Abstract
BACKGROUND
We report on the long-term results of a phase II study of pre-irradiation temozolomide followed by concurrent temozolomide and radiotherapy (RT) in patients with newly diagnosed anaplastic oligodendroglioma (AO) and mixed anaplastic oligoastrocytoma (MOA).
METHODS
Pre-RT temozolomide was given for up to 6 cycles. RT with concurrent temozolomide was administered to patients with less than a complete radiographic response.
RESULTS
Forty eligible patients were entered and 32 completed protocol treatment. With a median follow-up time of 8.7 years (range: 1.1 to 10.1), median progression-free survival (PFS) is 5.8 years (95% C.I. 2.0, NR) and median overall survival (OS) has not been reached (5.9, NR). 1p/19q data are available in 37 cases; 23 tumors had codeletion while 14 tumors had no loss or loss of only 1p or 19q (non-codeleted). In codeleted patients, 9 patients have progressed and 4 have died; neither median PFS nor OS have been reached and two patients who received only pre-RT temozolomide and no RT have remained progression-free for over 7 years. 3-year PFS and 6-year OS are 78% (95% CI: 61-95%) and 83% (95% CI: 67-98%), respectively. Codeleted patients show a trend towards improved 6-year survival when compared to the codeleted procarbazine/CCNU/vincristrine (PCV) and RT cohort in RTOG 9402 (67%, 95% CI:55-79%). For non-codeleted patients, median PFS and OS are 1.3 and 5.8 years, respectively.
CONCLUSIONS
These updated results suggest that the regimen of dose intense, pre-RT temozolomide followed by concurrent RT/temozolomide has significant activity, particularly in patients with 1p/19q codeleted AOs and MAOs.
Keywords: oligodendroglioma, temozolomide, 1p/19q loss of heterozygosity, MGMT, RTOG
INTRODUCTION
While anaplastic oligodendroglioma (AO) and mixed anaplastic oligoastrocytoma (MOA) represent only about 5% of primary brain tumors, their management has been the subject of multiple prospective clinical trials [1]. Subsets of these tumors have genetic alterations which have been associated with an improved prognosis, and more significantly, an improved response to currently available adjuvant medical therapies [2-4]. One such alteration is loss of heterozygosity (LOH) of chromosomes 1p and 19q, referred to as “codeletion” [5,6] The presence of 1p/19q codeletion, which is likely the result of a chromosomal translocation [7], is associated with radiosensitivity, chemosensitivity, and prolonged survival [5,6,8-11]. Other alterations observed in these tumors include methylation of the MGMT promoter, and mutation of the IDH-1 gene [3,12]. These changes also are associated with an improved prognosis, although their role as predictors of response to treatment remains to be established.
Radiotherapy (RT) has been the most widely used adjuvant therapy; more recently chemotherapy has become a part of the standard treatment of these tumors. The addition of procarbazine, CCNU and vincristine (PCV) chemotherapy, either before or after RT, has been investigated in two large phase III randomized trials (RTOG 9402 and EORTC 26951) [13,14]. Both of these trials recently underwent updated analyses and for the first time it has been demonstrated in codeleted patients that the addition of chemotherapy to adjuvant radiotherapy is associated with an improved OS, thereby establishing the new standard of care for these patients [2,4]. A current conundrum in the neuro-oncology community is whether temozolomide (TMZ) can be substituted for the more toxic PCV regimen, and no prospective data are available to support this.
RTOG BR0131 examined the use of pre-radiotherapy chemotherapy with TMZ followed by RT with concurrent TMZ in patients with newly diagnosed AO and MOA. An initial report on this study showed that this regimen was safe and appeared to demonstrate activity against these tumors [15]. In this report we update the toxicity observations, biomarker evaluations, and the long-term outcomes of patients treated under this protocol.
PATIENTS AND METHODS
Patients aged ≥18 years with a newly diagnosed, supratentorial AO or MOA were eligible. The detailed criteria for anaplasia, and patient eligibility and exclusion were described in a previous publication [15]. Pathology was confirmed by central pathology review. Patients with prior suspected or proven low grade glioma were eligible provided they had a currently biopsy-proven pure or mixed anaplastic oligodendroglioma and had not received either RT or chemotherapy. Women of childbearing potential and sexually active men were required to agree to use a reliable form of birth control. All study centers had institutional review board approval, and all patients signed a study-specific informed consent prior to study entry.
Study Design and Treatment
For the first phase of the study, all protocol-eligible patients were treated with pre-irradiation temozolomide. Within six weeks following the completion of pre-irradiation chemotherapy and the evaluation of response, all patients were re-registered for the second phase of the study (RT plus temozolomide for those patients not achieving a complete response during the first phase of treatment). The treatment details were summarized in a previous publication [15] and the salient details regarding the RT and TMZ treatments are included in the current report.
Pre-irradiation TMZ chemotherapy was administered orally at 150 mg/m2/day on days 1-7 and days 15-21 of a 28-day cycle (7-day on/7-day off). The planned duration of pre-irradiation therapy was 6 cycles, and CT/MRI scans were obtained every 8 weeks. Non-progressing patients (i.e. responders, stable disease or absence of evaluable disease) continued to receive treatment until 2 cycles after maximum response (up to 6 cycles). Temozolomide was discontinued (and RT started) for treatment delays in excess of 8 weeks between cycles, unacceptable toxicity, CT or MRI documented tumor progression, clinical deterioration, which in the judgment of the treating physician was due to disease progression, or patient withdrawal from the study. Dose reductions but not escalations were permitted for toxicity. Patients achieving a radiographic complete response did not proceed to the next phase of treatment, i.e. concomitant chemoradiotherapy and instead underwent observation.
The dose of TMZ during RT was 75 mg/m2/day for 42 days. RT was given in 1.8 Gy per fraction (to isocenter), 1 fraction per day, 5 days per week, to a total of 59.4 Gy in 33 fractions. RT was required to begin no later than 6 weeks after completing the final cycle of TMZ, (i.e., within 6 weeks of day 28 of the final cycle of TMZ) blood counts permitting. During concurrent radiation-TMZ therapy, patients received prophylaxis against Pneumocystis jirovecii pneumonia with trimethoprim/sulfamethoxazole double strength tablets (DS) three times per week, or alternatively with either dapsone 50 mg bid or aerosolized pentamidine, 300 mg by nebulizer every 4 weeks (i.e., two doses during radiation).
If there was no measurable disease on MRI (including FLAIR/T2 sequences) following the completion of 6 cycles of TMZ (complete response), then RT was not given and the patient was observed until the study endpoint (post-chemotherapy pre-irradiation therapy progression) was reached. Verification of complete response was performed by central review.
Surveillance and Follow-up
Tumor progression was determined from the modified Macdonald et al [16] criteria and included assessment of residual enhancing, non-enhancing, or minimally enhancing tumor; progression was defined as a > 25% increase in tumor area (two diameters) provided that the patient had not had his/her dose of steroids decreased since the last evaluation period. Complete response (CR), defined as resolution of enhancing disease, required a confirmatory imaging exam at a minimum of 4 weeks after the first determination of CR. The RANO criteria were not used as this study pre-dated their publication (17).
Fluorescence In Situ Hybridization (FISH) Analysis
Locus-specific FISH probes mapping to 1p36, 1q24, 19p13.1, and 19q13.3 used for these analyses were previously developed by the Mayo Clinic group [9,18], and the method for analysis, which was performed at Mayo Clinic, was described in a previous publication [15].
Methylation Specific PCR Analysis of MGMT
DNA methylation patterns in the CpG island of the MGMT gene were determined by chemical modification of unmethylated, but not the methylated, cytosines to uracil and subsequent PCR using primers specific for either methylated or the modified unmethylated DNA [19]. Primer sequences and methodology for analysis were described previously [15].
Statistical Analysis
This phase II study was designed to have 80% power to determine if pre-irradiation chemotherapy with TMZ achieves a six-month progression rate of 5% for patients with newly diagnosed pure and mixed anaplastic oligodendrogliomas. The null hypothesis was a rate of progression of 20%, based upon the results of RTOG 9402 which included a similar population of patients [13]. Based upon this design, it was concluded that if ≤ 4 of the first 35 evaluable patients had progressed within six months, then the null hypothesis would be rejected in favor of the alternative, and we would conclude that pre-irradiation TMZ merits further investigation in a phase III trial.
Frequency tables with counts and percentages were used to describe pretreatment characteristics, adverse events, and compliance review results. Overall survival (OS) and progression-free survival (PFS) were estimated using the Kaplan-Meier method [20]. An OS event was defined as death due to any cause. A PFS event was defined as death due to any cause or radiographic progression (determined by the local investigator). OS and PFS were estimated from the date of registration. The log-rank test [21] was used to compare the survival curves of 1p/19q codeleted versus one or neither deleted. Two-sided Fisher's exact tests were used to compare the distributions of progression status (progression vs. no progression) by 1p/19q deletion status (1p/19q codeleted vs. one or neither deleted).
RESULTS
Patients
An initial report from this study was published previously; [15] this report includes updated information regarding patient disposition, treatment, biomarker analysis and outcome. A total of 42 patients from 18 institutions were enrolled on this study between July 30, 2002 and April 30, 2004. Two patients were deemed ineligible; one site that had enrolled one patient did not submit data and resigned from the study, and one patient refused protocol treatment prior to the start of therapy. For the remaining 40 patients, 22 (55%) were male; the median age was 45 years (range = 18 – 71); 20 (50%) had minor, 4 (10%) had moderate, and 16 (40%) had no neurological symptoms; 33 (82%) had a normal mental status; 36 (90%) had at least a partial resection; and 26 (65%) had an oligodendroglioma or an oligo-dominant mixed glioma (see Table 1). FISH analysis for 1p/19q deletion could be performed on 37 (92%) of 39 cases.
TABLE 1.
Pretreatment Characteristics (n=40)
| Age | |
| Median | 45 |
| Range | 18 – 71 |
| n | % | |
|---|---|---|
| Gender | ||
| Male | 22 | 55 |
| Female | 18 | 45 |
| Zubrod performance status | ||
| 0 | 27 | 68 |
| 1 | 13 | 32 |
| Prior surgery of disease site | ||
| Stereotactic biopsy | 1 | 3 |
| Open (craniotomy) biopsy | 3 | 8 |
| Partial resection | 18 | 45 |
| Total Tumor Resection | 18 | 45 |
| Neurologic function | ||
| No neurologic symptoms | 16 | 40 |
| Minor neurologic symptoms | 20 | 50 |
| Moderate neurologic symptoms | 4 | 10 |
| Mental status | ||
| Normal Function | 33 | 83 |
| Minor mental confusion | 7 | 18 |
| Histology | ||
| Oligodendroglioma | 13 | 33 |
| Mixed Glioma, Oligo dominant | 13 | 33 |
| Mixed Glioma, Oligo = Astro | 11 | 28 |
| Mixed Glioma, Astro dominant | 1 | 3 |
| Mixed Glioma, Not specified | 2 | 5 |
Note: Total of percentages may exceed 100 due to rounding.
Patient Disposition and Treatment Delivery
Pre-irradiation chemotherapy was delivered per protocol to all 40 patients. Twenty-six (67%) patients received all 6 cycles of pre-RT TMZ, including one patient who received 8 cycles of pre-irradiation chemotherapy. Ten patients (25%) received fewer than 6 cycles secondary to tumor progression (2 patients, 5%), withdrawal from the protocol (4 patients, 10%), unacceptable toxicity or concurrent illness (3 patients, 7.5%), or other (1 patient, 2.5%, hospitalized for pneumonia). An additional 4 patients (10%) were reported as having completed 6 cycles but documentation of the date or dose of the final cycle was incomplete. There were no grade 5 toxicities. A summary of the toxicities associated with pre-RT chemotherapy is shown in Table 2.
TABLE 2.
Pre-RT Temozolomide Toxicity (n=40)
| Grade |
|||||
|---|---|---|---|---|---|
| Category | 1 | 2 | 3 | 4 | 5 |
| Allergy/immunology | 0 | 1 | 0 | 0 | 0 |
| Blood/bone marrow | 7 | 7 | 14 | 0 | 0 |
| Cardiovascular (Arrhythmia) | 0 | 1 | 0 | 0 | 0 |
| Cardiovascular (General) | 3 | 0 | 1 | 1 | 0 |
| Constitutional symptoms | 13 | 16 | 1 | 0 | 0 |
| Dermatology/skin | 8 | 3 | 2 | 0 | 0 |
| Endocrine | 2 | 0 | 0 | 0 | 0 |
| Gastrointestinal | 17 | 14 | 1 | 0 | 0 |
| Hemorrhage | 1 | 0 | 0 | 0 | 0 |
| Hepatic | 16 | 2 | 0 | 0 | 0 |
| Infection Febrile Neutropenia | 1 | 3 | 1 | 0 | 0 |
| Metabolic/laboratory | 8 | 2 | 0 | 0 | 0 |
| Musculoskeletal | 1 | 2 | 1 | 0 | 0 |
| Neurology | 4 | 9 | 0 | 0 | 0 |
| Ocular/visual | 2 | 1 | 1 | 0 | 0 |
| Pain | 6 | 5 | 2 | 0 | 0 |
| Pulmonary | 1 | 0 | 2 | 1 | 0 |
| Renal/genitourinary | 3 | 0 | 0 | 0 | 0 |
| Second malignancy | 0 | 0 | 0 | 1 | 0 |
| Sexual/reproductive function | 0 | 0 | 2 | 0 | 0 |
| Syndromes | 0 | 0 | 1 | 0 | 0 |
| Pending clarification of term | 0 | 0 | 1 | 0 | 0 |
Twenty-four patients completed concurrent chemotherapy and RT per protocol. Of the original 40 patients, 3 withdrew due to patient refusal to continue therapy or toxicity, 3 due to physician preference, 2 due to progression of disease, 1 had stable disease (SD) and did not receive RT due to incarceration in prison, 2 had CR and 2 had absence of evaluable disease (AED) and therefore did not receive RT. Withholding of RT for the 4 patients (10%) with CR or AED after pre-RT temozolomide was specified by the protocol. Three additional patients did not receive concurrent chemoRT despite being eligible to do so after evaluation of their response to pre-RT chemotherapy. All 24 patients who started concurrent chemoRT completed the regimen as specified. This therapy was well tolerated; there were no grade 4 or 5 toxicities. A summary of the toxicities observed during concurrent chemoRT is shown in Table 3.
TABLE 3.
Concurrent RT & Temozolomide Toxicity (n=24)
| Grade |
|||||
|---|---|---|---|---|---|
| Category | 1 | 2 | 3 | 4 | 5 |
| Auditory/Hearing | 1 | 1 | 0 | 0 | 0 |
| Blood/bone marrow | 5 | 4 | 4 | 0 | 0 |
| Cardiovascular (General) | 1 | 0 | 0 | 0 | 0 |
| Constitutional symptoms | 6 | 8 | 0 | 0 | 0 |
| Dermatology/skin | 4 | 8 | 0 | 0 | 0 |
| Endocrine | 0 | 1 | 0 | 0 | 0 |
| Gastrointestinal | 2 | 7 | 1 | 0 | 0 |
| Hepatic | 6 | 1 | 0 | 0 | 0 |
| Metabolic/laboratory | 2 | 0 | 0 | 0 | 0 |
| Neurology | 2 | 4 | 2 | 0 | 0 |
| Ocular/visual | 1 | 1 | 0 | 0 | 0 |
| Pain | 3 | 3 | 0 | 0 | 0 |
| Pulmonary | 0 | 1 | 0 | 0 | 0 |
| Renal/genitourinary | 3 | 0 | 0 | 0 | 0 |
| Sexual/reproductive function | 0 | 0 | 1 | 0 | 0 |
| Pending clarification of term | 0 | 1 | 0 | 0 | 0 |
Late toxicity from radiotherapy included only one grade 3, three grade 2 and ten grade 1 toxicities.
Biomarker Analysis and Primary Endpoint Evaluation (6 month PFS)
FISH analysis for 1p/19q deletion could be performed on 37 (93%) of 40 cases; 3 cases had insufficient material for evaluation. Of the 37 evaluable cases, 14 tumors (38% of evaluable) had LOH of either chromosome 1p or 19q or neither, and 23 tumors (62% of evaluable) had LOH of both 1p and 19q.
MGMT analysis revealed informative data for 26 cases (65%); 4 cases (15% of evaluable) had an unmethylated and 22 cases (85% of evaluable) had a methylated MGMT gene promoter. Of the 23 patients with 1p/19q codeletion, 17 were evaluable for MGMT promoter methylation; 16 (94.1%) were methylation positive.
Twenty-nine cases had informative 1p/19q data and were evaluable for progression at 6 months. Of these 29 cases, 18 (62%) had codeletion of 1p/19q. In terms of the correlation of 1p/19q status to response, one of the 2 patients with CR and 2 of the two patients with AED had codeletion of 1p/19q. 6 of 7 cases with PR (of 9 total) evaluated for 1p/19q had codeletion of 1p/19q. Nine of the 16 patients with SD also had codeletion of 1p/19q. Two patients who progressed within 6 months of starting treatment were intact at both loci; the third patient who progressed was not evaluable for 1p/19q deletion. Hence, all 18 of the patients found to have combined 1p/19q deletion were free from progression at 6 months (Table 4). However, the relationship between 1p/19q codeletion and 6-month progression was not statistically significant (p-value = 0.14, Two-sided Fisher's Exact test) possibly due to the small number of cases. When progression at any time was taken into consideration, there were a total of 37 cases with informative 1p/19q data. Progression occurred in 18 patients, of whom 10 (56%) had noncodeleted tumors. Nineteen patients remain free from progression; 15 (79%) of whom had codeleted tumors.
TABLE 4.
1p/19q Deletion Status vs. 6-Month Scan
| Progression N (%) | No Progression N (%) | Total (%) | |
|---|---|---|---|
| Both 1p and 19q deleted (N) | 0 | 18 (67%) | 18 (62%) |
| One or neither deleted (N) | 2 (100%) | 9 (33%) | 11 (38%) |
| Total (N, %) | 2 ( 7%) | 27 (93%) | 29 |
Two-sided Fisher's Exact test, p-value = 0.14
Of the 26 cases with MGMT promoter methylation data, 21 also had evaluable scans at 6 months; none of these 21 patients showed progression at 6 months. Seventeen (81% of evaluable) of these 21 patients had tumors with MGMT promoter methylation.
Long Term Outcome Evaluation
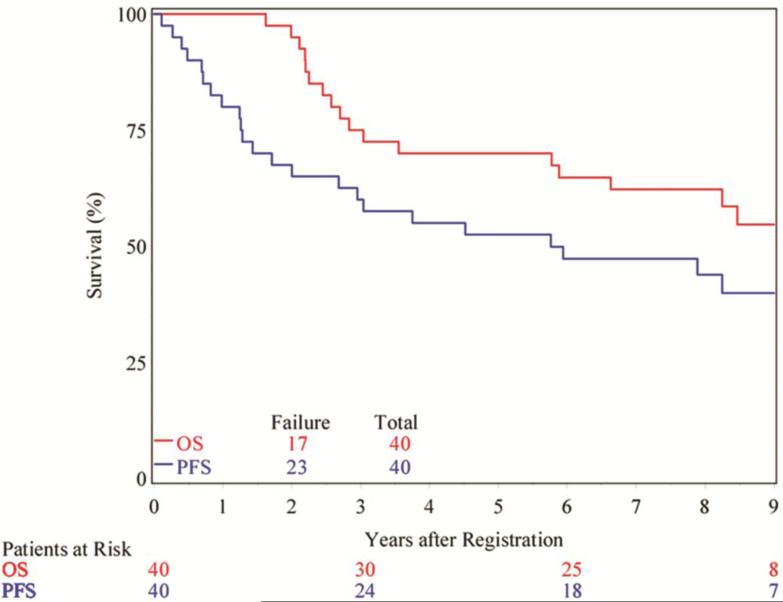
The median duration of follow-up for all patients is 8.7 years (range = 4.7 to 10). Evaluation of the entire patient cohort revealed that 17 of 40 patients (42%) had died, and hence the median survival time has not yet been reached at the time of this analysis. Twenty-three patients (58%) have died or had tumor progression and the median PFS is 5.8 years (95% CI; 2.0 – ND years). Figure 1 shows the Kaplan-Meier curves for overall survival and PFS for the entire cohort.
Figure 1.
Kaplan-Meier curves showing the overall survival (red) and progression-free survival (blue) of all patients.
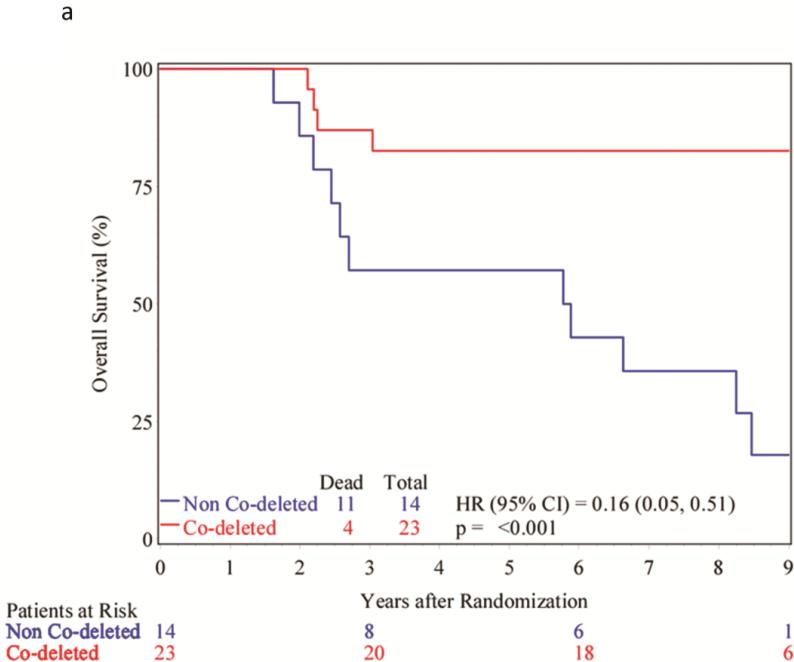
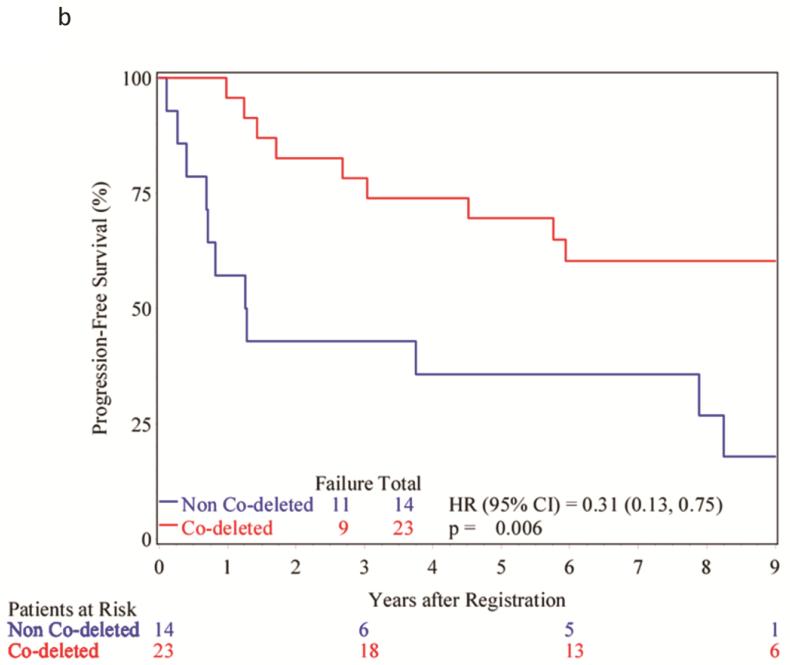
Evaluation of outcomes of the patients whose tumors demonstrated 1p/19q codeletion (N=23) reveals that only 4 (17%) patients have died, 17 (74%) remain alive after 7 years and 2 (9%) were alive but lost to follow up between 4 and 7 years; the median survival time has not been reached. For the 14 patients whose tumors had deletion of either 1p or 19q, or neither, 9 (64%) have died, 5 (36%) remain alive after 7-year follow up with a median survival of 5.8 years (2.2 – 8.5 years). PFS for patients with 1p/19q codeletion has not been reached (9 of 23 patients). For those whose tumors were not codeleted, the PFS was 1.3 years (0.4 – 8.3 years) (11 of 14 patients).
Cox proportional hazard analysis reveals that the hazard ratio associated with 1p/19q codeletion was 0.16 (0.05 – 0.51, p<0.001, Figure 2A) for overall survival and 0.31 (0.13 – 0.75, p=0.006, Figure 2B) for PFS.
Figure 2.
A. Kaplan-Meier curves showing the overall survival of patients based upon tumor 1p/19q status. B. Kaplan-Meier curves showing the progression-free survival of patients based upon tumor 1p/19q status.
DISCUSSION
Two phase III trials that evaluated the role and timing of PCV in the treatment of AO and MAO initially published results showing a favorable impact of the addition of PCV to RT on PFS, particularly in tumors with 1p/19q codeletion, but no statistically significant impact on survival compared to initial therapy with RT alone [13,14]. Updated analyses subsequently confirmed the survival benefit of PCV in addition to RT for patients with codeleted AO and MAO, and this regimen has been proposed as the new standard of care for these patients [2,4]. Significant concern has been raised, however, regarding the routine use of the PCV regimen for these patients due to the clinically meaningful, and often irreversible toxicity that may occur [22-24]. TMZ regimens appear to produce less toxicity than PCV in patients with high grade gliomas, and further can be combined with radiotherapy with relative ease; some investigators have suggested that TMZ could reasonably substitute for PCV as standard therapy for codeleted AO and MAO [10,25-28]. On the other hand, one study that compared PCV to TMZ in recurrent anaplastic glioma did not detect significant differences in toxicity [29]. To date, no prospective, randomized data have compared the efficacy, and relative toxicity, of PCV versus TMZ for codeleted AO and MAO.
RTOG BR0131 investigated the use of pre-RT dose intense TMZ, and the report of the primary analysis (6-month PFS) has been published previously [15]. The initial report indicated that pre-RT TMZ produced a 6-month PFS outcome in codeleted patients that was at least similar to, if not better, than that observed in the 9402 codeleted patient cohort, with a lower rate of severe toxicity. This updated analysis of RTOG BR0131 provides initial data regarding the long-term outcomes of patients treated with a dose intense regimen of TMZ prior to and concurrent with RT.
With a median follow-up time of nearly 9 years, the codeleted cohort of patients treated with the dose intense pre-RT TMZ regimen has reached neither median PFS nor OS. In comparison, the codeleted patients treated with pre-RT PCV in RTOG 9402 had a median PFS of 8.4 years and median OS of 14.7 years [2]. In the updated analysis of EORTC 26951 trial, in which PCV was adjuvant to RT, median PFS for the codeleted cohort was 13.1 years and median OS was not reached after approximately 12 years follow-up time [4]. While this updated analysis of RTOG BR0131 is not a direct comparison to the prospectively evaluated regimens involving the use of PCV, our long-term outcome data need to be considered in the routine management of patients with codeleted AO and MAO, and could serve the basis for a future prospective evaluation of these two chemotherapy regimens. A multigroup, international clinical trial (NCT00887146) will directly compare the use of PCV or TMZ as adjuvants to radiation therapy for newly diagnosed WHO grade II and III 1p/19q codeleted gliomas.
There are a few notable limitations to this study. It was a relatively small, single arm study, which makes it difficult to perform direct comparisons to the 2 phase III trials [2,4,13,14]. Also, while the primary analysis of response and 6-month PFS was centrally reviewed, overall progression was not centrally reviewed and hence those results may be impacted by the effects of pseudoprogression, which may be more frequent in cases with MGMT promoter methylation [30]. Finally, this study was performed prior to the discovery of IDH1/2 mutations and hence tumors were not tested for these mutations [31]. That said, the correlation between IDH1/2 mutation and 1p/19q codeletion is quite high and hence evaluation of these mutations may not provide meaningful additional information [32].
Particularly in the setting of the recently published long term analyses of phase III study results, which showed that combinations of PCV chemotherapy with RT produced prolonged survival of patients with codeleted AO and MAO, the newly defined role of chemotherapy for these patients has raised new questions regarding the use and timing of RT. RTOG BR0131 demonstrated that immediately following a 6-month course of neoadjuvant TMZ, all 18 patients with codeleted tumors were free from progression. With the exception of the 2 patients who had CRs, the other 16 codeleted patients subsequently underwent chemoRT and their excellent outcomes must be attributed to both the neoadjuvant and adjuvant therapies. Taken together with our finding of 100% 6-month PFS for codeleted patients, it would be of value to determine whether a more prolonged course of neoadjuvant chemotherapy would provide more durable tumor control.
CONCLUSION
These updated results suggest that the regimen of dose intense, pre-RT temozolomide followed by concurrent RT/temozolomide appears to have significant activity, indirectly comparable to that observed with PCV/RT in RTOG 9402 in patients with 1p/19q codeleted AOs and MAOs.
Acknowledgments
FUNDING
This project was supported by grants U10CA21661, U10CA180868, U10CA180822, U10 CA37422, U24CA180803 from the National Cancer Institute (NCI).
Footnotes
CONFLICT OF INTEREST
The following authors have disclosed financial relationships with commercial entities that may be impacted by this work:
DMP – Merck (research support, honoraria)
DRM – Merck Canada (honoraria, travel support), Roche Canada (honoraria, travel support)
JHS – Varian Medical System (consultant)
The following authors declare no conflicts of interests with respect this work: MAV, CH, CG, RBJ, NNL, DCS, LS, DB, MPM
Portions of this work have been presented in abstract form (Society for NeuroOncology, 2012)
REFERENCES
- 1.Mork SJ, Lindegaard KF, Halvorsen TB, Lehmann EH, Solgaard T, Hatlevoll R, Harvei S, Ganz J. Oligodendroglioma: incidence and biological behavior in a defined population. J Neurosurg. 1985;63(6):881–889. doi: 10.3171/jns.1985.63.6.0881. [DOI] [PubMed] [Google Scholar]
- 2.Cairncross G, Wang M, Shaw E, Jenkins R, Brachman D, Buckner J, Fink K, Souhami L, Laperriere N, Curran W, Mehta M. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol 20. 2013;31(3):337–343. doi: 10.1200/JCO.2012.43.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cairncross JG, Wang M, Jenkins RB, Shaw EG, Giannini C, Brachman DG, Buckner JC, Fink KL, Souhami L, Laperriere NJ, Huse JT, Mehta MP, Curran WJ., Jr Benefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDH. J Clin Oncol 10. 2014;32(8):783–790. doi: 10.1200/JCO.2013.49.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van den Bent MJ, Brandes AA, Taphoorn MJ, Kros JM, Kouwenhoven MC, Delattre JY, Bernsen HJ, Frenay M, Tijssen CC, Grisold W, Sipos L, Enting RH, French PJ, Dinjens WN, Vecht CJ, Allgeier A, Lacombe D, Gorlia T, Hoang-Xuan K. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol 20. 2013;31(3):344–350. doi: 10.1200/JCO.2012.43.2229. [DOI] [PubMed] [Google Scholar]
- 5.Cairncross JG, Ueki K, Zlatescu MC, Lisle DK, Finkelstein DM, Hammond RR, Silver JS, Stark PC, Macdonald DR, Ino Y, Ramsay DA, Louis DN. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90(19):1473–1479. doi: 10.1093/jnci/90.19.1473. [DOI] [PubMed] [Google Scholar]
- 6.Ino Y, Betensky RA, Zlatescu MC, Sasaki H, Macdonald DR, Stemmer-Rachamimov AO, Ramsay DA, Cairncross JG, Louis DN. Molecular subtypes of anaplastic oligodendroglioma: implications for patient management at diagnosis. Clin Cancer Res. 2001;7(4):839–845. [PubMed] [Google Scholar]
- 7.Jenkins RB, Blair H, Ballman KV, Giannini C, Arusell RM, Law M, Flynn H, Passe S, Felten S, Brown PD, Shaw EG, Buckner JC. A t(1;19)(q10;p10) mediates the combined deletions of 1p and 19q and predicts a better prognosis of patients with oligodendroglioma. Cancer Res. 2006;66(20):9852–9861. doi: 10.1158/0008-5472.CAN-06-1796. [DOI] [PubMed] [Google Scholar]
- 8.Bauman GS, Ino Y, Ueki K, Zlatescu MC, Fisher BJ, Macdonald DR, Stitt L, Louis DN, Cairncross JG. Allelic loss of chromosome 1p and radiotherapy plus chemotherapy in patients with oligodendrogliomas. Int J Radiat Oncol Biol Phys. 2000;48:825–830. doi: 10.1016/s0360-3016(00)00703-3. [DOI] [PubMed] [Google Scholar]
- 9.Smith JS, Perry A, Borell TJ, Lee HK, O'Fallon J, Hosek SM, Kimmel D, Yates A, Burger PC, Scheithauer BW, Jenkins RB. Alterations of chromosome arms 1p and 19q as predictors of survival in oligodendrogliomas, astrocytomas, and mixed oligoastrocytomas. J Clin Oncol. 2000;18:636–645. doi: 10.1200/JCO.2000.18.3.636. [DOI] [PubMed] [Google Scholar]
- 10.Chahlavi A, Kanner A, Peereboom D, Staugaitis SM, Elson P, Barnett G. Impact of chromosome 1p status in response of oligodendroglioma to temozolomide: Preliminary results. J Neurooncol. 2003;61:267–273. doi: 10.1023/a:1022580610598. [DOI] [PubMed] [Google Scholar]
- 11.Thiessen B, Maguire JA, McNeil K, Huntsman D, Martin MA, Horsman D. Loss of heterozygosity for loci on chromosome arms 1p and 10q in oligodendroglial tumors: Relationship to outcome and chemosensitivity. J Neurooncol. 2003;64:271–278. doi: 10.1023/a:1025689004046. [DOI] [PubMed] [Google Scholar]
- 12.van den Bent MJ, Gravendeel LA, Gorlia T, Kros JM, Lapre L, Wesseling P, Teepen JL, Idbaih A, Sanson M, Smitt PA, French PJ. A hypermethylated phenotype is a better predictor of survival than MGMT methylation in anaplastic oligodendroglial brain tumors: a report from EORTC study 26951. Clin Cancer Res. 2011;17(22):7148–7155. doi: 10.1158/1078-0432.CCR-11-1274. [DOI] [PubMed] [Google Scholar]
- 13.Cairncross G, Berkey B, Shaw E, Jenkins R, Scheithauer B, Brachman D, Buckner J, Fink K, Souhami L, Laperierre N, Mehta M, Curran W. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol. 2006;24(18):2707–2714. doi: 10.1200/JCO.2005.04.3414. [DOI] [PubMed] [Google Scholar]
- 14.van den Bent MJ, Carpentier AF, Brandes AA, Sanson M, Taphoorn MJ, Bernsen HJ, Frenay M, Tijssen CC, Grisold W, Sipos L, Haaxma-Reiche H, Kros JM, van Kouwenhoven MC, Vecht CJ, Allgeier A, Lacombe D, Gorlia T. Adjuvant Procarbazine, Lomustine and Vincristine Improves Progression-Free Survival but Not Overall Survival in Newly Diagnosed Anaplastic Oligodendrogliomas and Oligoastrocytomas: A Randomized European Organization for Research and Treatment of Cancer Phase III Trial. J Clin Oncol. 2006;24:2715. doi: 10.1200/JCO.2005.04.6078. [DOI] [PubMed] [Google Scholar]
- 15.Vogelbaum MA, Berkey B, Peereboom D, Macdonald D, Giannini C, Suh JH, Jenkins R, Herman J, Brown P, Blumenthal DT, Biggs C, Schultz C, Mehta M. Phase II trial of preirradiation and concurrent temozolomide in patients with newly diagnosed anaplastic oligodendrogliomas and mixed anaplastic oligoastrocytomas: RTOG BR0131. Neuro Oncol. 2009;11(2):167–175. doi: 10.1215/15228517-2008-073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macdonald DR, Cascino TL, Schold SC, Cairncross JG. Response criteria for phase II studies of malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 17.Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J. Clin Oncol. 2010;28(11):1963–72. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 18.Smith JS, Alderete B, Minn Y, Borell TJ, Perry A, Mohapatra G, Hosek SM, Kimmel D, O'Fallon J, Yates A, Feuerstein BG, Burger PC, Scheithauer BW, Jenkins RB. Localization of common deletion regions on 1p and 19q in human gliomas and their association with histological subtype. Oncogene. 1999;18:4144–4152. doi: 10.1038/sj.onc.1202759. [DOI] [PubMed] [Google Scholar]
- 19.Esteller M, Risques RA, Toyota M, Capella G, Moreno V, Peinado MA, Baylin SB, Herman JG. Promoter hypermethylation of the DNA repair gene O(6)-methylguanine-DNA methyltransferase is associated with the presence of G:C to A:T transition mutations in p53 in human colorectal tumorigenesis. Cancer Res. 2001;61(12):4689–4692. [PubMed] [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 21.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemotherapy Reports. 1966;50:163–170. [PubMed] [Google Scholar]
- 22.Roth P, Wick W, Weller M. Anaplastic oligodendroglioma: a new treatment paradigm and current controversies. Curr Treat Options Oncol. 2013;14(4):505–513. doi: 10.1007/s11864-013-0251-7. [DOI] [PubMed] [Google Scholar]
- 23.Cairncross G, Berkey B, Shaw E, Jenkins R, Scheithauer B, Brachman D, Buckner J, Fink K, Souhami L, Laperierre N, Mehta M, Curran W. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol. 2006;2024(18):2707–2714. doi: 10.1200/JCO.2005.04.3414. [DOI] [PubMed] [Google Scholar]
- 24.Villano JL, Wen PY, Lee EQ, Nayak L, Reardon DA, Rosenfeld MR. PCV for anaplastic oligodendrogliomas: back to the future or a step backwards? A point/counterpoint discussion. J Neurooncol. 2013;113(1):143–147. doi: 10.1007/s11060-013-1100-z. [DOI] [PubMed] [Google Scholar]
- 25.Chinot OL, Honore S, Dufour H, Barrie M, Figarella-Branger D, Muracciole X, Braguer D, Martin PM, Grisoli F. Safety and efficacy of temozolomide in patients with recurrent anaplastic oliodendrogliomas after standard radiotherapy and chemotherapy. J Clin Oncol. 2001;19:2449–2455. doi: 10.1200/JCO.2001.19.9.2449. [DOI] [PubMed] [Google Scholar]
- 26.van den Bent MJ, Keime-Guibert F, Brandes AA, Taphoorn MJ, Kros JM, Eskens FA, Carpentier AF. Temozolomide chemotherapy in recurrent oligodendroglioma. Neurology. 2001;57(2):340–342. doi: 10.1212/wnl.57.2.340. [DOI] [PubMed] [Google Scholar]
- 27.van den Bent MJ, Taphoorn MJB, Brandes AA, Menten J, Stupp R, Frenay M, Chinot O, Kros JM, van der Rijt CC, Vecht ChJ, Allgeier A, Gorlia T, European Organization for Research and Treatment of Cancer Brain Tumor Group Phase II study of first line chemotherapy with temozolomide in recurrent oligodendroglial tumors: The European Organization for the Research and Treatment of Cancer Brain Tumor Group Study 26971. J Clin Oncol. 2003;21:2525–2528. doi: 10.1200/JCO.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 28.Anderson MD, Gilbert MR. Treatment recommendations for anaplastic oligodendrogliomas that are codeleted. Oncology (Williston Park) 2013;27(4):315–320. [PubMed] [Google Scholar]
- 29.Brada M, Stenning S, Gabe R, Thompson LC, Levy D, Rampling R, Erridge S, Saran F, Gattamaneni R, Hopkins K, Beall S, Collins VP, Lee SM. Temozolomide versus procarbazine, lomustine, and vincristine in recurrent high-grade glioma. J. Clin Oncol. 2010;28(30):4601–8. doi: 10.1200/JCO.2009.27.1932. [DOI] [PubMed] [Google Scholar]
- 30.Brandes AA, Franceschi E, Tosoni A, Blatt V, Pession A, Tallini G, Bertorelle R, Bartolini S, Calbucci F, Andreoli A, Frezza G, Leonardi M, Spagnolli F, Ermani M. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26(13):2192–7. doi: 10.1200/JCO.2007.14.8163. [DOI] [PubMed] [Google Scholar]
- 31.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, Friedman H, Friedman A, Reardon D, Herndon J, Kinzler KW, Velculescu VE, Vogelstein B, Bigner DD. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–73. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yip S, Butterfield YS, Morozova O, Chittaranjan S, Blough MD, An J, Birol I, Chesnelong C, Chiu R, Chuah E, Corbett R, Docking R, Firme M, Hirst M, Jackman S, Karsan A, Li H, Louis DN, Maslova A, Moore R, Moradian A, Mungall KL, Perizzolo M, Qian J, Roldan G, Smith EE, Tamura-Wells J, Thiessen N, Varhol R, Weiss S, Wu W, Young S, Zhao Y, Mungall AJ, Jones SJ, Morin GB, Chan JA, Cairncross JG, Marra MA. Concurrent CIC mutations, IDH mutations, and 1p/19q loss distinguish oligodendrogliomas from other cancers. J Pathol. 2012;226(1):7–16. doi: 10.1002/path.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]