothelial Growth Factor Receptor Tyrosine Kinase Inhibitors: A Retrospective Analysis. Clinical genitourinary cancer. 2014 doi: 10.1016/j.clgc.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Culp SH, Karam JA, Wood CG. Population-based analysis of factors associated with survival in patients undergoing cytoreductive nephrectomy in the targeted therapy era. Urologic oncology. 2014;32:561–8. doi: 10.1016/j.urolonc.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Merrill MMWC, Tannir NM, Slack RS, Babaian KN, Jonasch E, Pagliaro LC, Compton Z, Tamboli P, Sircar K, Pisters LL, Matin SF, Karam JA. Clinically Non-Metastatic Renal Cell Carcinoma With Sarcomatoid Dedifferentiation: Natural History and Outcomes after Surgical Resection with Curative Intent. Urologic Oncology: Seminars and Original Investigations. 2015 doi: 10.1016/j.urolonc.2014.11.021. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sella A, Logothetis CJ, Ro JY, Swanson DA, Samuels ML. Sarcomatoid renal cell carcinoma. A treatable entity. Cancer. 1987;60:1313–8. doi: 10.1002/1097-0142(19870915)60:6<1313::aid-cncr2820600625>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 11.Haas NB, Lin X, Manola J, Pins M, Liu G, McDermott D, et al. A phase II trial of doxorubicin and gemcitabine in renal cell carcinoma with sarcomatoid features: ECOG 8802. Med Oncol. 2012;29:761–7. doi: 10.1007/s12032-011-9829-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golshayan AR, George S, Heng DY, Elson P, Wood LS, Mekhail TM, et al. Metastatic sarcomatoid renal cell carcinoma treated with vascular endothelial growth factor-targeted therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:235–41. doi: 10.1200/JCO.2008.18.0000. [DOI] [PubMed] [Google Scholar]
- 13.Molina AM, Tickoo SK, Ishill N, Trinos MJ, Schwartz LH, Patil S, et al. Sarcomatoid-variant renal cell carcinoma: treatment outcome and survival in advanced disease. American journal of clinical oncology. 2011;34:454–9. doi: 10.1097/COC.0b013e3181f47aa4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.SBE, Byrd DR. Compton CCea. AJCC Cancer Staging Manual. 7. Springer; New York: 2010. [Google Scholar]
- 15.Delahunt B, Cheville JC, Martignoni G, Humphrey PA, Magi-Galluzzi C, McKenney J, et al. The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. The American journal of surgical pathology. 2013;37:1490–504. doi: 10.1097/PAS.0b013e318299f0fb. [DOI] [PubMed] [Google Scholar]
- 16.Breiman L, Friedman J, Olshen R, et al. Classification and Regression Trees. Wadsworth International Group; Belmont, CA: 1984. [Google Scholar]
- 17.Therneau T, Atkinson E. Technical Report 61. Mayo Clinic, Section of Statistics; 1997. An introduction to recursive partitioning using the RPART routine. [Google Scholar]
- 18.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer chemotherapy reports Part 1. 1966;50:163–70. [PubMed] [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assn. 1958;53:457–81. [Google Scholar]
- 20.Mian BM, Bhadkamkar N, Slaton JW, Pisters PW, Daliani D, Swanson DA, et al. Prognostic factors and survival of patients with sarcomatoid renal cell carcinoma. The Journal of urology. 2002;167:65–70. [PubMed] [Google Scholar]
- 21.de Peralta-Venturina M, Moch H, Amin M, et al. Sarcomatoid Differentiation in Renal Cell Carcinoma A Study of 101 Cases. Am J Path. 2001;25:275–84. doi: 10.1097/00000478-200103000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Shuch B, Bratslavsky G, Shih J, Vourganti S, Finley D, Castor B, et al. Impact of pathological tumour characteristics in patients with sarcomatoid renal cell carcinoma. BJU international. 2012;109:1600–6. doi: 10.1111/j.1464-410X.2011.10785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim T, Zargar-Shoshtari K, Dhillon J, Lin HY, Yue B, Fishman M, et al. Using Percentage of Sarcomatoid Differentiation as a Prognostic Factor in Renal Cell Carcinoma. Clinical genitourinary cancer. 2014 doi: 10.1016/j.clgc.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Zhang BY, Thompson RH, Lohse CM, Leibovich BC, Boorjian SA, Cheville JC, et al. Novel prognostic model for patients with sarcomatoid renal cell carcinoma. BJU international. 2014 doi: 10.1111/bju.12781. [DOI] [PubMed] [Google Scholar]
- 25.Shuch B, Said J, LaRochelle JC, Zhou Y, Li G, Klatte T, et al. Histologic evaluation of metastases in renal cell carcinoma with sarcomatoid transformation and its implications for systemic therapy. Cancer. 2010;116:616–24. doi: 10.1002/cncr.24768. [DOI] [PMC free article] [PubMed] [Google Scholar]