Abstract
Objective
To retrospectively validate and compare a modified frailty index predicting adverse outcomes to other risk stratification tools among patients undergoing urologic oncological surgeries.
Materials and Methods
The American College of Surgeons National Surgical Quality Improvement Program was queried from 2005–2013 to identify patients undergoing cystectomy, prostatectomy, nephrectomy, and nephroureterectomy. Using the Canadian Study of Health & Aging Frailty Index, 11 variables were matched to the database; 4 were also added due to their relevance in oncology patients. The incidence of mortality, Clavien-Dindo IV complications, and adverse events were assessed with patients grouped according to their modified frailty index score.
Results
A total of 41,681 cases of patients were identified undergoing surgery for presumed urological malignancy. Patients with a high frailty index score of >0.20 had a 3.70 odds of a Clavien-Dindo IV event (CI: 2.865–4.788, p<0.0005) and a 5.95 odds of 30-day mortality (CI: 3.72–9.51, p<0.0005) in comparison to non-frail patients after adjusting for race, gender, age, smoking history and procedure. Using C-statistics to compare the sensitivity and specificity of the predictive ability of different models per risk stratification tool and Akaiki Information Criteria to assess for the fit of the models with the data, the modified frailty index was comparable or superior to the Charlson Comorbidity Index but inferior to the American Society of Anesthesiologists Risk Class in predicting 30-day mortality or Clavien-Dindo IV events. When the modified frailty index was augmented with the American Society of Anesthesiologists Risk Class, the new index was superior in all regards in comparison to risk stratification tools.
Conclusion
Existing risk stratification tools may be improved by incorporating variables in our 15 point modified frailty index as well as other factors such as walking speed, exhaustion, and sarcopenia to fully assess frailty. This is relevant in diseases like kidney and prostate cancer, where surveillance and other non-surgical interventions exist as alternatives to a potentially complicated surgery. In these scenarios, our modified frailty index augmented by the American Society of Anesthesiologists Risk Class may help inform which patients do not benefit from surgery although this index needs prospective validation.
Keywords: Frail elderly, surgical outcomes, urologic oncology, pre-operative evaluation, patient survival
Introduction
Frailty is a growing issue for surgeons as frail patients have worse health outcomes with increased mortality rates, hospitalizations, and institutionalization rates [1]. Frailty is a medical syndrome with multiple contributors and characterized by diminished strength, endurance, and reduced physiologic function increasing an individual’s vulnerability to dependency and death [2]. Frailty is associated with poor oncological outcomes like disease progression and diseasespecific mortality [3].
The Canadian Study of Health & Aging Frailty Index (CSHA-FI) is a clinically validated measure of frailty that includes the extent of comorbidities and quality of life variables in an accumulating deficit model of frailty [4]. Rockland, et al defined frailty as a function of the severity of a patient’s comorbidities and declines in activities of daily living[4]. They validated their accumulating deficit model of frailty showing that it was equivalent to the phenotypic frailty model defined by the Fried Frailty Index, which takes into account factors like walking speed and weight loss[5]. Abbreviated versions of the CSHA-FI have been validated as preoperative risk stratification tools in prospective and retrospective fashion in general surgery, gynecological oncology, and orthopedic surgery [6–11]. An abbreviated version has been validated retrospectively using the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) dataset among vascular surgery patients; patients undergoing colectomy; emergency and elective general surgery patients; and cardiothoracic patients undergoing lobectomies [11–15]. In all cases, frailty measured by increasing score in the frailty index was associated with adverse outcomes.
We used the variables from CSHA-FI mapped to the ACS-NSQIP dataset to create a modified 15-point frailty index (mFI) with additional variables pertinent to our patient population in a model of frailty that measures accumulating deficits [4, 5, 16]. We validated our modified FI in genitourinary patients to see how frailty and comorbidities impacts patients across the most common oncological surgeries in urology: prostatectomy, cystectomy, nephrectomy, and nephroureterectomy.
Material and Methods
Under the data use agreement of the ACS, we reviewed the NSQIP participant use files from 2005–13. The NSQIP database is a national, validated, outcomes-based dataset managed by the ACS. The hospitals participating in the consortium are the source of the data used herein; they have not verified, and are not responsible, for the statistical validity of the data analysis or the conclusions we have derived.
We collected 11 variables from the CSHA-FI matched to preoperative variables in the NSQIP database of patients who were identified by the primary Current Procedure Terminology (CPT) as having undergone prostatectomy, cystectomy, nephrectomy, and nephroureterectomy. Non-oncological cases were excluded. Four additional variables were added to create our mFI: history of metastasis, chemotherapy/radiation exposure, weight loss, and renal failure (Table 1). History of metastasis and treatment with chemotherapy/radiation both denote the severity of a patient’s cancer. Weight loss is a marker of frailty validated by the Fried Frailty Index [1]. Renal failure with creatinine > 3 mg/mL predisposes patients to adverse outcomes [17]. The mFI index score was calculated using the sum of risk factors per patients and divided by the amount of total risk factors. Variables in the frailty index with no mention of severity were dichotomized as absent (0) or present (1); other variables were trichotomized with 1 being most severe similar to Mitnitski, et al [5].
Table 1.
Eleven ACS-NSQIP variables were similar to 11 variables in the CSHA-FI. Four ACS-NSQIP variables related to oncology patients were added to make the FI consisting of 15 variables in total. The number of positive factors in the FI was recorded for each patient and divided by 15 to create a frailty index value.
| ACS-NSQIP Variables | CSHA-FI Variables |
|---|---|
| 1.Diabetes mellitus | History of Diabetes Mellitus |
| 2.Functional Status | Impaired mobility, problems dressing oneself |
| 3.History of severe COPD | Lung Problems |
| 4.CHF exacerbation in 30 days before surgery | Congestive Heart Failure |
| 5.History of MI 6 months prior to surgery | MI |
| 6.Previous PCI, cardiac surgery, or history of angina | Cardiac Problems |
| 7.Hypertension requiring medication | Arterial Hypertension |
| 8.Peripheral vascular disease or rest pain | Peripheral Pulses |
| 9.Impaired sensorium | Clouding/Delirium/Changes in mental function |
| 10.History of TIA or CVA without neurologic deficit | Cerebrovascular Problems |
| 11.History of CVA with neurologic deficit | History of stroke |
| 12.Weight loss within last 6 months greater than 10% | |
| 13.Chemotherapy or radiation prior to surgery | |
| 14.History of Metastasis | |
| 15. Severe Renal Failure or currently on dialysis |
The following adverse events were recorded in binary fashion: 30-day mortality, septic shock (SS), failure to extubate (ventilator dependence), unplanned re-intubation, myocardial infarction (MI), acute renal failure (ARF), cardiac arrest requiring cardiopulmonary resuscitation (CA), surgical site infection or dehiscence, deep vein thrombosis (DVT), and pulmonary embolism (PE) as defined in the ACS-NSQIP participant user file. Complications were classified as Clavien-Dindo IV as Webb, et al. has done by including the following ACS-NSQIP variables: SS, MI, CA, PE, ARF, unplanned re-intubations, and ventilator dependence [18, 19].
Pearson’s χ2 test was used for categorical comparisons. Age, sex, race, smoking status, procedure, and the mFI were placed in a multivariate logistic regression model looking at mortality and Clavien-Dindo IV complications with the mFI included as an ordinal variable instead of continuous variable to improve the stability of the final model. Odds ratios (OR) with 95% confidence intervals (CI) were recorded. Two-tailed tests were used in all cases with significance defined as p < 0.05.
A modified Charlson Comorbidity Index (CCI) was calculated from variables in the ACS-NSQIP[20]. The American Society of Anesthesiologists Class Risk Group (ASA), functional status, work relative value unit (wRVU), and age were obtained from the ACS-NSQIP database. Different risk stratification tools were analyzed via an Area Under the Receiver Operator Characteristics Curve (ROC) comparing the area under the curve defined as the C-statistic between the different models. We compared our mFI to the previously cited 11-point CSHA-FI, CCI and ASA by assessing the Akaike Information Criterion (AIC)—a measure of the relative quality of a model with lower values being better— and C-statistic—a measure of assessing the optimization of sensitivity and specificity for a given outcome—for each model while adjusting for age, surgical procedure and approach, smoking history, and gender for the outcomes of mortality and Clavien-Dindo IV complications. A further modified model combining ASA physical status with our 15 variable mFI with a weight of 0 for the lowest group of 0 and 5 points for those with > 4 physical status was also assessed. Statistical analyses were performed using SPSS version 20.0 or higher (IBM Corp, Armonk, NY).
Results
The ACS-NSQIP database was queried for a total of 41,681 patients who met our selection criteria with the following clinical and demographic characteristics (Table 2). Cystectomy patients had the highest 30-day mortality rate (2.6%) and Clavien-Dindo IV complications (9.5%); prostatectomy patients had the lowest 30-day mortality (0.2%) and Clavien-Dindo IV complications (1.1%).
Table 2.
Demographic and clinical characteristics for the different patients undergoing urologic surgeries for malignancy. Elderly patients were concentrated in the groups undergoing nephroureterectomy and radical cystectomy. The patients undergoing radical prostatectomy were a lower risk group overall with a larger proportion being ASA Class II or less in comparison to the groups undergoing other surgeries. Mortality after surgery was highest in those undergoing radical cystectomy (2.6%) while it was lowest for those undergoing radical prostatectomy (0.2%).
| Prostatectomy | Partial Nephrectomy |
Radical Nephrectomy |
Nephro- ureterectomy |
Cystectomy | |
|---|---|---|---|---|---|
| Total number of cases | 23350 | 5709 | 7791 | 1443 | 3388 |
| Mean Age, y (SD) | 62(7) | 59 (12) | 62 (13) | 62 (12) | 59 (12) |
| Males | 100% 23350/23350 |
60.8% 3466/5709 |
61.1% 4760/7791 |
61.3% 883/1443 |
80.4% 2722/3388 |
| Nonwhite race or Hispanic | 24.1% 5421/23350 |
21.1% 1155/5709 |
24.0% 1778/7791 |
17.8% 247/1443 |
7.6% 228/3388 |
| Diabetes Mellitus | 11.3% 2644/23350 |
19.4% 1107/5709 |
20.2% 1576/7791 |
19.9% 287/1443 |
19.6% 665/3388 |
| Current Smoker | 12.5% 2926/23350 |
21.0% 1198/5709 |
19.4% 1514/7791 |
24.3% 351/1443 |
24.7% 836/3388 |
| Hypertension | 50.4% 11784/23350 |
60.9% 3479/5709 |
64.7% 5044/7791 |
68.0% 981/1443 |
60.8% 2061/3388 |
| End stage renal disease | 0.3% 66/23350 |
0.4% 24/5709 |
1.0% 396/7791 |
2.4% 34/1143 |
1.0% 34/3388 |
| Charlson Comorbidity Index | |||||
| 0 | 8 (<0.1%) | 94 (1.6%) | 78 (1.0%) | 3 (0.2%) | 5 (0.1%) |
| 1 | 522 (2.2%) | 238 (4.2%) | 230 (3.0%) | 11 (0.8%) | 43 (1.3%) |
| 2 | 2916 (12.5%) | 351 (6.2%) | 507 (6.5%) | 54 (3.7%) | 154 (4.5%) |
| 3 | 4206 (18.0%) | 434 (7.6%) | 600 (7.7%) | 106 (7.3%) | 289 (8.5%) |
| 4 | 1481 (6.3%) | 560 (9.8%) | 827 (10.6%) | 173 (11.9%) | 381 (11.2%) |
| 5 | 851(3.6%) | 709 (12.4%) | 781 (10.1%) | 161 (11.2%) | 280 (8.3%) |
| 6 | 4372 (18.7%) | 972 (17.1%) | 1100 (14.1%) | 149 (10.3%) | 336 (10.0%) |
| 7 | 6288(27.0%) | 1170 (20.5%) | 1318 (17.0%) | 198 (13.7%) | 592 (17.5%) |
| 8 | 2309 (9.9%) | 798 (14.0%) | 1135 (14.6%) | 268 (18.6%) | 719 (21.3%) |
| >9 | 406 (1.7%) | 380 (6.7%) | 1212 (15.6%) | 320 (22.2%) | 589 (17.4%) |
| ASA Class | |||||
| 1 | 964 (4.1%) | 135 (2.4%) | 164 (2.1%) | 16 (1.1%) | 20 (0.6%) |
| 2 | 14654 (62.8%) | 2432 (42.7%) | 2584 (33.2%) | 430 (29.8%) | 844 (24.9%) |
| 3 | 7521(32.2%) | 2981 (52.3%) | 4448 (57.1%) | 908 (63.0%) | 2325 (68.7%) |
| 4 | 186 (0.8%) | 153 (2.7%) | 588 (7.5%) | 87 (6.1%) | 195 (5.8%) |
| Frailty Score out of 15 | |||||
| 0–0.05 | 11312 (48.4%) | 2101 (36.8%) | 2289 (29.4%) | 410 (28.4%) | 1108 (32.7%) |
| 0.05–0.10 | 9526 (40.8%) | 2377 (41.6%) | 3169 (40.7%) | 634 (43.9%) | 1330 (39.3%) |
| 0.10–0.15 | 1656 (7.1%) | 701 (12.3%) | 833 (10.7%) | 181 (12.5%) | 423 (12.%) |
| 0.15–0.20 | 637 (2.7%) | 407 (7.1%) | 942 (12.1%) | 130 (9.0%) | 351 (10.4%) |
| > 0.20 | 219 (0.9%) | 123 (2.2%) | 558 (7.2%) | 88 (6.1%) | 176 (5.2%) |
| Functional Status | |||||
| Independent | 23241 (99.5%) | 5633 (98.7%) | 7583 (97.3%) | 1400 (97.0%) | 3314 (97.8%) |
| Limited Dependence | 50 (0.2%) | 50 (0.9%) | 160 (2.1%) | 30 (2.1%) | 48 (1.4%) |
| Totally Dependent | 5 (<0.1%) | 0 (0%) | 15 (0.2%) | 0 (0%) | 22 (0.6%) |
| Unknown | 62 (0.3%) | 26 (0.5%) | 33 (0.4%) | 13 (0.9%) | 4 (0.1%) |
| Procedure subtypes | |||||
| Open | 5333 (22.8%) | 2284 (40.0%) | 3128 (40.1%) | 434 (30.1%) | 3388 (100%) |
| Minimally Invasive | 18026 (77.2%) | 3425 (60.0%) | 4663 (59.9%) | 1009 (69.9%) | - |
| 30-Day Mortality | 0.2% 37/23350 |
0.4% 20/5709 |
1.0% 79/7791 |
1.7% 25/1443 |
2.6% 88/3388 |
| Clavien-Dindo IV Complications | 1.1% 246/23350 |
2.3% 129/5709 |
3.9% 306/7791 |
5.3% 76/1443 |
9.5% 322/3388 |
| Septic Shock or Sepsis | 0.8% 191/23350 |
1.2% 67/5709 |
1.6% 127/7791 |
3.0% 43/1443 |
12.5% 422/3388 |
| Ventilator Dependence | 0.1% 34/23350 |
0.4% 21/5709 |
1.2% 93/7791 |
1.3% 19/1443 |
2.6% 89/3388 |
| Unplanned re-intubation | 0.2% 54/23350 |
0.8% 44/5709 |
1.5% 116/7791 |
1.7% 25/1443 |
3.3% 112/3388 |
| Myocardial Infarction | 0.2% 41/23350 |
0.5% 26/5709 |
0.5% 40/7791 |
1.0% 15/1443 |
1.7% 58/3388 |
| Renal Failure | 0.4% 98/23350 |
1.4% 79/5709 |
1.8% 142/7791 |
3.0% 43/1443 |
3.5% 120/3388 |
| Cardiac arrest | 0.1% 23/23350 |
0.4% 21/5709 |
0.4% 35/7791 |
0.6% 8/1443 |
1.0% 33/3388 |
| Pulmonary Embolism | 0.5% 113/23350 |
0.4% 23/5709 |
0.6% 47/7791 |
0.5% 7/1443 |
2.6% 87/3388 |
| Deep Venous thrombosis | 0.7% 171/23350 |
0.5% 27/5709 |
0.8% 61/7791 |
1.4% 20/1443 |
3.4% 116/3388 |
| Surgical site infection or dehiscence | 1.4% 334/23350 |
1.7% 96/5709 |
2.4% 190/7791 |
2.8% 40/1443 |
14.4% 487/3388 |
| Bleeding requiring blood transfusion | 4.1% 952/23350 |
7.4% 423/5709 |
14.7% 1147/7791 |
13.2% 191/1443 |
39.1% 1326/3388 |
| Readmission | 4.2% 272/6534 |
5.6% 146/2625 |
6.5% 210/3223 |
8.3% 46/553 |
17.6% 469/2659 |
| Re-operation | 0.9% 62/6534 |
1.8% 48/2625 |
2.0% 64/3223 |
3.8% 21/553 |
5.8% 155/2694 |
For prostatectomy patients, increasing mFI was associated with increased rates of Clavien-Dindo IV events, SS, ventilator dependence, unplanned re-intubations, ARF, CA, bleeding requiring blood transfusion, surgical site infections and dehiscence, re-operations and readmissions (Table 3.a, χ2 p < 0.01 for all). For nephrectomies, increasing mFI was associated with 30 day mortality, Clavien-Dindo IV events, SS, ventilator dependence, unplanned re-intubations, MI, ARF, CA, DVT, bleeding requiring blood transfusion, and readmissions (Table 3.a χ2 p< 0.0005 for all). For nephroureterectomies, increasing mFI was associated with increasing incidence of 30-day mortality, Clavien-Dindo IV complications, SS, ventilator dependence, re-intubations, MI, ARF, CA, DVT, bleeding requiring blood transfusion, and reoperations (Table 3.b, χ2 p<0.05). Finally, for cystectomy, increasing mFI was associated with increased incidence of 30-day mortality, Clavien-Dindo IV complications, ventilator dependence, re-intubations, ARF, and bleeding requiring blood transfusions (Table 3.b, χ2 p< 0.01).
Table 3.
Outcomes grouped by type of surgery and their modified frailty index score.
| Table 3.a | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Radical Prostatectomy | Radical and Partial Nephrectomy | ||||||||||||
| Frailty Index | 0–0.05 | 0.05–0.10 | 0.10–0.15 | 0.15– 0.20 |
>0.20 | p-value | Frailty Index | 0–0.05 | 0.05–0.10 | 0.10–0.15 | 0.15– 0.20 |
>0.20 | p-value |
| 30-day Mortality* | 0.1% 6/11312 |
0.1% 14/9526 |
0.4% 7/1656 |
1.1% 7/637 |
1.4% 3/219 |
<0.005 | 30-day Mortality* | 0.3% 12/4390 |
0.6% 36/5546 |
0.8% 12/1534 |
1.2% 16/1349 |
3.4% 23/681 |
<0.0005 |
| Clavien-Dindo IV* | 0.7% 81/11312 |
1.1% 109/9526 |
1.4% 24/1656 |
3.0% 19/637 |
5.9% 13/219 |
<0.0005 | Clavien-Dindo IV* | 1.5% 66/4390 |
3.0% 169/5546 |
4.0% 61/1534 |
6.1% 82/1349 |
8.4% 57/681 |
<0.0005 |
| Septic Shock/Sepsis* | 0.5% 61/11312 |
0.9% 82/9526 |
1.4% 24/1656 |
2.2% 14/637 |
4.6% 10/219 |
< 0.0005 | Septic Shock/Sepsis* | 0.9% 39/4390 |
1.5% 83/5546 |
0.8% 12/1534 |
2.7% 36/1349 |
3.5% 24/681 |
<0.0005 |
| Ventilator Dependent* | <0.1% 5/11312 |
0.2% 17/9526 |
0.3% 5/1656 |
0.5% 3/637 |
1.8% 4/219 |
<0.0005 | Ventilator Dependent* | 0.3% 13/4390 |
0.7% 38/5546 |
1.3% 20/1534 |
1.7% 23/1349 |
2.9% 20/681 |
<0.0005 |
| Re-Intubated* | 0.1% 10/11312 |
0.3% 27/9526 |
0.3% 5/1656 |
1.1% 7/637 |
2.3% 5/219 |
<0.0005 | Re-Intubated* | 0.5% 21/4390 |
1.0% 55/5546 |
1.5% 23/1534 |
2.7% 36/1349 |
3.7% 25/681 |
<0.0005 |
| Myocardial Infarction* | 0.1% 10/11312 |
0.2% 19/9526 |
0.2% 4/1656 |
0.6% 4/637 |
1.8% 4/219 |
< 0.0005 | Myocardial Infarction* | 0.2% 7/4390 |
0.5% 27/5546 |
0.5% 8/1534 |
1.0% 13/1349 |
1.6% 11/681 |
<0.0005 |
| Acute Renal Failure* | 0.1% 15/11312 |
0.6% 53/9526 |
0.7% 11/1656 |
1.7% 11/637 |
3.7% 8/219 |
<0.0005 | Acute Renal Failure* | 0.6% 28/4390 |
1.4% 80/5546 |
2.7% 42/1534 |
3.4% 46/1349 |
3.7% 25/681 |
<0.0005 |
| Cardiac Arrest* | <0.1% 5/11312 |
0.1% 10/9526 |
0.3% 5/1656 |
0.3% 2/637 |
0.5% 1/219 |
<0.0005 | Cardiac Arrest* | 0.2% 9/4390 |
0.4% 23/5546 |
0.3% 4/1534 |
1.0% 13/1349 |
1.0% 7/681 |
<0.0005 |
| Pulmonary embolism | 0.5% 56/11312 |
0.5% 43/9526 |
0.5% 9/1656 |
0.3% 2/637 |
1.4% 3/219 |
0.365 | Pulmonary embolism | 0.4% 16/4390 |
0.6% 31/5546 |
0.8% 12/1534 |
0.4% 6/1349 |
0.7% 5/681 |
0.285 |
| Deep venous thrombosis | 0.7% 77/11312 |
0.8% 73/9526 |
0.8% 14/1656 |
0.8% 5/637 |
0.9% 2/219 |
0.913 | Deep venous thrombosis * | 0.3% 15/4390 |
0.7% 41/5546 |
0.8% 12/1534 |
1.1% 15/1349 |
0.7% 5/681 |
0.017 |
| Surgical Site Infection or dehiscence* | 1.1% 125/11312 |
1.6% 150/9526 |
1.8% 30/1656 |
2.8% 18/637 |
5.0% 11/219 |
<0.0005 | Surgical Site Infection or dehiscence | 1.9% 83/4390 |
2.1% 114/5546 |
2.3% 36/1534 |
2.4% 32/1349 |
3.1% 21/681 |
0.280 |
| Bleeding requiring blood transfusion * | 3.7% 417/11312 |
4.2% 392/9526 |
4.2% 69/1656 |
8.0% 51/637 |
10.5% 23/219 |
<0.0005 | Bleeding requiring blood transfusion* | 7.2% 315/4390 |
11.1% 617/5546 |
13.0% 200/1534 |
20.9% 282/1349 |
22.9% 156/681 |
<0.0005 |
| Readmission (2011–2013)* | 3.4% 108/3201 |
4.5% 116/2596 |
5.4% 29/542 |
9.0% 16/178 |
17.6% 3/17 |
<0.0005 | Readmission (2011–2013)* | 4.0% 80/2021 |
5.9% 137/2304 |
6.8% 50/736 |
10.3% 60/583 |
14.2% 29/204 |
<0.0005 |
| Re-operation (2011–2013)* | 0.6% 19/3201 |
1.2% 32/2596 |
1.1% 6/542 |
2.8% 5/178 |
0% 0/17 |
0.010 | Re-operation (2011–2013) | 1.5% 30/2021 |
1.9% 14/2304 |
1.9% 14/736 |
2.7% 16/583 |
3.9% 8/204 |
0.075 |
| Table 3.b | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nephroureterectomy | Radical Cystectomy | ||||||||||||
| Frailty Index | 0–0.05 | 0.05–0.10 | 0.10– 0.15 |
0.15– 0.20 |
>0.20 | p-value | Frailty Index | 0–0.05 | 0.05–0.10 | 0.10– 0.15 |
0.15– 0.20 |
>0.20 | p-value |
| 30-day Mortality* | 0.5% 2/410 |
0.9% 6/634 |
3.3% 6/181 |
4.6% 6/130 |
5.7% 5/88 |
<0.0005 | 30-day Mortality* | 2.1% 21/1008 |
2.6% 34/1330 |
2.4% 10/423 |
3.1% 11/351 |
6.8% 12/176 |
0.005 |
| Clavien-Dindo IV* | 1.2% 5/410 |
5.0% 32/634 |
7.2% 13/181 |
11.5% 15/130 |
12.5% 11/88 |
<0.0005 | Clavien-Dindo IV* | 6.6% 73/1108 |
9.2% 122/1330 |
14.2% 60/423 |
10.5% 37/351 |
17.0% 30/176 |
<0.0005 |
| Septic Shock/Sepsis* | 1.2% 5/410 |
2.5% 16/634 |
7.2% 13/181 |
8.5% 11/130 |
4.5% 4/88 |
0.001 | Septic Shock/Sepsis | 10.6% 117/1108 |
13.5% 179/1330 |
14.7% 62/423 |
11.7% 41/351 |
13.1% 23/176 |
0.135 |
| Ventilator Dependent* | 0.2% 1/410 |
0.8% 5/634 |
3.9% 7/181 |
2.3% 3/130 |
5.7% 5/88 |
<0.0005 | Ventilator Dependent* | 1.4% 15/1108 |
2.4% 32/1330 |
4.7% 20/423 |
3.4% 12/351 |
5.7% 10/176 |
< 0.0005 |
| Re-Intubated* | 1.0% 4/410 |
0.9% 6/634 |
2.8% 5/181 |
4.6% 6/130 |
6.8% 6/88 |
<0.0005 | Re-Intubated* | 2.1% 23/1108 |
2.9% 39/1330 |
5.4% 23/423 |
4.3% 15/351 |
6.8% 12/176 |
0.001 |
| Myocardial Infarction* | 0% 0/410 |
1.1% 7/634 |
1.7% 3/181 |
1.5% 2/130 |
1.1% 1/88 |
0.043 | Myocardial Infarction | 1.1% 12/1108 |
1.7% 22/1330 |
2.4% 10/423 |
2.8% 10/351 |
2.3% 4/176 |
0.113 |
| Acute Renal Failure* | 1.0% 4/410 |
3.3% 21/634 |
2.8% 5/181 |
6.2% 8/130 |
4.5% 4/88 |
0.024 | Acute Renal Failure* | 1.6% 18/1108 |
3.5% 46/1330 |
4.7% 20/423 |
6.6% 23/351 |
7.4% 13/176 |
<0.0005 |
| Cardiac Arrest* | 0.2% 1/410 |
0.2% 1/634 |
3.3% 6/181 |
2.3% 3/130 |
2.3% 2/88 |
0.006 | Cardiac Arrest | 0.8% 9/1108 |
1.1% 15/1330 |
0.7% 3/423 |
1.1% 4/351 |
1.1% 2/176 |
0.870 |
| Pulmonary embolism | 0% 0/410 |
0.6% 4/634 |
0.6% 1/181 |
0.8% 1/130 |
1.1% 1/88 |
0.511 | Pulmonary embolism | 2.6% 29/1108 |
2.6% 35/1330 |
2.8% 12/423 |
2.0% 7/351 |
2.3% 4/176 |
0.969 |
| Deep venous thrombosis* | 0.7% 3/410 |
0.8% 5/634 |
3.9% 7/181 |
1.5% 2/130 |
3.4% 3/88 |
0.008 | Deep venous thrombosis | 3.0% 33/1108 |
3.8% 51/1330 |
4.0% 17/423 |
2.6% 9/351 |
3.4% 6/176 |
0.628 |
| Surgical Site Infection or dehiscence | 2.2% 9/410 |
2.8% 18/634 |
2.2% 4/181 |
3.8% 5/130 |
4.5% 4/88 |
0.679 | Surgical Site Infection or dehiscence | 12.5% 139/1108 |
15.0% 200/1330 |
16.8% 71/423 |
13.4% 47/351 |
17.0% 30/176 |
0.148 |
| Bleeding requiring Blood transfusion* | 7.1% 29/410 |
12.5% 79/634 |
17.1% 31/181 |
23.1% 30/130 |
25.0% 22/88 |
<0.0005 | Bleeding requiring blood transfusion* | 34.6% 383/1108 |
39.9% 531/1330 |
44.2% 187/423 |
42.5% 149/351 |
43.2% 76/176 |
0.002 |
| Readmission (2011–2013) | 8.2% 15/182 |
6.3% 14/221 |
7.3% 6/82 |
14.3% 7/49 |
21.1% 4/19 |
0.108 | Readmission (2011–2013) | 15.9% 138/866 |
17.4% 182/1046 |
21.1% 75/355 |
18.5% 50/270 |
19.7% 24/122 |
0.264 |
| Re-operation (2011–2013)* | 2.7% 5/182 |
2.7% 6/221 |
2.4% 2/82 |
8.2% 4/49 |
21.1% 4/19 |
0.001 | Re-operation (2011–2013) | 4.4% 39/882 |
6.6% 70/1057 |
7.5% 27/358 |
5.2% 14/271 |
4.0% 5/126 |
0.116 |
There are differences between the subgroups for the given adverse outcome (noted with an *). 30-day mortality and Clavien Dindo IV complications were significantly associated with increasing frailty index score for all surgeries. Readmission was significant with increasing frailty index score for all surgeries except radical cystectomy (p=0.264) and nephroureterectomy (p=0.108). Re-operation was significant with increasing frailty index score for all except radical cystectomy (p=0.116).
Our multivariate model (Hosmer and Lemeshow Test, p=0.358) controlling for smoking history, gender, procedure and race showed that increasing mFI was associated with increased OR of Clavien-Dindo IV complications with an OR of 3.704 for the frailest patients (mFI > 0.20, CI: 2.865–4.788, p<0.0005) in comparison to the non-frail patients (Table 4). The mFI was significantly associated with mortality with the subgroup of patients with mFI of >0.20 having an OR of 5.946 (CI: 3.718–9.509, p< 0.0005) in comparison to the non-frail patients (Table 4).
Table 4.
A multivsariate logistic regression model with the outcomes of mortality or Clavien IV outcomes for all surgeries was done with the results as shown after selection from univariate analysis. The model adjusts for age, gender, race, smoking status, and procedure and was a good fit for the data (Hosmer and Lemeshow Test, p=0.358). All variables were included as categorical variables with the exception of age, which was a continuous variable. There were increased odds of a Clavien-Dindo IV outcome for all the risk groups ranging from 1.553 in those with a mFI of 0.05–0.10 to 3.704 for those with a mFI > 0.20 in comparison to the reference group of mFI 0–0.05 (p<0.0005). There were increased odds of death for those with a mFI greater than 0.10–0.15 for Clavien-Dindo IV outcomes (p<0.0005) but not mortality when compared to the reference group of mFI 0–0.05 (p=0.066).
| Clavien-Dindo IV Complications |
95% Confidence Interval |
30-Day Mortality |
95% Confidence Interval |
|||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Odds Ratio | Lower Bound |
Upper Bound |
P-value | Odds Ratio |
Lower Bound |
Upper Bound |
P-value |
| Age (years) | 1.034 | 1.027 | 1.041 | <0.0005 | 1.060 | 1.044 | 1.076 | <0.0005 |
| Gender | ||||||||
| Male | Ref. | - | - | - | Ref. | - | - | - |
| Female | 1.228 | 1.038 | 1.453 | 0.017 | 0.892 | 0.651 | 1.223 | 0.479 |
| Modified FI | ||||||||
| 0–0.05 | Ref. | - | - | - | - | - | - | - |
| 0.05–0.10 | 1.553 | 1.305 | 1.848 | <0.0005 | 1.452 | 0.975 | 2.162 | 0.066 |
| 0.10–0.15 | 2.076 | 1.660 | 2.597 | <0.0005 | 1.939 | 1.183 | 3.176 | 0.009 |
| 0.15–0.20 | 2.761 | 2.199 | 3.466 | <0.0005 | 3.397 | 2.133 | 5.409 | <0.0005 |
| >0.20 | 3.704 | 2.865 | 4.788 | <0.0005 | 5.946 | 3.718 | 9.509 | <0.0005 |
| Race and Ethnicity | ||||||||
| White | Ref. | - | - | - | Ref. | - | - | - |
| Non-White or Hispanic | 1.150 | 0.978 | 1.352 | 0.091 | 1.192 | 0.848 | 1.676 | 0.312 |
| Smoking Status | ||||||||
| Non-Smoker | Ref. | - | - | - | Ref. | - | - | - |
| Smoker | 1.314 | 1.116 | 1.547 | 0.001 | 1.411 | 1.007 | 1.979 | 0.046 |
| Procedure | ||||||||
| MIS prostatectomy | Ref. | - | - | - | Ref. | - | - | - |
| Open prostatectomy | 1.485 | 1.119 | 1.969 | 0.006 | 1.758 | 0.872 | 3.545 | 0.115 |
| MIS radical nephrectomy | 1.756 | 1.344 | 2.295 | <0.0005 | 2.337 | 1.285 | 4.252 | 0.005 |
| Open radical nephrectomy | 5.759 | 4.595 | 7.216 | <0.0005 | 6.792 | 3.984 | 11.580 | <0.0005 |
| MIS partial nephrectomy | 1.482 | 1.059 | 2.073 | 0.022 | 1.535 | 0.676 | 3.484 | 0.306 |
| Open partial nephrectomy | 3.677 | 2.781 | 4.862 | <0.0005 | 2.606 | 1.219 | 5.573 | 0.013 |
| MIS nephroureterectomy | 3.402 | 2.402 | 4.817 | <0.0005 | 5.667 | 2.907 | 11.048 | <0.0005 |
| Open nephroureterectomy | 4.734 | 3.071 | 7.298 | <0.0005 | 6.236 | 2.684 | 14.493 | <0.0005 |
| Radical Cystectomy | 7.420 | 6.013 | 9.155 | <0.0005 | 9.550 | 5.794 | 15.743 | <0.0005 |
MIS: Minimally Invasive
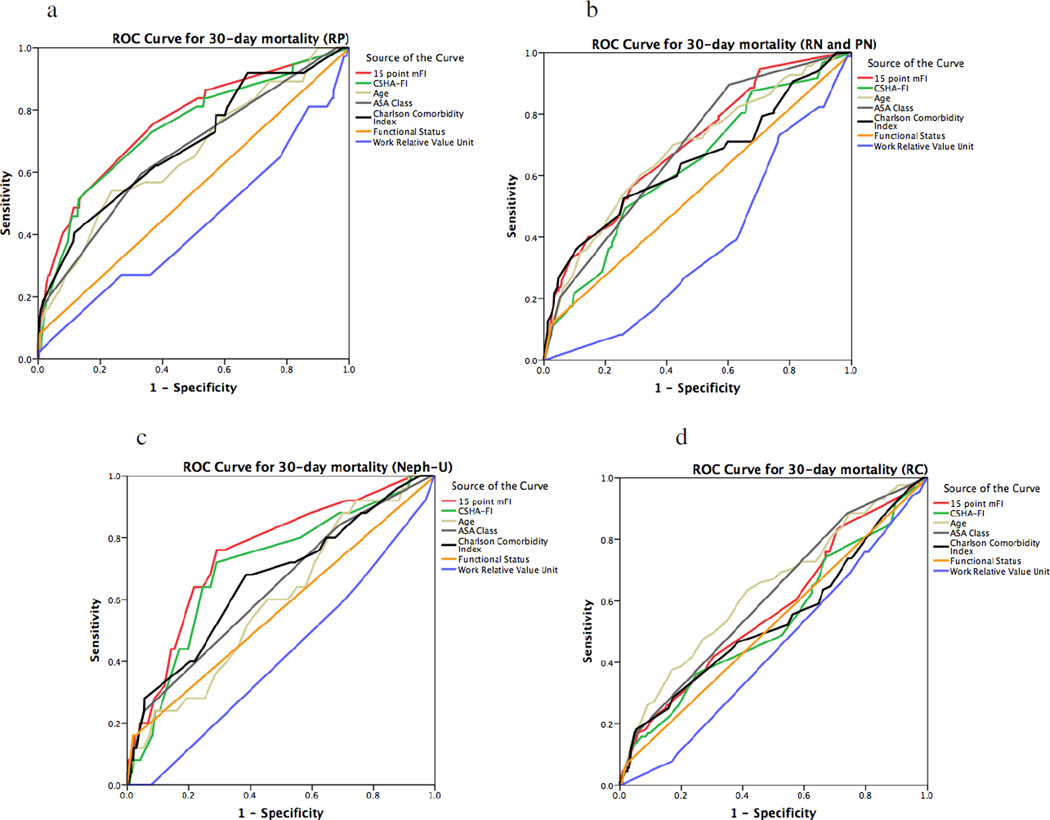
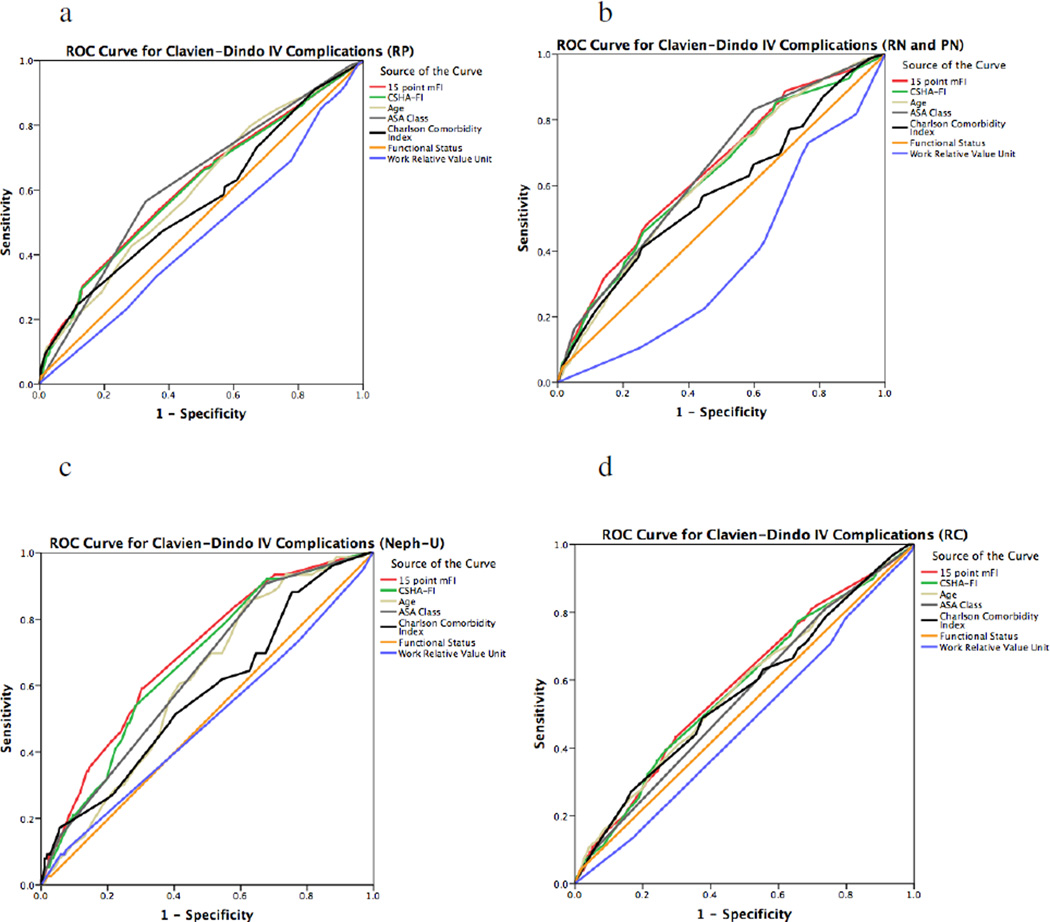
The ROC curve showed that our mFI had fair sensitivity and specificity for predicting death in radical prostatectomy (C-statistic 0.760, p<0.0005) and nephroureterectomy (C-statistic 0.753, p<0.0005) (Figure 1). With regards to the ROC curve for Clavien-Dindo IV complications, the modified FI had poor sensitivity and specificity for all outcomes of interest (Figure 2). In both assessing mortality and Clavien-Dindo IV outcomes in all surgeries, the 15-point mFI was superior to the 11-point CSHA-FI used in the literature.
Figure 1.
Receiver operator characteristics (ROC) curve for mortality using our mFI in comparison to the existing parameters of predicting adverse outcomes. Our mFI had very poor sensitivity and specificity for predicting death in radical cystectomy (RC, Fig. 1.d C-statistic 0.574, p< 0.0005), fair sensitivity and specificity for predicting death in radical prostatectomy (RP, Fig. 1.a, C-statistic 0.760, p<0.0005), fair sensitivity and specificity for predicting death in nephroureterectomy (Neph-U, Fig. 1.c, C-statistic 0.753, p<0.0005), and poor sensitivity and specificity for predicting death in partial and radical nephrectomy (PN and RN, Fig. 1.b, C-statistic 0.698, p<0.0005). In all cases except RC, our 15-point mFI performed better than the ASA Risk Class Stratification System and the Charlson Comorbidity Index. For RC, the ASA Class outperformed the mFI with a C-statistic of 0.612 (p<0.0005) in comparison to the 15-point mFI that had a C-statistic of 0.574 (p<0.0005). Our 15-point mFI was superior to the 11-point CSHA-FI in all cases.
Figure 2.
Receiver operator characteristics (ROC) curve for Clavien-Dindo IV outcomes using the mFI in comparison to the existing parameters of frailty. The mFI had poor sensitivity and specificity in radical prostatectomy (RP, Fig. 1.a, C-statistic 0.615, p<0.0005), very poor sensitivity and specificity in radical cystectomy (RC, Fig. 1.d, C-statistic 0.585, p< 0.0005), poor sensitivity and specificity in nephroureterectomy (Neph-U, Fig. 1.c, C-statistic 0.691, p<0.0005), and poor sensitivity and specificity in radical and partial nephrectomy (RN and PN, Fig. 1.b, C-statistic 0.646, p<0.0005). However, the mFI equaled or surpassed the Charlson Comorbidity Index or ASA Class Risk stratification in RN, PN and Neph-U. In RP, the ASA Class outcompeted the mFI with a higher C-statistic of 0.623 in comparison to 0.615. In RC, the ASA Class also outcompeted the mFI with a higher C-statistic of 0.612 in comparison to 0.585. The 15-point mFI was superior to the 11-point CSHA-FI in all the comparisons.
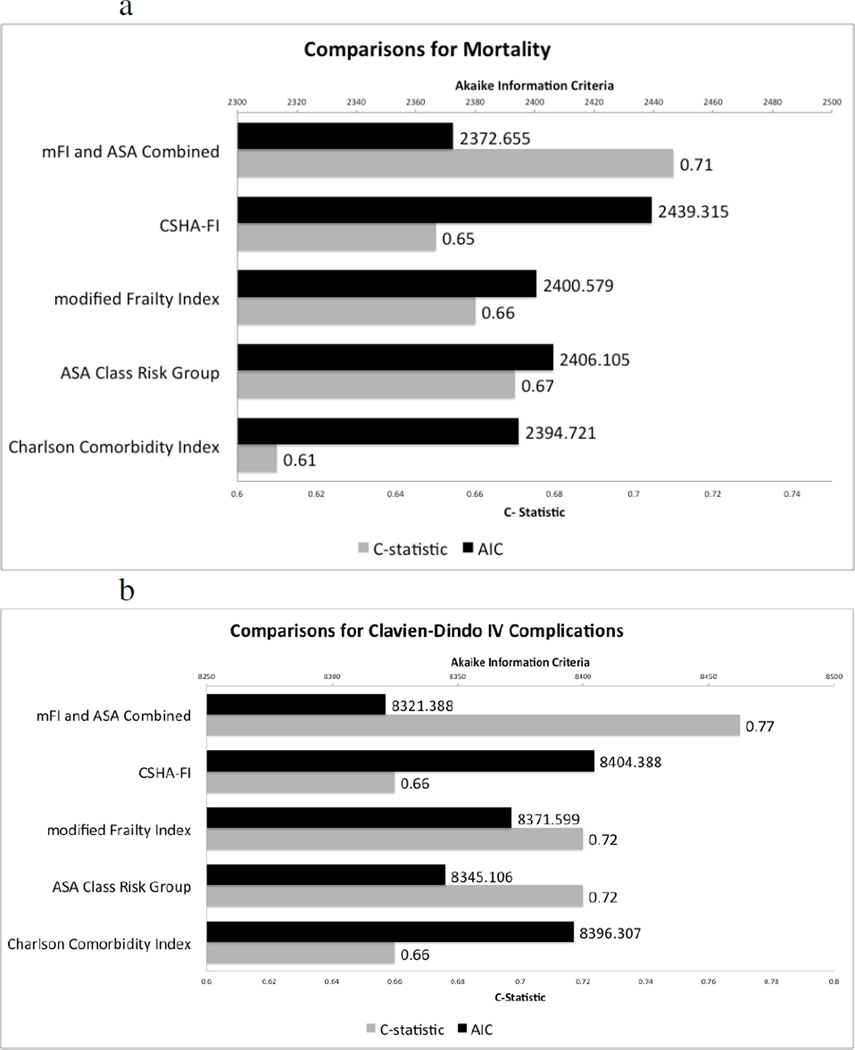
A multinomial logistic regression model was created using the multivariate model to assess the differences in AIC while also measuring the C-statistic for each model to compare our 15-point mFI with the 11-point CSHA-FI, ASA Class Risk, and CCI as well as a combined mFI + ASA index [21]. These each were compared as continuous variables. The mFI had fair sensitivity and specificity (C-statistic for mortality 0.66, for Clavien-Dindo IV Complications 0.72) while maintaining a low AIC (AIC for mortality= 2400.6, AIC for Clavien Dindo IV Complications=8371.6) although the ASA Class Risk groupings outperformed it in both outcomes with a higher sensitivity and specificity (C-statistic for mortality 0.67, for Clavien-Dindo IV Complications 0.72) and lower AIC (AIC mortality=2406.1, AIC Clavien-Dindo IV Complications =8345.1). The combined ASA and mFI was superior in all regards with the lowest AIC (Mortality=2372.7, Clavien Dindo IV Complications =8321.4) and the highest C-statistic (Mortality 0.71, Clavien Dindo IV Complications = 0.77) among models compared.
Discussion
Compared to healthy patients, frail patients who are exposed to stressors such as surgical intervention may suffer disproportionate decompensation due to a lack of physiological reserve [22]. Therefore the risk-benefit ratio of surgery should include frailty and severity of comorbidities to capture the full risk of a surgical candidate undergoing a surgical oncological intervention.
In this retrospective study, using the ACS-NSQIP dataset, we validated a FI, modified it for patients undergoing surgery for a primary urologic malignancy, show the frailty indexes inferiority to the ASA Risk stratification tool and superiority to the CCI. Combining the prospectively collected ASA with the mFI, we created a superior risk stratification tool that predicts adverse events. The ASA Risk Stratification likely added elements not discernible from history alone at the day of surgery.
For cystectomy patients, the mFI was not as good of a predictor of 30-day mortality as other measures as it had a very poor sensitivity and specificity with a C-statistic < 0.6. This suggests that surgeries with high underlying risks like cystectomies may be harder to predict adverse events based on frailty or comorbidities alone. This may also be explained by the presentations of the different underlying diseases driving the need for surgery. Bladder cancer patients requiring surgery usually present with high-risk muscle invasive bladder cancer patients after failing local management. These patients tend to be at a higher tumor stage and underlying risk of death in comparison to patients undergoing partial nephrectomy or those with low and intermediate risk prostate cancer undergoing radical prostatectomy. For all surgeries, our mFI was superior or comparable to the CCI in predicting mortality or Clavien-Dindo IV outcomes but it was not superior to the ASA, which had a higher C-statistic and lower AIC. Hence, when the ASA was combined with our mFI, it was the best predictor of morbidity and mortality. Although this frailty index has been studied before, this study is the first to rigorously compare it to other risk indices used in clinical practice while creating a novel frailty index with potential clinical utility.
In the literature, frailty is associated with postoperative complications especially in older adults with comorbidities, across surgical specialties [11, 23]. However, little has been done to disentangle the relationship of the comorbidities measured by existing risk stratification tools, and the different existing indexes for frailty. In a prospective study of patients below 65 years of age undergoing elective operations, Robinson et al. used walking speed as a surrogate for frailty. Decreased walking speed was associated with increased mortality at 1 year post-operation, but this test was not compared to other popular risk stratification tools [24]. Revenig, et al., in a prospective study assessing frailty by measuring shrinking, weakness, exhaustion, low activity, and slow walking speed showed that increasingly frail patients had increased complications, but it was not compared to other risk stratification tools [25]. Courtney-Brooks, et al., focused on patients undergoing surgery for gynecological malignancy. Their prospectively measured frailty index predicted 30-day post-op complication but did not detect deaths or readmissions [9]. Makary, et al. prospectively collected the Fried Frailty Index on patients undergoing elective general surgery while augmenting this index with the ASA Class Risk Groups, Lee’s revised cardiac risk index, and the Eagle Score. This modified Fried Frailty index predicted worse outcomes and higher post-operative complications by increasing the sensitivity of other risk stratification tools, capturing more adverse outcomes [6]. No information was provided in comparing the performance of their modified Fried’s Index with other validated risk stratification tools like the CCI.
There may be better ways to quantify frailty that do not depend on history and patient narratives. A novel method by Waits, et al. in Michigan used a surrogate for frailty called morphometric age, created from imaging characteristics of patients 90-days prior to undergoing surgery. Increasing morphometric age predicted an increased number of complications, and worse outcomes after liver transplantation [26]. Furthermore, a study by Psutka, et al. looked at patients with sarcopenia, determined by imaging, and who underwent cystectomy. They showed that patients with sarcopenia had worse survival and worse cancer specific outcomes [27].
Our study has several limitations including its retrospective nature. Patient cancer specific information, treatment history, and longitudinal follow-up after 30 days were not available. Those who received non-cytotoxic chemotherapeutic agents in the case of renal cell carcinoma were not recorded but given the decreased morbidity of these treatments in comparison to cytotoxic chemotherapeutic agents we believe these can be included in our mFI with a lower penalty. Another limitation is that we do not have information about institutions or surgeons performing the procedures in order to understand in which situations warrant the use of a frailty index calculation before surgery. This is important especially in prostate cancer where alternate non-surgical curative treatments exist such as radiation. Moreover, the 15 variables in our frailty index may only constitute a portion of the frailty syndrome with more variables needed to capture the spectrum of frailty. However, much of the literature has already used this modified 11 variable frailty index with similar results to our study although few have compared it to existing risk stratification tools as we have and our 15 variable modified frailty index was superior in predicting patients at higher risk of mortality (11 point CSHA-FI AUC= 0.659 vs. 15 point FI AUC= 0.716, p< 0.0005 for both) and Clavien-Dindo IV complications (11 point CSHA-FI AUC= 0.645 versus 15 point FI AUC= 0.665, p< 0.0005 for both). When combined with ASA Risk Stratification, its predictive ability was even more pronounced. Finally, 2.8% of patients were considered vulnerable with mFI > 0.20 and modifying Rockland’s, et al definition of vulnerable in their frailty index spectrum [4]. This implies that surgeons may prospectively identify extremely frail patients who are not surgical candidates, refusing to operate them. This may explain the low amount of adverse events observed although it is in line with the literature.
Conclusion
There has been a growing need for a structured, evidence based preoperative evaluation for frail patients undergoing oncological genitourinary surgery [28, 29]. Our modified FI was associated with worse outcomes comparable to existing risk stratification tools when looking at 30-day mortality and Clavien-Dindo IV outcomes. When our mFI was combined with the ASA Class risk stratification, it was superior to all existing risk stratification tools indicating potential clinical application. We plan to apply this mFI to our active surveillance population in both renal cell carcinoma and prostate cancer to see whether it can predict who fails active surveillance or expires from competing causes of mortality.
Figure 3.
A comparison of different risk stratification tools with the modified frailty index in our multivariate model. The parameters measured to assess the different models were Akaiki information criteria (AIC) and the C- Statistic. A low AIC indicates better goodness of fit while a higher C-statistic value indicates an optimized model with both good sensitivity and specificity for a given outcome. The outcomes assessed were mortality (Figure 3.a) and Clavien-Dindo IV complications (Figure 3.b). The modified frailty index had fair sensitivity and specificity (C-statistic for mortality 0.66, for Clavien Dindo IV Complications 0.72) while maintaining a low AIC (AIC for mortality= 2400.6, AIC for Clavien Dindo IV Complications=8371.6) although the ASA Class Risk groupings outperformed it in both outcomes with an equal or higher sensitivity and specificity (C-statistic for mortality 0.67, for Clavien Dindo IV Complications 0.72) and lower AIC (AIC mortality=2406.1, AIC Clavien Dindo IV Complications =8345.1). However, when the ASA Class Risk Group and the mFI were combined, it was superior in all regards with lowest AIC (Mortality=2372.7, Clavien Dindo IV Complications =8321.4) and the highest C-statistic (Mortality 0.71, Clavien Dindo IV Complications = 0.77).
Acknowledgements
Funding for the lead author and primary investigator was awarded through the National Institute of Diabetes and Digestive and Kidney Diseases via a National Institute of Health T35 Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fried LP. Frailty in older adults: Evidence for a phenotype. The journals of gerontology Series A. Biological sciences and medical sciences. 2001;56(3) doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 2.Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, et al. Frailty Consensus: A Call to Action. J Am Med Dir Assoc. 2013 Jun;14(6):392–397. doi: 10.1016/j.jamda.2013.03.022. PubMed PMID: WOS:000320613100004. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Handforth C, Clegg A, Young C, Simpkins S, Seymour MT, Selby PJ, et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2014 Nov 17; doi: 10.1093/annonc/mdu540. PubMed PMID: 25403592. [DOI] [PubMed] [Google Scholar]
- 4.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2005 Aug 30;173(5):489–495. doi: 10.1503/cmaj.050051. PubMed PMID: 16129869. Pubmed Central PMCID: 1188185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol a-Biol. 2007 Jul;62(7):738–743. doi: 10.1093/gerona/62.7.738. PubMed PMID: WOS:000253828700008. English. [DOI] [PubMed] [Google Scholar]
- 6.Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, et al. Frailty as a Predictor of Surgical Outcomes in Older Patients. Journal of the American College of Surgeons. 2010 Jun;210(6):901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 7.Joseph B, Pandit V, Zangbar B, Kulvatunyou N, Hashmi A, Green DJ, et al. Superiority of frailty over age in predicting outcomes among geriatric trauma patients: a prospective analysis. JAMA Surg. 2014 Aug;149(8):766–772. doi: 10.1001/jamasurg.2014.296. PubMed PMID: 24920308. [DOI] [PubMed] [Google Scholar]
- 8.Saxton A, Velanovich V. Preoperative frailty and quality of life as predictors of postoperative complications. Ann Surg. 2011 Jun;253(6):1223–1229. doi: 10.1097/SLA.0b013e318214bce7. PubMed PMID: 21412145. [DOI] [PubMed] [Google Scholar]
- 9.Courtney-Brooks M, Tellawi AR, Scalici J, Duska LR, Jazaeri AA, Modesitt SC, et al. Frailty: an outcome predictor for elderly gynecologic oncology patients. Gynecologic oncology. 2012 Jul;126(1):20–24. doi: 10.1016/j.ygyno.2012.04.019. PubMed PMID: 22522190. [DOI] [PubMed] [Google Scholar]
- 10.Patel KV, Brennan KL, Brennan ML, Jupiter DC, Shar A, Davis ML. Association of a Modified Frailty Index With Mortality After Femoral Neck Fracture in Patients Aged 60 Years and Older. Clin Orthop Rel Res. 2014 Mar;472(3):1010–1017. doi: 10.1007/s11999-013-3334-7. PubMed PMID: WOS:000330976400037. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farhat JS, Velanovich V, Falvo AJ, Horst HM, Swartz A, Patton JH, Jr, et al. Are the frail destined to fail? Frailty index as predictor of surgical morbidity and mortality in the elderly. The journal of trauma and acute care surgery. 2012 Jun;72(6):1526–1530. doi: 10.1097/TA.0b013e3182542fab. discussion 30-1. PubMed PMID: 22695416. [DOI] [PubMed] [Google Scholar]
- 12.Karam J, Tsiouris A, Shepard A, Velanovich V, Rubinfeld I. Simplified Frailty Index to Predict Adverse Outcomes and Mortality in Vascular Surgery Patients. Annals of Vascular Surgery. 2013 Oct;27(7):904–908. doi: 10.1016/j.avsg.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Obeid NM, Azuh O, Reddy S, Webb S, Reickert C, Velanovich V, et al. Predictors of critical care-related complications in colectomy patients using the National Surgical Quality Improvement Program: exploring frailty and aggressive laparoscopic approaches. The journal of trauma and acute care surgery. 2012 Apr;72(4):878–883. doi: 10.1097/TA.0b013e31824d0f70. PubMed PMID: 22491599. [DOI] [PubMed] [Google Scholar]
- 14.Velanovich V, Antoine H, Swartz A, Peters D, Rubinfeld I. Accumulating deficits model of frailty and postoperative mortality and morbidity: its application to a national database. Journal of Surgical Research. 2013 Jul;183(1):104–110. doi: 10.1016/j.jss.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 15.Tsiouris A, Hammoud ZT, Velanovich V, Hodari A, Borgi J, Rubinfeld I. A modified frailty index to assess morbidity and mortality after lobectomy. The Journal of surgical research. 2013 Jul;183(1):40–46. doi: 10.1016/j.jss.2012.11.059. PubMed PMID: 23273884. [DOI] [PubMed] [Google Scholar]
- 16.Pena FG, Theou O, Wallace L, Brothers TD, Gill TM, Gahbauer EA, et al. Comparison of alternate scoring of variables on the performance of the frailty index. BMC geriatrics. 2014;14:25. doi: 10.1186/1471-2318-14-25. PubMed PMID: 24559204. Pubmed Central PMCID: 3938909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang F, Dupuis JY, Nathan H, Williams K. An analysis of the association between Preoperative renal dysfunction and outcome in cardiac surgery - Estimated creatinine clearance or plasma creatinine level as measures of renal function. Chest. 2003 Nov;124(5):1852–1862. doi: 10.1378/chest.124.5.1852. PubMed PMID: WOS:000186591600039. English. [DOI] [PubMed] [Google Scholar]
- 18.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo Classification of Surgical Complications Five-Year Experience. Ann Surg. 2009 Aug;250(2):187–196. doi: 10.1097/SLA.0b013e3181b13ca2. PubMed PMID: WOS: 000268873400002. English. [DOI] [PubMed] [Google Scholar]
- 19.Webb S, Rubinfeld I, Velanovich V, Horst HM, Reickert C. Using National Surgical Quality Improvement Program (NSQIP) data for risk adjustment to compare Clavien 4 and 5 complications in open and laparoscopic colectomy. Surgical endoscopy. 2012 Mar;26(3):732–737. doi: 10.1007/s00464-011-1944-2. PubMed PMID:22038161. [DOI] [PubMed] [Google Scholar]
- 20.Alberts BD, Woldu SL, Weinberg AC, Danzig MR, Korets R, Badani KK. Venous thromboembolism after major urologic oncology surgery: a focus on the incidence and timing of thromboembolic events after 27,455 operations. Urology. 2014 Oct;84(4):799–806. doi: 10.1016/j.urology.2014.05.055. PubMed PMID: 25156513. [DOI] [PubMed] [Google Scholar]
- 21.Kates M, Swanson S, Wisnivesky JP. Survival following lobectomy and limited resection for the treatment of stage I non-small cell lung cancer<=1 cm in size: a review of SEER data. Chest. 2011 Mar;139(3):491–496. doi: 10.1378/chest.09-2547. PubMed PMID: 20576736. [DOI] [PubMed] [Google Scholar]
- 22.Bergman H, Ferrucci L, Guralnik J, Hogan DB, Hummel S, Karunananthan S, et al. Frailty: an emerging research and clinical paradigm--issues and controversies. J Gerontol A Biol Sci Med Sci. 2007 Jul;62(7):731–737. doi: 10.1093/gerona/62.7.731. PubMed PMID: 17634320. Pubmed Central PMCID:2645660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dasgupta M, Rolfson DB, Stolee P, Borrie MJ, Speechley M. Frailty is associated with postoperative complications in older adults with medical problems. Arch Gerontol Geriatr. 2009 Jan-Feb;48(1):78–83. doi: 10.1016/j.archger.2007.10.007. PubMed PMID: 18068828. [DOI] [PubMed] [Google Scholar]
- 24.Robinson TN, Wu DS, Sauaia A, Dunn CL, Stevens-Lapsley JE, Moss M, et al. Slower walking speed forecasts increased postoperative morbidity and 1-year mortality across surgical specialties. Ann Surg. 2013 Oct;258(4):582–588. doi: 10.1097/SLA.0b013e3182a4e96c. discussion 8–90. PubMed PMID: 23979272. Pubmed Central PMCID: 3771691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Revenig LM, Canter DJ, Master VA, Maithel SK, Kooby DA, Pattaras JG, et al. A Prospective Study Examining the Association Between Preoperative Frailty and Postoperative Complications in Patients Undergoing Minimally Invasive Surgery. J Endourol. 2014 Apr;28(4):476–480. doi: 10.1089/end.2013.0496. PubMed PMID: WOS:000337304100014. English. [DOI] [PubMed] [Google Scholar]
- 26.Waits SA, Kim EK, Terjimanian MN, Tishberg LM, Harbaugh CM, Sheetz KH, et al. Morphometric Age and Mortality After Liver Transplant. JAMA Surg. 2014 Apr;149(4):335–340. doi: 10.1001/jamasurg.2013.4823. PubMed PMID: WOS:000334609900005. English. [DOI] [PubMed] [Google Scholar]
- 27.Psutka SP, Carrasco A, Schmit GD, Moynagh MR, Boorjian SA, Frank I, et al. Sarcopenia in patients with bladder cancer undergoing radical cystectomy: impact on cancer-specific and all-cause mortality. Cancer. 2014 Sep 15;120(18):2910–2918. doi: 10.1002/cncr.28798. PubMed PMID: 24840856. [DOI] [PubMed] [Google Scholar]
- 28.Amrock LG, Deiner S. The implication of frailty on preoperative risk assessment. Curr Opin Anesthesiol. 2014 Jun;27(3):330–335. doi: 10.1097/ACO.0000000000000065. PubMed PMID: WOS:000335957100012. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bettelli G. Anaesthesia for the elderly outpatient: preoperative assessment and evaluation, anaesthetic technique and postoperative pain management. Current opinion in anaesthesiology. 2010 Dec;23(6):726–731.. doi: 10.1097/ACO.0b013e3283400b6c. PubMed PMID: 20930621. [DOI] [PubMed] [Google Scholar]