Abstract
Heterologous sensitization of adenylyl cyclase (also referred to as superactivation, sensitization, or supersensitization of adenylyl cyclase) is a cellular adaptive response first described 40 years ago in the laboratory of Dr. Marshall Nirenberg. This apparently paradoxical cellular response occurs following persistent activation of Gαi/o-coupled receptors and causes marked enhancement in the activity of adenylyl cyclases, thereby increasing cAMP production. Since our last review in 2005, significant progress in the field has led to a better understanding of the relevance of, and the cellular biochemical processes that occur, during the development and expression of heterologous sensitization. In this review we will discuss the recent advancements in the field and the mechanistic hypotheses on heterologous sensitization.
Keywords: Heterologous sensitization, adenylyl cyclase, G protein-coupled receptor, superactivation, G protein subunit, protein kinase
1. Background
1.1 Introduction to heterologous sensitization
The history of and basic concepts involving heterologous sensitization of adenylyl cyclase were extensively discussed in our previous review (Watts and Neve, 2005). Thus, the present review will provide a limited introduction describing the history of this topic, and then incorporate recent findings into our current understanding of this paradoxical phenomenon, first described forty years ago in the laboratory of Dr. Marshall Nirenberg (Sharma et al., 1975).
G protein-coupled receptors (GPCRs) are seven transmembrane domain proteins involved in the transmission of extracellular signals into intracellular signaling cascades. Because of their diversity and prominent physiological relevance, GPCRs represent the most targeted class of proteins by FDA-approved drugs (Overington et al., 2006; Rask-Andersen et al., 2011). Various hormones, neurotransmitters, and drugs interact with GPCRs to elicit their cellular and physiological responses (Hanson and Stevens, 2009; Lefkowitz, 2004). Activation of GPCRs leads to conformational changes in the receptor that ultimately cause activation of heterotrimeric G proteins through the exchange of GDP for GTP in the Gα subunit (Nygaard et al., 2013; Rasmussen et al., 2011). The Gα subunit and Gβγ subunits are thought to then dissociate/undergo rearrangement, leading to diverse signaling events through a variety of effectors (Rasmussen et al., 2011). Following the activation of associated G proteins, the receptor can be phosphorylated by G protein-coupled receptor kinases (GRK), allowing for the recruitment of β-arrestin to the GPCR (Lefkowitz and Shenoy, 2005; Nygaard et al., 2013). Recruitment of β-arrestin can lead to receptor desensitization and receptor downregulation from the membrane, as well as diverse β-arrestin signaling events (Lefkowitz and Shenoy, 2005).
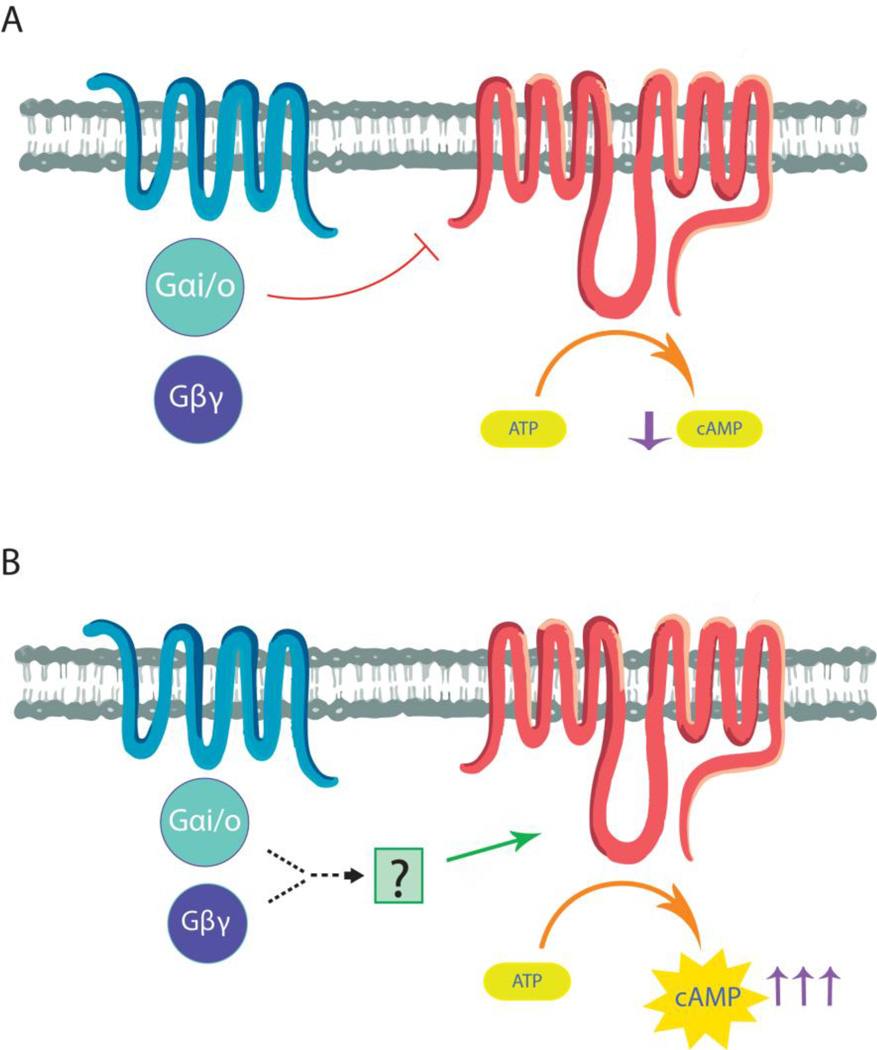
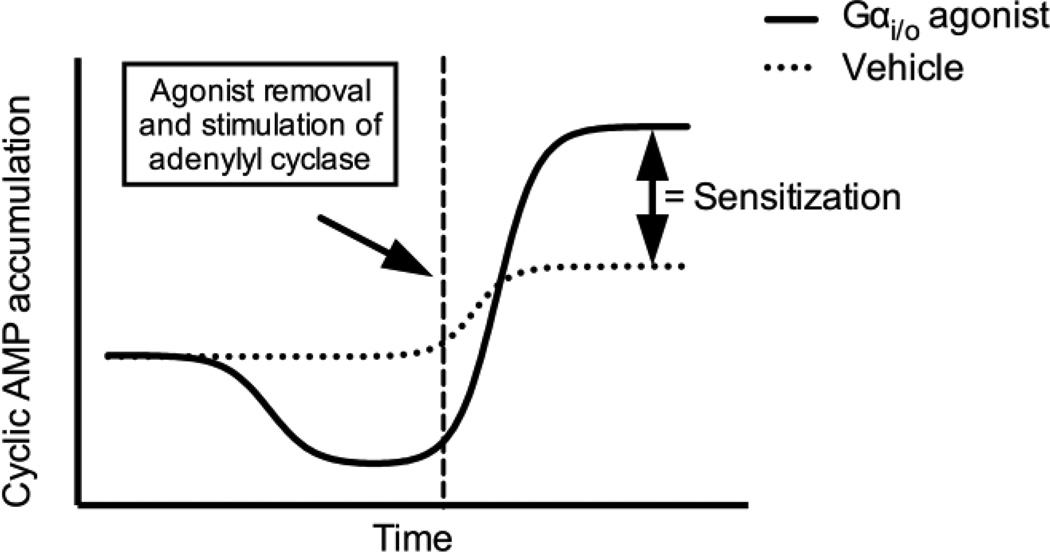
There are four major types of Gα subunits of G proteins (Gαs, Gαi/o, Gαq/11, and Gα12/13) that lead to distinct cellular signaling events (Moreira, 2014). The Gαs family of proteins activate adenylyl cyclases to increase intracellular cAMP concentrations (Rasmussen et al., 2011; Sunahara and Taussig, 2002). Intracellular cAMP levels are reduced following receptor activation of Gαi/o proteins, which acutely inhibit adenylyl cyclases (figure 1A) (Sunahara and Taussig, 2002; Watts et al., 1998). In contrast, prolonged stimulation of Gαi/o-coupled receptors leads to a cellular adaptive response known as heterologous sensitization of adenylyl cyclase (also referred to as superactivation, sensitization, or supersensitization of adenylyl cyclase) (figure 1B). This Gαi/o-coupled receptor-mediated enhancement of cAMP signaling is most readily observed following the addition of receptor antagonist and subsequent activation of adenylyl cyclase (figure 2). Heterologous sensitization was first reported for the δ-opioid receptor in the laboratory of Dr. Marshall Nirenberg (Sharma et al., 1975). Since then, it has been demonstrated for a number of GPCRs and is believed to be a signaling event shared by most Gαi/o-coupled receptors (Watts and Neve, 2005).
Figure 1.
Differential regulation of adenylyl cyclase by Gαi/o-coupled receptors. A. Acute activation of Gαi/o-coupled receptors leads to activation of Gαi/o proteins, which inhibit adenylyl cyclases and, therefore, decrease cAMP production. B. In heterologous sensitization persistent activation of Gαi/o-coupled receptors causes a cellular adaptive response that enhances drug-stimulated adenylyl cyclase activity and increased cAMP accumulation.
Figure 2.
Temporal relationship of cAMP accumulation as a result of the activation of Gαi/o-coupled receptors. Initial activation of Gαi/o-coupled receptors leads to a decrease in cAMP production, however, persistent activation leads to heterologous sensitization and a subsequent enhancement in cAMP production that is readily observed by removing the Gαi/o agonist and stimulating the adenylyl cyclase.
1.2 Adenylyl cyclases
Adenylyl cyclases are responsible for catalyzing the synthesis of cAMP from ATP and serve as the downstream effector for mediating the sensitized cAMP response. They are also acutely modulated by GPCRs that signal through Gαs, Gαq/11, and Gαi/o proteins. The membrane-bound adenylyl cyclases share an overall similar structure, which is composed of three intracellular domains (N terminus, C1 domain, and C2 domain) and two transmembrane clusters (M1 and M2), each containing six transmembrane helixes (Cooper and Crossthwaite, 2006; Sadana and Dessauer, 2009; Wang et al., 2009). There are nine different isoforms of membrane-bound adenylyl cyclases (Cooper and Crossthwaite, 2006; Sadana and Dessauer, 2009), and the expression patterns and regulatory properties of the adenylyl cyclases are unique for each isoform. All membranous adenylyl cyclase isoforms are stimulated by Gαs; however, the other regulatory properties allow them to be divided into four groups (Patel et al., 2001; Sunahara and Taussig, 2002). Group 1 adenylyl cyclases include AC1, AC3, and AC8 and are stimulated by calcium/calmodulin. AC2, AC4, and AC7 represent group 2 adenylyl cyclases, which are conditionally activated by Gβγ subunits. Group 3 adenylyl cyclases comprise AC5 and AC6, which are inhibited by calcium. AC9 is the lone adenylyl cyclase isoform in group 4 and is relatively insensitive to the small molecule adenylyl cyclase activator, forskolin (Hacker et al., 1998). These unique regulatory properties of the adenylyl cyclases provide isoform-specific mechanisms for the regulation of adenylyl cyclases, and also provide for group and isoform-specific mechanisms for the expression of heterologous sensitization (Watts and Neve, 2005). For example, prolonged activation of Gαi/o-coupled GPCRs elevates the calcium-stimulated activity of AC1 and AC8, but not AC3 (Avidor-Reiss et al., 1997; Cumbay and Watts, 2001; Nevo et al., 1998; Watts and Neve, 1996). Group 2 adenylyl cyclases are not sensitized to stimulation by Gαs, but persistent dopamine D2 receptor activation sensitizes AC2 to activation by PKC (Avidor-Reiss et al., 1997; Cumbay and Watts, 2001; Nevo et al., 1998; Rhee et al., 2000; Thomas and Hoffman, 1996; Watts and Neve, 1996). Other proteins that interact with and regulate activity of specific isoforms of adenylyl cyclase include calmodulin, A-kinase-anchoring-proteins (AKAPs), Snapin, Ric8a, protein phosphatase 2A (PP2A), and the protein associated with Myc (PAM) (Masada et al., 2012; Sadana and Dessauer, 2009; Wang et al., 2009). The regulatory properties and binding partners specific to each adenylyl cyclase isoform add to the complexity of heterologous sensitization and suggest that even though it is likely that some mechanistic features of sensitization are shared by all adenylyl cyclases, there are likely isoform-specific mechanisms as well.
2. Mechanistic insights on heterologous sensitization
2.1 Gαi/o subunits
Heterologous sensitization is a phenomenon shared by numerous Gαi/o-coupled receptors and, because of this, is linked to activation of Gαi/o proteins. Mechanistically, inactivation of Gαi/o with pertussis toxin treatment blunts receptor-mediated heterologous sensitization (Watts, 2002). Pretreatment with pertussis toxin leads to ADP-ribosylation of Gαi/o subunits, ultimately preventing their activation by receptors. Because pertussis toxin inhibits all isoforms of Gαi (i.e. Gαi1, Gαi2, Gαi3), and Gαo (Gαoa and Gαob), pertussis toxin insensitive (PTXi) isoforms of inhibitory Gα have been employed to determine the isoforms involved in heterologous sensitization for a number of receptors (Clark et al., 2004; Taussig et al., 1992; Tso and Wong, 2000, 2001; Watts et al., 1998; Zhang et al., 2006). Despite receptor dependency, PTXi-Gαo robustly rescued sensitization through the dopamine D2 receptor and µ-opioid receptor.(Clark et al., 2004; Watts et al., 1998). These observations are consistent with studies of dopamine D2 and µ-opioid receptor coupling to Gαo in the central nervous system (Chalecka-Franaszek et al., 2000; Jiang et al., 2001; Sternweis and Robishaw, 1984). On the other hand, there is also evidence that other inhibitory Gα subunits (i.e. Gαi1 or Gαi2) are also involved in heterologous sensitization (Tso and Wong, 2000, 2001; Zhang et al., 2006). This and other research (Clark et al., 2004; Watts et al., 1998) revealed only a partial rescue by a single PTXi Gα protein, suggesting that heterologous sensitization may involve multiple inhibitory Gα subunits.
There are two other inhibitory Gα subunits, Gαt and Gαz, of which only Gαt is sensitive to inactivation by pertussis toxin (Wong et al., 1992; Yamaguchi et al., 1997). Expression of Gαt blunts heterologous sensitization through µ and δ-opioid receptors (Avidor-Reiss et al., 1996; Rubenzik et al., 2001; Thomas and Hoffman, 1996), and heterologous sensitization mediated by Gαz seems to be dependent on the isoform of adenylyl cyclase expressed in the cells. For example, following pertussis toxin treatment and overexpression of Gαz, heterologous sensitization through the µ-opioid receptor was observed in cells expressing AC5, but not AC6 (Ammer and Christ, 2002).
The acute activity of Gαi/o can also modulate the sensitized adenylyl cyclase response. Specifically, acute activation of the µ-opioid receptor reduced δ-opioid receptor-mediated heterologous sensitization (Levitt et al., 2011). Selective acute activation of δ-opioid, α2 adrenergic, or nociception/orphanin FQ peptide receptors also reduced µ-opioid receptor-mediated heterologous sensitization (Levitt et al., 2011). These results suggest that even when sensitized, adenylyl cyclases are still subject to acute inhibition by Gαi/o, and that during sensitization Gαi/o subunits retain their ability to be activated by receptors as well as inhibit cAMP. Such observations are consistent with divergent mechanisms for the acute inhibition response when compared to the sensitized response (Watts and Neve, 1996).
2.2 Gβγ subunits
Interaction with agonists promotes GPCR structural/conformational changes that lead to the activation of Gα and also allow Gβγ subunits to modulate their effectors (Nygaard et al., 2013). Signaling through Gβγ subunits is diverse and can lead to a variety of cellular events including ERK phosphorylation, modulation of adenylyl cyclase isoforms (e.g. AC1 and AC2), conditional activation of glycine receptors, activation of phospholipase C β2/β3, GIRK (G protein-coupled inwardly rectifying potassium) and N-type calcium channels, phosphoinositide 3 kinase, and stabilization of microtubules (Cooper and Crossthwaite, 2006; Khan et al., 2013; Lin and Smrcka, 2011). Therefore, there are a number of different mechanisms through which Gβγ subunits may be involved with heterologous sensitization. To determine whether Gβγ subunits are required for heterologous sensitization, Gβγ scavengers have been employed. The C-terminus of G protein receptor kinase (βARK-Ct) and Gαt (α-transducin) are Gβγ subunit sequestering-proteins. Overexpression of either of these prevented sensitization through CB1 cannabinoid, dopamine D2, and µ-opioid receptors (Avidor-Reiss et al., 1996; Ejendal et al., 2012; Rhee et al., 2000; Rubenzik et al., 2001; Thomas and Hoffman, 1996). These data suggest that Gβγ subunits perform an essential role in the development of heterologous sensitization. That Gβγ subunits directly inhibit AC1 activity and conditionally activate AC2 (Cooper and Crossthwaite, 2006; Sunahara and Taussig, 2002) further suggests that the involvement of Gβγ subunits in heterologous sensitization may be dependent on the model system and adenylyl cyclase under analysis. For example, heterologous sensitization of AC2 by the dopamine D2 receptor has been reported in HEK cells, but it has also been reported that chronic activation of the µ-opioid receptor in COS-7 cells leads to an inhibition of AC2 activity (Conley and Watts, 2013; Schallmach et al., 2006). It was also revealed that Gβγ scavengers partially rescued the reduced activity of AC2 following chronic activation of the µ-opioid receptor in COS-7 cells (Schallmach et al., 2006). Additionally, there are five Gβ subunits and twelve Gγ subunits, specific forms of which are associated with specific receptors and Gα proteins (Khan et al., 2013). It has further been hypothesized that different Gβγ dimers may have distinct functions (Dupre et al., 2009; Khan et al., 2013; McIntire et al., 2001), a hypothesis in agreement with the knockout of specific Gγ subunits resulting in unique phenotypes in mice (Moon et al., 2014; Schwindinger et al., 2012). Together, this research suggests that Gβγ subunits play an important role in the development of heterologous sensitization. That Gβγ scavengers inhibit sensitization of AC5, and that an AC5 mutant lacking interaction with Gβγ subunits still undergoes sensitization by the dopamine D2 receptor (Ejendal et al., 2012; Sadana et al., 2009), suggest that one or more of the Gβγ effectors may be associated with sensitization. However, the possibility of dependence on a direct interaction is not excluded, especially for group 2 adenylyl cyclases, which are conditionally activated by Gβγ subunits through a direct interaction (Boran et al., 2011; Chen et al., 1995; Diel et al., 2008; Diel et al., 2006; Weitmann et al., 2001).
2.3 Gαs subunits
Gαs subunits activate all isoforms of adenylyl cyclases and, as several lines of evidence indicate, play an essential role in heterologous sensitization of specific isoforms of adenylyl cyclase. For example, heterologous sensitization has been associated with enhanced Gαs coupling to both receptors and adenylyl cyclases (Ammer and Schulz, 1995, 1996, 1998; Chakrabarti et al., 2005; Chen and Rasenick, 1995; Watts and Neve, 1996). It has been shown that persistent activation of the dopamine D2 receptor leads to enhanced potency of forskolin and relative efficacy of isoproterenol (β-adrenergic receptor agonist) in C6 glioma cells (Watts and Neve, 1996). These findings are consistent with an enhancement in the activity of Gαs subunits, which can synergistically activate adenylyl cyclases in the presence of forskolin (Sunahara and Taussig, 2002). Accordingly, forskolin-stimulated sensitization of adenylyl cyclases has been reported in the presence of GTP (reflecting Gαs activity) in CHO cells following chronic activation of the adenosine A3 receptor (Palmer et al., 1997). Notably, uncoupling of Gαs from adenylyl cyclases using manganese chloride blunted sensitization; furthermore, this enhanced adenylyl cyclase activity was not linked to changes in the expression levels of G proteins (Palmer et al., 1997).
The above findings suggest that during the sensitization process there is an enhancement of the interactions between Gαs subunits and adenylyl cyclases, leading to enhanced activity of the adenylyl cyclases. These observations, the fact that all isoforms of adenylyl cyclases are stimulated by Gαs, and that heterologous sensitization has been demonstrated for most isoforms of adenylyl cyclases, prompted us to hypothesize that heterologous sensitization is a result of enhanced interactions between Gαs and adenylyl cyclases (Avidor-Reiss et al., 1997; Cooper and Crossthwaite, 2006; Watts and Neve, 2005; Watts et al., 2001; Watts and Neve, 1996). In order to test this hypothesis, two complementary approaches were employed. The first used a small series of mutant adenylyl cyclases (i.e. AC1 and AC5), which were insensitive to stimulation by Gαs (Zimmermann et al., 1998). The three Gαs-insensitive AC5 mutants failed to support dopamine D2 receptor-induced sensitization, which was consistent with our hypothesis (Watts et al., 2001). On the other hand, the Gαs-insensitive mutants of AC1 could still be sensitized by the persistent activation of the dopamine D2 receptor, although the magnitude of sensitization was reduced compared to wild-type AC1 (Vortherms et al., 2004). The second complementary approach used mouse embryonic fibroblasts that were Gαs deficient (Bastepe et al., 2002). In agreement with the previous studies, AC5 was not sensitized by the dopamine D2 receptor in Gαs-deficient cells; moreover, sensitization was rescued by expression of Gαs (Vortherms et al., 2006). The results with AC1 were also consistent with the previous report using Gαs-insensitive mutants of AC1 (Vortherms et al., 2004). Specifically, chronic activation of the dopamine D2 receptor led to a significant sensitized response of AC1 in the Gαs-deficient cells that was less than the response in the presence of Gαs (Vortherms et al., 2006). These data suggest that there are both Gαs-dependent and -independent mechanisms of heterologous sensitization, as well as adenylyl cyclase isoform-specific requirements for the expression of the sensitized response. It is also worth noting the effects of prolonged activation of Gαi/o-coupled receptors on the Gαs-stimulated AC2 response. It has been reported that chronic activation of such receptors leads to either superinhibition or has no effects on the Gαs-mediated AC2 response (Avidor-Reiss et al., 1997; Nevo et al., 1998; Rhee et al., 2000; Schallmach et al., 2006; Thomas and Hoffman, 1996).
The mechanisms responsible for the Gαs-dependent enhancements of adenylyl cyclase activity in heterologous sensitization have not yet been elucidated. However, posttranslational modifications on both Gαs and adenylyl cyclases, as well as membrane compartmentalization of Gαs, have been suggested (for more discussion see Watts and Neve, 2005). One of our previous hypotheses was that a change in palmitoylation of Gαs might lead to greater colocalization and interaction of Gαs with adenylyl cyclase. However, Gαs-deficient cells overexpressing a palmitoylation-deficient Gαs mutant underwent sensitization to the same extent as cells expressing wild type Gαs, suggesting that Gαs palmitoylation is not required for heterologous sensitization (Ejendal et al., 2012). The interaction between Gαs and Gβγ subunits has also been investigated. These experiments revealed that a Gβγ binding-deficient Gαs mutant can also lead to sensitization of AC5 by the dopamine D2 receptor in the same model mentioned above (Ejendal et al., 2012). Because sequestration of Gβγ subunits with βARKct blunts sensitization of AC5 by the dopamine D2 receptor, it seems that the mechanism by which Gβγ subunits participate in heterologous sensitization does not involve direct coupling to Gαs (Ejendal et al., 2012). It has been shown that inhibition of small GTPases involved in signalosome assembly, such as Sar1, inhibited heterologous sensitization of AC5 (Ejendal et al., 2012). Because inhibition of Sar1 does not interfere with the interaction of AC5 with Gβγ subunits, these data suggest that Gαs and Gβγ subunits play their roles at different steps or signaling complexes of heterologous sensitization (Dupre et al., 2007; Dupre et al., 2009; Ejendal et al., 2012).
2.4 AGS proteins
Regulation of G protein activity is important for the modulation of acute GPCR signaling and may also be associated with heterologous sensitization. One group of proteins that regulate G protein activity is the AGS (activator of G protein signaling) proteins, which interact with Gα subunits and were first identified in a yeast-based genetic screen as activators of Gβγ signaling (Cismowski et al., 2001; Cismowski et al., 1999; Takesono et al., 1999). Although AGS1 enhances GTPγS binding to Gαi in vitro (Cismowski et al., 1999), in intact HEK cells, overexpression of AGS1 had no effect on acute dopamine D2 receptor-mediated inhibition of AC1 activity (Nguyen and Watts, 2005). It seems that the effects of AGS1 on inhibitory GPCRs are linked to Gβγ subunits. For example, overexpression of AGS1 leads to an inhibition of M2 muscarinic receptor-mediated activation of GIRK channels (Luscher and Slesinger, 2010; Takesono et al., 2002). Additionally, for both the dopamine D2 and the formyl peptide receptors, overexpression of AGS1 inhibited ERK phosphorylation (Graham et al., 2002; Nguyen and Watts, 2005). Consistent with the inhibitory effects on the signaling of Gβγ subunits, overexpression of AGS1 inhibited dopamine D2 receptor-mediated sensitization of AC1 in HEK cells (Nguyen and Watts, 2005).
AGS3 is another AGS protein that is expressed throughout the brain (Blumer et al., 2005). ASG3 interacts preferentially with Gαi/o proteins in the GDP bound state and inhibits GTPγS binding (Blumer et al., 2005; Cismowski et al., 2000; Cismowski et al., 2001), and has been implicated in withdrawal following administration of drugs of abuse. For instance, Bowers et al. found increased levels of expression of AGS3 in the nucleus accumbens of rodents in cocaine and ethanol withdrawal models (2003; 2008). In these studies, knockdown of AGS3 reduced ethanol-seeking behavior during ethanol withdrawal (Bowers et al., 2008) and could also inhibit the behaviors associated with cocaine withdrawal (Bowers et al., 2003). Consistent with the animal models, in rat primary nucleus accumbens/striatal neurons, morphine withdrawal caused an increase in the expression of AGS3 (Fan et al., 2009). The effects of AGS3 on acute and sensitized signaling of Gαi/o-coupled GPCRs have also been studied in cell models. In HEK cells, overexpression of AGS3 led to an enhancement of both acute dopamine D2 receptor-mediated inhibition of AC1 and the potency of dopamine D2 receptor-mediated conditional activation of AC2, a signaling event that is attributed to activation of Gβγ subunits (Conley and Watts, 2013; Federman et al., 1992; Watts and Neve, 1997). For heterologous sensitization it has been shown that AGS3 attenuates α2 adrenergic receptor-mediated sensitization in CHO cells (Sato et al., 2004). It has also been shown that overexpression of AGS3 inhibits dopamine D2 receptor-mediated sensitization of AC1 and AC2 in HEK cells (Conley and Watts, 2013). In contrast, it was reported that a siRNA-mediated reduction of AGS3 suppressed morphine-induced sensitization in nucleus accumbens/striatal neurons (Fan et al., 2009). These studies suggest a potential role for AGS proteins in the development of heterologous sensitization, although their functions appear to be both isoform- and model-specific.
2.5 RGS proteins
Another group of important modulators of G protein activity that may have a role in heterologous sensitization are the RGS (regulator of G protein signaling) proteins. This group of proteins regulate the kinetics of GPCR signaling by accelerating the GTPase activity of Gα proteins (Neubig and Siderovski, 2002), and can also regulate GPCR signaling by modulating the interaction of Gα proteins with their effectors independent of their GTPase accelerating activity (Hooks et al., 2008). Moreover, RGS proteins can also interact with additional signaling proteins, and may be part of larger signaling complexes with scaffolding proteins, receptors and other signaling proteins, including adenylyl cyclases and G proteins (Abramow-Newerly et al., 2006; Hooks et al., 2008; Roy et al., 2006).
Because RGS proteins interact with several components of the signaling cascade of GPCRs, they may play a role in heterologous sensitization. It has been shown that overexpression of Gαi/o-selective RGS proteins reduces receptor-mediated inhibition of cAMP production (Chatterjee et al., 1997; Huang et al., 1997; Wang et al., 2002), but also that RGS proteins may positively regulate signaling of Gαi/o-coupled receptors in cell lines (Boutet-Robinet et al., 2003). Additionally, overexpression of RGS2 and Gαs with different isoforms of adenylyl cyclase in HEK cells causes a decrease in basal cAMP production by AC1, AC2, AC3, AC4, AC5, and AC6, compared with control cells that were not transfected with RGS2 (Roy et al., 2006). In rats, several drugs of abuse (i.e. amphetamine, morphine, and cocaine), when administered acutely, reduce the levels of RGS4 mRNA in the locus coeruleus, whereas chronic treatments lead to increased RGS4 mRNA levels (Bishop et al., 2002; Gold et al., 2003). In contrast, it was shown that in the nucleus accumbens of mice, acute morphine leads to an increase in the levels of RGS9-2, whereas chronic morphine treatments lead to a decrease in the levels of RGS9-2 (Zachariou et al., 2003). These findings indicate that different isoforms of RGS proteins are distinctly regulated by drugs of abuse. Individual isoforms of RGS proteins may therefore have different roles in heterologous sensitization, possibly due to interactions with distinct signaling proteins (Hooks et al., 2008). Indeed, studies indicate a role for RGS proteins in heterologous sensitization. For example, expression of RGS-insensitive Gαo leads to enhanced potency and efficacy of heterologous sensitization through the µ-opioid receptor in C6 glioma cells (Clark et al., 2004). Furthermore, there is evidence that RGS9-2/Gβ5 complexes inhibit µ-opioid receptor-mediated heterologous sensitization of AC5 in mouse striatal neurons and HEK cells stably expressing the µ-opioid receptor and AC5 (Xie et al., 2012). Activation of RGS proteins could lead to disinhibition of adenylyl cyclases through regulation (i.e. GTPase enhancement) of Gαi/o proteins; however, studies to date suggest an inhibitory function for RGS proteins in heterologous sensitization, which is consistent with the inhibitory acute effects on isoforms of adenylyl cyclases (Clark et al., 2004; Roy et al., 2006; Xie et al., 2012).
2.6 Protein kinases and phosphatases
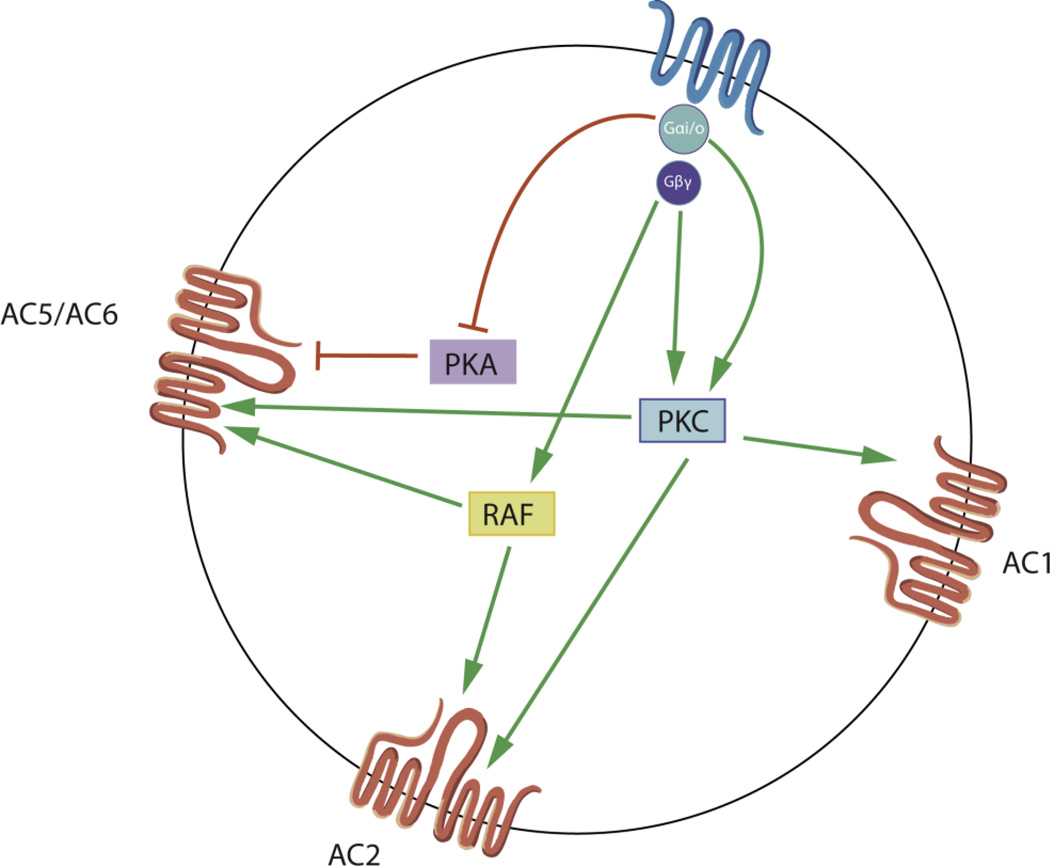
A role for phosphorylation in heterologous sensitization can be supported by the fact that the activity of different isoforms of adenylyl cyclases can be regulated by direct phosphorylation (Sadana and Dessauer, 2009; Watts and Neve, 2005). Moreover, protein kinases and phosphatases can also indirectly regulate the activity of adenylyl cyclases (Defer et al., 2000; Sadana and Dessauer, 2009). It has been shown that direct phosphorylation of AC5 and AC6 by protein kinase A (PKA) has inhibitory effects on the activity of these adenylyl cyclase isoforms (Chen et al., 1997; Iwami et al., 1995). Adenylyl cyclases can also be modulated by different isoforms of protein kinase C (PKC), which display stimulatory effects on AC1, AC2, AC3, AC5, and AC7, and inhibitory effects on AC4, AC6, and AC9 (Defer et al., 2000; Sadana and Dessauer, 2009). Adenylyl cyclases can also be regulated by calmodulin kinases (CaMK); CaMK IV has inhibitory effects on AC1 and CaMK II inhibits AC3 (Wayman et al., 1996; Wei et al., 1996). Furthermore, activation of the calcium-activated phosphatase calcineurin leads to inhibition of AC9 (Antoni et al., 1995). Together, these data suggest that a mechanism for heterologous sensitization may occur through the modulation of the phosphorylation state of adenylyl cyclases or its regulatory proteins (figure 3).
Figure 3.
Isoform-specific regulation of adenylyl cyclase by protein kinases that are activated by Gαi/o-coupled GPCRs. One of the proposed mechanisms for heterologous sensitization is that the phosphorylation state of the adenylyl cyclase is modified due to prolonged activation of Gαi/o-coupled receptors. Activation of inhibitory GPCRs leads to active Gαi/o and Gβγ subunits that can activate PKC, which phosphorylates and activates AC1, AC2, and AC5. Activation of Gαi/o inhibits PKA, leading to disinhibition of AC5 and AC6. Gβγ subunits can also activate Raf-1, which phosphorylates and activates AC2, AC5, and AC6.
Activation of Gαi/o-coupled receptors leads to downstream activation of protein kinases, some of which can regulate the activity of adenylyl cyclases, such as PKA, PKC, and Raf-1 kinase (figure 3) (Gordon et al., 2001; Johnston et al., 2002; Kotecha et al., 2002; Liu et al., 2003; Oak et al., 2001; Varga et al., 2003a; Varga et al., 2003b; Yoon et al., 2011). Prolonged activation of Gαi/o-coupled receptors sensitizes the response of AC2 and AC5 to PKC activation in cellular models, indicating that the phosphorylated state of these adenylyl cyclases can also be potentiated, and that there are additional mechanisms other than PKC-mediated phosphorylation of adenylyl cyclases for these specific examples of heterologous sensitization (Avidor-Reiss et al., 1997; Conley and Watts, 2013; Cumbay and Watts, 2001; Watts and Neve, 1996). Notably, it has been shown that chronic activation of opioid receptors leads to increased phosphorylation of adenylyl cyclases, and this phosphorylated state is associated with enhanced cAMP production (Chakrabarti et al., 1998; Varga et al., 2002; Varga et al., 2003a; Varga et al., 1999). These observations suggest that phosphorylation of adenylyl cyclases might regulate heterologous sensitization through at least two different mechanisms, one in which phosphorylation is part of the acute stimulatory signal, and another in which phosphorylation occurs during the development of sensitization to increase the activity of the adenylyl cyclases. One protein that might be involved in phosphorylation of adenylyl cyclases during sensitization is Raf-1. In CHO cells, heterologous sensitization via the δ-opioid receptor leads to phosphorylation of AC6, and inhibition of Raf-1 attenuates heterologous sensitization (Varga et al., 2002; Varga et al., 1999; Yue et al., 2006). Moreover, prolonged morphine treatments in primary rat dorsal root ganglion neurons lead to enhanced cAMP production, which is prevented by inhibition of Raf-1 (Yue et al., 2008). It has also been shown that Raf-1 can phosphorylate AC2 and AC5, but not AC1 (Ding et al., 2004). Notably, Raf-1-mediated phosphorylation of adenylyl cyclases has been linked to the activity of receptors tyrosine kinases (Beazely et al., 2005; Ding et al., 2004; Tan et al., 2001; Varga et al., 1998; Varga et al., 2003a).
Several lines of evidence suggest that GPCRs can mediate transactivation of receptors tyrosine kinases (Oak et al., 2001; Wang et al., 2005; Yoon et al., 2011). In CHO cells that stably express either the dopamine D2 or D4 receptor, trans-activation of platelet-derived growth factor receptors seems to be dependent on G proteins, since it can be completely inhibited by pertussis toxin and partially inhibited by βARKct (Oak et al., 2001). Transactivation of epidermal growth factor receptors by Gαi/o-coupled dopamine receptors is a required event for quinpirole-mediated ERK phosphorylation in primary striatal neurons (Wang et al., 2005). Recent studies have suggested a mechanism for heterologous sensitization involving a signaling switch from G proteins to receptor tyrosine kinase-like signaling. This mechanism has been shown for the µ-opioid receptor in HEK cells, where prolonged activation of the receptor caused Src kinase-mediated receptor phosphorylation at a tyrosine residue. This results in the recruitment and activation of growth factor receptor-bound protein/son of sevenless, further leading to the activation of Ras/Raf-1, which in turn phosphorylates the adenylyl cyclases and causes enhanced cAMP production (Zhang et al., 2013; Zhang et al., 2009). These studies suggest that the conversion of a Gαi/o-coupled receptor into a receptor tyrosine kinase-like signaling complex may be associated with the development of heterologous sensitization. However, because inhibition of Ras and disruption of the receptor tyrosine kinase-like signaling complex did not fully inhibit heterologous sensitization, it is likely that there are additional events underlying the development of sensitization (Zhang et al., 2013). Further, AC1 is not phosphorylated by Raf-1, which supports the existence of isoform-dependent mechanisms (Ding et al., 2004).
That protein kinases can also negatively regulate the activity of adenylyl cyclases suggests that protein phosphatases may also have a function in heterologous sensitization. However, to our knowledge phosphatases have not been directly linked to heterologous sensitization thus far. The current data indicates that phosphorylation has a function in heterologous sensitization; nevertheless, it is likely that this role depends on both the isoform of adenylyl cyclase and the protein kinase/phosphatase under investigation.
3. Heterologous sensitization in animals
Demonstration in animal models was an important step in the establishment of heterologous sensitization as a more physiologically relevant receptor response following prolonged activation of Gαi/o-coupled receptors. For example, in 1988 Nestler and Tallman observed that following a five-day chronic treatment of rats with subcutaneous morphine pellets, there was an increase in the amounts of active PKA in the locus coeruleus. These results are consistent with the known outcomes of heterologous sensitization. Also in 1988, Duman et al. used a similar approach to show that chronic morphine treatments in rats resulted in increased cAMP levels in the locus coeruleus. Surprisingly, both manuscripts reported no change in cAMP/PKA activity in other brain regions known to express opioid receptors, such as the striatum (Duman et al., 1988; Nestler and Tallman, 1988).
Heterologous sensitization has long been associated with drug tolerance and dependence (Sharma et al., 1975). However, a report by Bohn et al. in 2000 further investigated these phenomena and provided evidence that the recruitment of β-arrestin was associated with opioid tolerance while heterologous sensitization appeared to be related to dependence (Bohn et al., 2000). Using β-arrestin knockout mice, they first showed that those mice did not display the signs of tolerance that are observed in wild type mice following chronic morphine treatments. On the other hand, the knockout mice still displayed behaviors consistent with drug dependence. Notably, neurons from the striatum of both wild type and β-arrestin knockout mice underwent heterologous sensitization following chronic treatment of those animals with morphine (Bohn et al., 2000). These data suggest that β-arrestin is not required for heterologous sensitization and that heterologous sensitization appears to be associated with drug dependence in mice (Bohn et al., 2000). These results are consistent with a recent report by our group that used quantitative bias analyses to show that the agonist-mediated recruitment of β-arrestin to the dopamine D2 receptor is not correlated with heterologous sensitization in CHO cells (Brust et al., 2015). The G protein-coupled receptor kinases (GRK) that phosphorylate GPCRs allowing for the recruitment of β-arrestins (Lefkowitz and Shenoy, 2005) were also implicated in heterologous sensitization. It has been shown that overexpression of GRK2 and GRK3 inhibit D2 dopamine receptor-mediated heterologous sensitization in HEK cells (Namkung et al., 2009). Nevertheless, this effect was associated with sequestration of Gβγ subunits (both GRK2 and GRK3 bind Gβγ subunits) and not with enhanced β-arrestin recruitment, since overexpression of GRK5 and GRK6 (which do not bind Gβγ subunits) had no significant effects (Namkung et al., 2009).
Agonists of the µ-opioid receptor are commonly used in clinics for their analgesic effects (Dworkin et al., 2007; Pasternak, 2014). It has been recently shown that tissue injury in mice results in increased µ-opioid receptor constitutive activity, which inhibits signaling events associated with nociception in neurons of the spinal cord (Corder et al., 2013). In the injured mice, inhibition of the constitutive activity of the µ-opioid receptor with naltrexone in the post-hyperalgesia state results in pain reinstatement, induction of behaviors consistent with opioid dependence, and also causes heterologous sensitization of AC1 in the spinal cord (Corder et al., 2013). Consistent with previous reports, these studies also showed a link between heterologous sensitization and the appearance of behaviors analogous to opioid dependence in mice. Furthermore, the results suggest that injury-induced physiological concentrations of endogenous opioids or constitutive receptor activity are enough to develop µ-opioid receptor-mediated heterologous sensitization of AC1 in the spinal cord of mice (Corder et al., 2013).
Heterologous sensitization has also been shown in animals through the activation of dopamine receptors. For instance, chronic i.p. injections of the dopamine D2 receptor agonist quinpirole results in an enhancement of the cAMP response of striatal neurons to activation of dopamine D1 receptors (Gαs-coupled) in hamsters (Chester et al., 2006). Additionally, chronic treatment of mice with quinpirole or pramipexole for five weeks sensitizes neurons from the nucleus accumbens to dopamine-activated cAMP production (Aloisi et al., 2011). These studies indicate that prolonged activation of the dopamine D2 receptor in rodents leads to heterologous sensitization of the dopamine D1 receptor-mediated activation of adenylyl cyclases. Together with the reports of heterologous sensitization through opioid receptors, these findings suggest that the cellular adaptive responses observed in cell models following prolonged activation of Gαi/o-coupled receptors (i.e. heterologous sensitization) are also manifested in animals.
4. Conclusions and future perspectives
Heterologous sensitization is characterized by a seemingly paradoxical increase in the activity of adenylyl cyclases following prolonged activation of Gαi/o-coupled receptors. Increasing evidence suggests that several proteins are involved in the development and expression of heterologous sensitization of adenylyl cyclase isoforms. Moreover, there appear to be adenylyl cyclase-isoform specific mechanisms. The reports discussed here suggest that during persistent activation of Gαi/o-coupled receptors, either an activation or a disinhibition (or perhaps both) of adenylyl cyclases drives the sensitized response. Despite progress in the last forty years, the precise mechanism of this receptor-mediated response remains unknown. It is also important to note that 1) the specificity of the mechanistic clues reported for heterologous sensitization in terms of type of receptor and adenylyl cyclase isoform and 2) methodological and model system differences likely influence the overall outcomes of the experiments and contribute to the difficulties associated with drawing a generalized mechanism for heterologous sensitization.
In addition to G proteins and kinases, several adenylyl cyclase-interacting proteins have been identified (Sadana and Dessauer, 2009; Wang et al., 2009). One relatively simple hypothesis for the general mechanism of heterologous sensitization is that the observed enhanced adenylyl cyclase activity results from a protein interaction that is induced (or inhibited) by prolonged receptor activation. These interactions can be part of a larger signaling complex and may involve translocation of signaling components or even a rearrangement of pre-existing signaling complexes; these could influence important signaling features, such as the presence of adenylyl cyclases in microdomains and cellular compartmentalization (Wang et al., 2009; Willoughby and Cooper, 2007). We are exploring approaches to examine the heterologous sensitization interactome using a bimolecular fluorescence complementation (BiFC) screening approach. BiFC is a method used to measure and visualize protein-protein interactions. In BiFC, proteins are fused with opposite termini of a fluorescent protein (e.g. N- and C-termini of Green Fluorescent Protein). Upon physical interaction between the fusion proteins, the two termini of the fluorescent protein complement to form a full fluorescent protein that can be detected by a fluorescence microscope, a flow cytometer, or a fluorescence plate reader (Hu and Kerppola, 2003; Vidi et al., 2010). Adenylyl cyclase isoforms tagged with a BiFC terminus and a library of proteins tagged with the complementary BiFC terminus can be employed for the screen. To further study their roles in heterologous sensitiziation, we anticipate identifying proteins that have increased/decreased interactions with adenylyl cyclases as a result of persistent activation of Gαi/o-coupled receptors. These studies may also be useful to identify additional adenylyl cyclase isoform-specific interacting partners and understand where in the receptor signaling cascade heterologous sensitization branches out and becomes specific for individual isoforms of adenylyl cyclase. A second strategy to achieve this and discover additional proteins involved in heterologous sensitization is siRNA library screening. We have recently developed a high-throughput assay platform to examine the effects of siRNA-mediated knockdown of proteins on heterologous sensitization (Conley et al., 2014). These methods can be adapted to screen a siRNA genome-wide library, pursuing genes that modulate heterologous sensitization of different isoforms of adenylyl cyclase. It is anticipated that these experiments will lead to the identification of genes and proteins that are shared by different adenylyl cyclase isoforms, as well as those that are specific modulators of individual isoforms of adenylyl cyclases.
Heterologous sensitization of adenylyl cyclases is associated with the physiological responses observed following chronic treatment with agonists of Gαi/o-coupled receptors. For example, the mechanisms may be relevant to the side effects of pharmacological treatments that lead to increased Gαi/o-coupled receptor activity. It is anticipated that continued research to unveil the molecular mechanisms of heterologous sensitization in cell and animal models will lead to a better understanding of the molecular basis for adaptive pathological changes associated with drug dependence and neurological disorders.
Acknowledgements
The authors wish to acknowledge Ms. Isabelle Verona Brust for preparing the figures in the manuscript and Ms. Stacy O. Nall for editorial assistance.
This work was supported by the National Institute of Mental Health [Grants MH060397 and MH101673] and by Purdue University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
The authors have no conflicts of interest to disclose.
References
- Abramow-Newerly M, Roy AA, Nunn C, Chidiac P. RGS proteins have a signalling complex: interactions between RGS proteins and GPCRs, effectors, and auxiliary proteins. Cell Signal. 2006;18:579–591. doi: 10.1016/j.cellsig.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Aloisi G, Silvano E, Rossi M, Millan MJ, Maggio R. Differential induction of adenylyl cyclase supersensitivity by antiparkinson drugs acting as agonists at dopamine D1/D2/D3 receptors vs D2/D3 receptors only: parallel observations from co-transfected human and native cerebral receptors. Neuropharmacology. 2011;60:439–445. doi: 10.1016/j.neuropharm.2010.10.018. [DOI] [PubMed] [Google Scholar]
- Ammer H, Christ TE. Identity of adenylyl cyclase isoform determines the G protein mediating chronic opioid-induced adenylyl cyclase supersensitivity. J Neurochem. 2002;83:818–827. doi: 10.1046/j.1471-4159.2002.01188.x. [DOI] [PubMed] [Google Scholar]
- Ammer H, Schulz R. Chronic activation of inhibitory delta-opioid receptors cross-regulates the stimulatory adenylate cyclase-coupled prostaglandin E1 receptor system in neuroblastoma x glioma (NG108-15) hybrid cells. J Neurochem. 1995;64:2449–2457. doi: 10.1046/j.1471-4159.1995.64062449.x. [DOI] [PubMed] [Google Scholar]
- Ammer H, Schulz R. Morphine dependence in human neuroblastoma SH-SY5Y cells is associated with adaptive changes in both the quantity and functional interaction of PGE1 receptors and stimulatory G proteins. Brain Res. 1996;707:235–244. doi: 10.1016/0006-8993(95)01265-6. [DOI] [PubMed] [Google Scholar]
- Ammer H, Schulz R. Adenylyl cyclase supersensitivity in opioid-withdrawn NG108-15 hybrid cells requires Gs but is not mediated by the Gsalpha subunit. J Pharmacol Exp Ther. 1998;286:855–862. [PubMed] [Google Scholar]
- Antoni FA, Barnard RJ, Shipston MJ, Smith SM, Simpson J, Paterson JM. Calcineurin feedback inhibition of agonist-evoked cAMP formation. J Biol Chem. 1995;270:28055–28061. doi: 10.1074/jbc.270.47.28055. [DOI] [PubMed] [Google Scholar]
- Avidor-Reiss T, Nevo I, Levy R, Pfeuffer T, Vogel Z. Chronic opioid treatment induces adenylyl cyclase V superactivation. Involvement of Gbetagamma. J Biol Chem. 1996;271:21309–21315. doi: 10.1074/jbc.271.35.21309. [DOI] [PubMed] [Google Scholar]
- Avidor-Reiss T, Nevo I, Saya D, Bayewitch M, Vogel Z. Opiate-induced adenylyl cyclase superactivation is isozyme-specific. J Biol Chem. 1997;272:5040–5047. doi: 10.1074/jbc.272.8.5040. [DOI] [PubMed] [Google Scholar]
- Bastepe M, Gunes Y, Perez-Villamil B, Hunzelman J, Weinstein LS, Juppner H. Receptor-mediated adenylyl cyclase activation through XLalpha(s), the extra-large variant of the stimulatory G protein alpha-subunit. Mol Endocrinol. 2002;16:1912–1919. doi: 10.1210/me.2002-0054. [DOI] [PubMed] [Google Scholar]
- Beazely MA, Alan JK, Watts VJ. Protein kinase C and epidermal growth factor stimulation of Raf1 potentiates adenylyl cyclase type 6 activation in intact cells. Mol Pharmacol. 2005;67:250–259. doi: 10.1124/mol.104.001370. [DOI] [PubMed] [Google Scholar]
- Bishop GB, Cullinan WE, Curran E, Gutstein HB. Abused drugs modulate RGS4 mRNA levels in rat brain: comparison between acute drug treatment and a drug challenge after chronic treatment. Neurobiol Disease. 2002;10:334–343. doi: 10.1006/nbdi.2002.0518. [DOI] [PubMed] [Google Scholar]
- Blumer JB, Cismowski MJ, Sato M, Lanier SM. AGS proteins: receptor-independent activators of G-protein signaling. Trends Pharmacol Sci. 2005;26:470–476. doi: 10.1016/j.tips.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Muopioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408:720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- Boran AD, Chen Y, Iyengar R. Identification of new Gbetagamma interaction sites in adenylyl cyclase 2. Cell Signal. 2011;23:1489–1495. doi: 10.1016/j.cellsig.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutet-Robinet EA, Finana F, Wurch T, Pauwels PJ, De Vries L. Endogenous RGS proteins facilitate dopamine D(2S) receptor coupling to G(alphao) proteins and Ca2+ responses in CHO-K1 cells. FEBS letters. 2003;533:67–71. doi: 10.1016/s0014-5793(02)03753-5. [DOI] [PubMed] [Google Scholar]
- Bowers MS, Hopf FW, Chou JK, Guillory AM, Chang SJ, Janak PH, Bonci A, Diamond I. Nucleus accumbens AGS3 expression drives ethanol seeking through G betagamma. Proc Natl Acad Sci U S A. 2008;105:12533–12538. doi: 10.1073/pnas.0706999105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers MS, Lake RW, McFarland K, Peterson YK, Lanier SM, Lapish CC, Kalivas PW. AGS3: a G-Protein regulator of addiction-associated behaviors. Ann N Y Acad Sci. 2003;1003:356–357. doi: 10.1196/annals.1300.025. [DOI] [PubMed] [Google Scholar]
- Brust TF, Hayes MP, Roman DL, Burris KD, Watts VJ. Bias Analyses of Preclinical and Clinical D2 Dopamine Ligands: Studies with Immediate and Complex Signaling Pathways. J Pharmacol Exp Ther. 2015;352:480–493. doi: 10.1124/jpet.114.220293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S, Regec A, Gintzler AR. Biochemical demonstration of mu-opioid receptor association with Gsalpha: enhancement following morphine exposure. Brain Res Mol Brain Res. 2005;135:217–224. doi: 10.1016/j.molbrainres.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Wang L, Tang WJ, Gintzler AR. Chronic morphine augments adenylyl cyclase phosphorylation: relevance to altered signaling during tolerance/dependence. Mol Pharmacol. 1998;54:949–953. doi: 10.1124/mol.54.6.949. [DOI] [PubMed] [Google Scholar]
- Chalecka-Franaszek E, Weems HB, Crowder AT, Cox BM, Cote TE. Immunoprecipitation of high-affinity, guanine nucleotide-sensitive, solubilized muopioid receptors from rat brain: coimmunoprecipitation of the G proteins G(alpha o), G(alpha i1), and G(alpha i3) J Neurochem. 2000;74:1068–1078. doi: 10.1046/j.1471-4159.2000.0741068.x. [DOI] [PubMed] [Google Scholar]
- Chatterjee TK, Eapen AK, Fisher RA. A truncated form of RGS3 negatively regulates G protein-coupled receptor stimulation of adenylyl cyclase and phosphoinositide phospholipase C. J Biol Chem. 1997;272:15481–15487. doi: 10.1074/jbc.272.24.15481. [DOI] [PubMed] [Google Scholar]
- Chen J, DeVivo M, Dingus J, Harry A, Li J, Sui J, Carty DJ, Blank JL, Exton JH, Stoffel RH, et al. A region of adenylyl cyclase 2 critical for regulation by G protein beta gamma subunits. Science. 1995;268:1166–1169. doi: 10.1126/science.7761832. [DOI] [PubMed] [Google Scholar]
- Chen J, Rasenick MM. Chronic treatment of C6 glioma cells with antidepressant drugs increases functional coupling between a G protein (Gs) and adenylyl cyclase. J Neurochem. 1995;64:724–732. doi: 10.1046/j.1471-4159.1995.64020724.x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Harry A, Li J, Smit MJ, Bai X, Magnusson R, Pieroni JP, Weng G, Iyengar R. Adenylyl cyclase 6 is selectively regulated by protein kinase A phosphorylation in a region involved in Galphas stimulation. Proc Natl Acad Sci U S A. 1997;94:14100–14104. doi: 10.1073/pnas.94.25.14100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester JA, Mullins AJ, Nguyen CH, Watts VJ, Meisel RL. Repeated quinpirole treatments produce neurochemical sensitization and associated behavioral changes in female hamsters. Psychopharmacology. 2006;188:53–62. doi: 10.1007/s00213-006-0468-2. [DOI] [PubMed] [Google Scholar]
- Cismowski MJ, Ma C, Ribas C, Xie X, Spruyt M, Lizano JS, Lanier SM, Duzic E. Activation of heterotrimeric G-protein signaling by a ras-related protein. Implications for signal integration. J Biol Chem. 2000;275:23421–23424. doi: 10.1074/jbc.C000322200. [DOI] [PubMed] [Google Scholar]
- Cismowski MJ, Takesono A, Bernard ML, Duzic E, Lanier SM. Receptor-independent activators of heterotrimeric G-proteins. Life Sci. 2001;68:2301–2308. doi: 10.1016/s0024-3205(01)01019-0. [DOI] [PubMed] [Google Scholar]
- Cismowski MJ, Takesono A, Ma C, Lizano JS, Xie X, Fuernkranz H, Lanier SM, Duzic E. Genetic screens in yeast to identify mammalian nonreceptor modulators of G-protein signaling. Nat Biotechnol. 1999;17:878–883. doi: 10.1038/12867. [DOI] [PubMed] [Google Scholar]
- Clark MJ, Neubig RR, Traynor JR. Endogenous regulator of G protein signaling proteins suppress Galphao-dependent, mu-opioid agonist-mediated adenylyl cyclase supersensitization. J Pharmacol Exp Ther. 2004;310:215–222. doi: 10.1124/jpet.103.064824. [DOI] [PubMed] [Google Scholar]
- Conley JM, Brust TF, Xu R, Burris KD, Watts VJ. Drug-induced sensitization of adenylyl cyclase: assay streamlining and miniaturization for small molecule and siRNA screening applications. Journal of visualized experiments : JoVE. 2014:e51218. doi: 10.3791/51218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley JM, Watts VJ. Differential effects of AGS3 expression on D(2L) dopamine receptor-mediated adenylyl cyclase signaling. Cell Mol Neurobiol. 2013;33:551–558. doi: 10.1007/s10571-013-9925-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D, Crossthwaite A. Higher-order organization and regulation of adenylyl cyclases. Trends Pharmacol Sci. 2006;27:426–431. doi: 10.1016/j.tips.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Corder G, Doolen S, Donahue RR, Winter MK, Jutras BL, He Y, Hu X, Wieskopf JS, Mogil JS, Storm DR, Wang ZJ, McCarson KE, Taylor BK. Constitutive mu-opioid receptor activity leads to long-term endogenous analgesia and dependence. Science. 2013;341:1394–1399. doi: 10.1126/science.1239403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumbay M, Watts V. Heterologous sensitization of recombinant adenylate cyclases by activation of D(2) dopamine receptors. J Pharmacol Exp Ther. 2001;297 297(293) [PubMed] [Google Scholar]
- Defer N, Best-Belpomme M, Hanoune J. Tissue specificity and physiological relevance of various isoforms of adenylyl cyclase. Am J of Physiol. Renal Physiol. 2000;279:F400–F416. doi: 10.1152/ajprenal.2000.279.3.F400. [DOI] [PubMed] [Google Scholar]
- Diel S, Beyermann M, Llorens JM, Wittig B, Kleuss C. Two interaction sites on mammalian adenylyl cyclase type I and II: modulation by calmodulin and G(betagamma) Biochem J. 2008;411:449–456. doi: 10.1042/BJ20071204. [DOI] [PubMed] [Google Scholar]
- Diel S, Klass K, Wittig B, Kleuss C. Gbetagamma activation site in adenylyl cyclase type II. Adenylyl cyclase type III is inhibited by Gbetagamma. J Biol Chem. 2006;281:288–294. doi: 10.1074/jbc.M511045200. [DOI] [PubMed] [Google Scholar]
- Ding Q, Gros R, Gray ID, Taussig R, Ferguson SS, Feldman RD. Raf kinase activation of adenylyl cyclases: isoform-selective regulation. Mol Pharmacol. 2004;66:921–928. [PubMed] [Google Scholar]
- Duman RS, Tallman JF, Nestler EJ. Acute and chronic opiate-regulation of adenylate cyclase in brain: specific effects in locus coeruleus. J Pharmacol Exp Ther. 1988;246:1033–1039. [PubMed] [Google Scholar]
- Dupre DJ, Baragli A, Rebois RV, Ethier N, Hebert TE. Signalling complexes associated with adenylyl cyclase II are assembled during their biosynthesis. Cell Signal. 2007;19:481–489. doi: 10.1016/j.cellsig.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Dupre DJ, Robitaille M, Rebois RV, Hebert TE. The role of Gbetagamma subunits in the organization, assembly, and function of GPCR signaling complexes. Annu Rev Pharmacol Toxicol. 2009;49:31–56. doi: 10.1146/annurev-pharmtox-061008-103038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin RH, O’Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, Kalso EA, Loeser JD, Miaskowski C, Nurmikko TJ, Portenoy RK, Rice AS, Stacey BR, Treede RD, Turk DC, Wallace MS. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132:237–251. doi: 10.1016/j.pain.2007.08.033. [DOI] [PubMed] [Google Scholar]
- Ejendal KF, Dessauer CW, Hebert TE, Watts VJ. Dopamine D(2) Receptor-Mediated Heterologous Sensitization of AC5 Requires Signalosome Assembly. J of Signal Transduct. 2012;2012:210324. doi: 10.1155/2012/210324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan P, Jiang Z, Diamond I, Yao L. Up-regulation of AGS3 during morphine withdrawal promotes cAMP superactivation via adenylyl cyclase 5 and 7 in rat nucleus accumbens/striatal neurons. Mol Pharmacol. 2009;76:526–533. doi: 10.1124/mol.109.057802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federman AD, Conklin BR, Schrader KA, Reed RR, Bourne HR. Hormonal-Stimulation of Adenylyl Cyclase through Gi-Protein Beta-Gamma-Subunits. Nature. 1992;356:159–161. doi: 10.1038/356159a0. [DOI] [PubMed] [Google Scholar]
- Gold SJ, Han MH, Herman AE, Ni YG, Pudiak CM, Aghajanian GK, Liu RJ, Potts BW, Mumby SM, Nestler EJ. Regulation of RGS proteins by chronic morphine in rat locus coeruleus. Eur J Neurosci. 2003;17:971–980. doi: 10.1046/j.1460-9568.2003.02529.x. [DOI] [PubMed] [Google Scholar]
- Gordon AS, Yao L, Jiang Z, Fishburn CS, Fuchs S, Diamond I. Ethanol acts synergistically with a D2 dopamine agonist to cause translocation of protein kinase C. Mol Pharmacol. 2001;59:153–160. doi: 10.1124/mol.59.1.153. [DOI] [PubMed] [Google Scholar]
- Graham TE, Prossnitz ER, Dorin RI. Dexras1/AGS-1 inhibits signal transduction from the Gi-coupled formyl peptide receptor to Erk-1/2 MAP kinases. J Biol Chem. 2002;277:10876–10882. doi: 10.1074/jbc.M110397200. [DOI] [PubMed] [Google Scholar]
- Hacker BM, Tomlinson JE, Wayman GA, Sultana R, Chan G, Villacres E, Disteche C, Storm DR. Cloning, chromosomal mapping, and regulatory properties of the human type 9 adenylyl cyclase (ADCY9) Genomics. 1998;50:97–104. doi: 10.1006/geno.1998.5293. [DOI] [PubMed] [Google Scholar]
- Hanson MA, Stevens RC. Discovery of new GPCR biology: one receptor structure at a time. Structure. 2009;17:8–14. doi: 10.1016/j.str.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks SB, Martemyanov K, Zachariou V. A role of RGS proteins in drug addiction. Biochem Pharmacol. 2008;75:76–84. doi: 10.1016/j.bcp.2007.07.045. [DOI] [PubMed] [Google Scholar]
- Hu C, Kerppola T. Simultaneous visualization of multiple protein interactions in living cells using multicolor fluorescence complementation analysis. Nat Biotechnol. 2003;21:539–545. doi: 10.1038/nbt816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Hepler JR, Gilman AG, Mumby SM. Attenuation of Gi- and Gq-mediated signaling by expression of RGS4 or GAIP in mammalian cells. Proc Natl Acad Sci U S A. 1997;94:6159–6163. doi: 10.1073/pnas.94.12.6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwami G, Kawabe J, Ebina T, Cannon PJ, Homcy CJ, Ishikawa Y. Regulation of adenylyl cyclase by protein kinase A. J Biol Chem. 1995;270:12481–12484. doi: 10.1074/jbc.270.21.12481. [DOI] [PubMed] [Google Scholar]
- Jiang M, Spicher K, Boulay G, Wang Y, Birnbaumer L. Most central nervous system D2 dopamine receptors are coupled to their effectors by Go. Proc Natl Acad Sci U S A. 2001;98:3577–3582. doi: 10.1073/pnas.051632598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston CA, Beazely MA, Vancura AF, Wang JK, Watts VJ. Heterologous sensitization of adenylate cyclase is protein kinase A-dependent in Cath.a differentiated (CAD)-D2L cells. J Neurochem. 2002;82:1087–1096. doi: 10.1046/j.1471-4159.2002.01033.x. [DOI] [PubMed] [Google Scholar]
- Khan SM, Sleno R, Gora S, Zylbergold P, Laverdure JP, Labbe JC, Miller GJ, Hebert TE. The expanding roles of Gbetagamma subunits in G protein-coupled receptor signaling and drug action. Pharmacol Rev. 2013;65:545–577. doi: 10.1124/pr.111.005603. [DOI] [PubMed] [Google Scholar]
- Kotecha SA, Oak JN, Jackson MF, Perez Y, Orser BA, Van Tol HH, MacDonald JF. A D2 class dopamine receptor transactivates a receptor tyrosine kinase to inhibit NMDA receptor transmission. Neuron. 2002;35:1111–1122. doi: 10.1016/s0896-6273(02)00859-0. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R, Shenoy S. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ. Historical review: a brief history and personal retrospective of seven-transmembrane receptors. Trends Pharmacol Sci. 2004;25:413–422. doi: 10.1016/j.tips.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Levitt ES, Purington LC, Traynor JR. Gi/o-coupled receptors compete for signaling to adenylyl cyclase in SH-SY5Y cells and reduce opioid-mediated cAMP overshoot. Mol Pharmacol. 2011;79:461–471. doi: 10.1124/mol.110.064816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Smrcka AV. Understanding molecular recognition by G protein betagamma subunits on the path to pharmacological targeting. Mol Pharmacol. 2011;80:551–557. doi: 10.1124/mol.111.073072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Ghahremani MH, Banihashemi B, Albert PR. Diacylglycerol and ceramide formation induced by dopamine D2S receptors via Gbeta gamma -subunits in Balb/c-3T3 cells. Am J Physiol Cell Physiol. 2003;284:C640–C648. doi: 10.1152/ajpcell.00190.2002. [DOI] [PubMed] [Google Scholar]
- Luscher C, Slesinger PA. Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat Rev Neurosci. 2010;11:301–315. doi: 10.1038/nrn2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masada N, Schaks S, Jackson SE, Sinz A, Cooper DM. Distinct mechanisms of calmodulin binding and regulation of adenylyl cyclases 1 and 8. Biochemistry. 2012;51:7917–7929. doi: 10.1021/bi300646y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire WE, MacCleery G, Garrison JC. The G protein beta subunit is a determinant in the coupling of Gs to the beta 1-adrenergic and A2a adenosine receptors. J Biol Chem. 2001;276:15801–15809. doi: 10.1074/jbc.M011233200. [DOI] [PubMed] [Google Scholar]
- Moon AM, Stauffer AM, Schwindinger WF, Sheridan K, Firment A, Robishaw JD. Disruption of G-protein gamma5 subtype causes embryonic lethality in mice. PLoS One. 2014;9:e90970. doi: 10.1371/journal.pone.0090970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira IS. Structural features of the G-protein/GPCR interactions. Biochim Biophys Acta. 2014;1840:16–33. doi: 10.1016/j.bbagen.2013.08.027. [DOI] [PubMed] [Google Scholar]
- Namkung Y, Dipace C, Urizar E, Javitch JA, Sibley DR. G protein-coupled receptor kinase-2 constitutively regulates D2 dopamine receptor expression and signaling independently of receptor phosphorylation. J Biol Chem. 2009;284:34103–34115. doi: 10.1074/jbc.M109.055707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Tallman JF. Chronic morphine treatment increases cyclic AMP-dependent protein kinase activity in the rat locus coeruleus. Mol Pharmacol. 1988;33:127–132. [PubMed] [Google Scholar]
- Neubig RR, Siderovski DP. Regulators of G-protein signalling as new central nervous system drug targets. Nat Rev Drug Discov. 2002;1:187–197. doi: 10.1038/nrd747. [DOI] [PubMed] [Google Scholar]
- Nevo I, Avidor-Reiss T, Levy R, Bayewitch M, Heldman E, Vogel Z. Regulation of adenylyl cyclase isozymes on acute and chronic activation of inhibitory receptors. Mol Pharmacol. 1998;54:419–426. doi: 10.1124/mol.54.2.419. [DOI] [PubMed] [Google Scholar]
- Nguyen CH, Watts VJ. Dexras1 blocks receptor-mediated heterologous sensitization of adenylyl cyclase 1. Biochem Biophys Res Commun. 2005;332:913–920. doi: 10.1016/j.bbrc.2005.05.041. [DOI] [PubMed] [Google Scholar]
- Nygaard R, Zou Y, Dror RO, Mildorf TJ, Arlow DH, Manglik A, Pan AC, Liu CW, Fung JJ, Bokoch MP, Thian FS, Kobilka TS, Shaw DE, Mueller L, Prosser RS, Kobilka BK. The dynamic process of beta(2)-adrenergic receptor activation. Cell. 2013;152:532–542. doi: 10.1016/j.cell.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oak JN, Lavine N, Van Tol HHM. Dopamine D-4 and D-2L receptor stimulation of the mitogen-activated protein kinase pathway is dependent on transactivation of the platelet-derived growth factor receptor. Mol Pharmacol. 2001;60:92–103. doi: 10.1124/mol.60.1.92. [DOI] [PubMed] [Google Scholar]
- Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006;5:993–996. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- Palmer TM, Harris CA, Coote J, Stiles GL. Induction of multiple effects on adenylyl cyclase regulation by chronic activation of the human A3 adenosine receptor. Mol Pharmacol. 1997;52:632–640. doi: 10.1124/mol.52.4.632. [DOI] [PubMed] [Google Scholar]
- Pasternak GW. Opiate pharmacology and relief of pain. J Clin Oncol. 2014;32:1655–1661. doi: 10.1200/JCO.2013.53.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel TB, Du Z, Pierre S, Cartin L, Scholich K. Molecular biological approaches to unravel adenylyl cyclase signaling and function. Gene. 2001;269:13–25. doi: 10.1016/s0378-1119(01)00448-6. [DOI] [PubMed] [Google Scholar]
- Rask-Andersen M, Almen MS, Schioth HB. Trends in the exploitation of novel drug targets. Nat Rev Drug Discov. 2011;10:579–590. doi: 10.1038/nrd3478. [DOI] [PubMed] [Google Scholar]
- Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, Thian FS, Chae PS, Pardon E, Calinski D, Mathiesen JM, Shah ST, Lyons JA, Caffrey M, Gellman SH, Steyaert J, Skiniotis G, Weis WI, Sunahara RK, Kobilka BK. Crystal structure of the beta2 adrenergic receptor-Gs protein complex. Nature. 2011;477:549–555. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee MH, Nevo I, Avidor-Reiss T, Levy R, Vogel Z. Differential superactivation of adenylyl cyclase isozymes after chronic activation of the CB(1) cannabinoid receptor. Mol Pharmacol. 2000;57:746–752. doi: 10.1124/mol.57.4.746. [DOI] [PubMed] [Google Scholar]
- Roy AA, Baragli A, Bernstein LS, Hepler JR, Hebert TE, Chidiac P. RGS2 interacts with Gs and adenylyl cyclase in living cells. Cell Signal. 2006;18:336–348. doi: 10.1016/j.cellsig.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Rubenzik M, Varga E, Stropova D, Roeske WR, Yamamura HI. Expression of alpha-transducin in Chinese hamster ovary cells stably transfected with the human delta-opioid receptor attenuates chronic opioid agonist-induced adenylyl cyclase superactivation. Mol Pharmacol. 2001;60:1076–1082. doi: 10.1124/mol.60.5.1076. [DOI] [PubMed] [Google Scholar]
- Sadana R, Dascal N, Dessauer CW. N terminus of type 5 adenylyl cyclase scaffolds Gs heterotrimer. Mol Pharmacol. 2009;76:1256–1264. doi: 10.1124/mol.109.058370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadana R, Dessauer CW. Physiological roles for G protein-regulated adenylyl cyclase isoforms: insights from knockout and overexpression studies. Neuro-Signals. 2009;17:5–22. doi: 10.1159/000166277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Gettys TW, Lanier SM. AGS3 and signal integration by Galpha(s)-and Galpha(i)-coupled receptors: AGS3 blocks the sensitization of adenylyl cyclase following prolonged stimulation of a Galpha(i)-coupled receptor by influencing processing of Galpha(i) J Biol Chem. 2004;279:13375–13382. doi: 10.1074/jbc.M312660200. [DOI] [PubMed] [Google Scholar]
- Schallmach E, Steiner D, Vogel Z. Adenylyl cyclase type II activity is regulated by two different mechanisms: implications for acute and chronic opioid exposure. Neuropharmacology. 2006;50:998–1005. doi: 10.1016/j.neuropharm.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Schwindinger WF, Mirshahi UL, Baylor KA, Sheridan KM, Stauffer AM, Usefof S, Stecker MM, Mirshahi T, Robishaw JD. Synergistic roles for G-protein gamma3 and gamma7 subtypes in seizure susceptibility as revealed in double knock-out mice. J Biol Chem. 2012;287:7121–7133. doi: 10.1074/jbc.M111.308395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Klee W, Nirenberg M. Dual regulation of adenylate cyclase accounts for narcotic dependence and tolerance. Proc Natl Acad Sci U S A. 1975;72:3092–3096. doi: 10.1073/pnas.72.8.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternweis PC, Robishaw JD. Isolation of two proteins with high affinity for guanine nucleotides from membranes of bovine brain. J Biol Chem. 1984;259:13806–13813. [PubMed] [Google Scholar]
- Sunahara RK, Taussig R. Isoforms of mammalian adenylyl cyclase: multiplicities of signaling. Mol Interv. 2002;2:168–184. doi: 10.1124/mi.2.3.168. [DOI] [PubMed] [Google Scholar]
- Takesono A, Cismowski MJ, Ribas C, Bernard M, Chung P, Hazard S, 3rd, Duzic E, Lanier SM. Receptor-independent activators of heterotrimeric G-protein signaling pathways. J Biol Chem. 1999;274:33202–33205. doi: 10.1074/jbc.274.47.33202. [DOI] [PubMed] [Google Scholar]
- Takesono A, Nowak MW, Cismowski M, Duzic E, Lanier SM. Activator of G-protein signaling 1 blocks GIRK channel activation by a G-protein-coupled receptor: apparent disruption of receptor signaling complexes. J Biol Chem. 2002;277:13827–13830. doi: 10.1074/jbc.M201064200. [DOI] [PubMed] [Google Scholar]
- Tan CM, Kelvin DJ, Litchfield DW, Ferguson SS, Feldman RD. Tyrosine kinase-mediated serine phosphorylation of adenylyl cyclase. Biochemistry. 2001;40:1702–1709. doi: 10.1021/bi0015818. [DOI] [PubMed] [Google Scholar]
- Taussig R, Sanchez S, Rifo M, Gilman AG, Belardetti F. Inhibition of the omega-conotoxin-sensitive calcium current by distinct G proteins. Neuron. 1992;8:799–809. doi: 10.1016/0896-6273(92)90100-r. [DOI] [PubMed] [Google Scholar]
- Thomas JM, Hoffman BB. Isoform-specific sensitization of adenylyl cyclase activity by prior activation of inhibitory receptors: role of beta gamma subunits in transducing enhanced activity of the type VI isoform. Mol Pharmacol. 1996;49:907–914. [PubMed] [Google Scholar]
- Tso PH, Wong YH. Deciphering the role of Gi2 in opioid-induced adenylyl cyclase supersensitization. Neuroreport. 2000;11:3213–3217. doi: 10.1097/00001756-200009280-00033. [DOI] [PubMed] [Google Scholar]
- Tso PH, Wong YH. Opioid-induced adenylyl cyclase supersensitization in human embryonic kidney 293 cells requires pertussis toxin-sensitive G proteins other than G(i1) and G(i3) Neurosci Lett. 2001;299:25–28. doi: 10.1016/s0304-3940(00)01772-9. [DOI] [PubMed] [Google Scholar]
- Varga E, Stropova D, Rubenzik M, Wang M, Landsman R, Roeske W, Yamamura H. Identification of adenylyl cyclase isoenzymes in CHO and B82 cells. Eur J Pharmacol. 1998;348:R1–R2. doi: 10.1016/s0014-2999(98)00258-1. [DOI] [PubMed] [Google Scholar]
- Varga EV, Rubenzik M, Grife V, Sugiyama M, Stropova D, Roeske WR, Yamamura HI. Involvement of Raf-1 in chronic delta-opioid receptor agonist-mediated adenylyl cyclase superactivation. Eur J Pharmacol. 2002;451:101–102. doi: 10.1016/s0014-2999(02)02220-3. [DOI] [PubMed] [Google Scholar]
- Varga EV, Rubenzik MK, Stropova D, Sugiyama M, Grife V, Hruby VJ, Rice KC, Roeske WR, Yamamura HI. Converging protein kinase pathways mediate adenylyl cyclase superactivation upon chronic delta-opioid agonist treatment. J Pharmacol Exp Ther. 2003a;306:109–115. doi: 10.1124/jpet.103.049643. [DOI] [PubMed] [Google Scholar]
- Varga EV, Stropova D, Rubenzik M, Waite S, Roeske WR, Yamamura HI. Phosphorylation of adenylyl cyclase VI upon chronic delta-opioid receptor stimulation. Eur J Pharmacol. 1999;364:R1–R3. doi: 10.1016/s0014-2999(98)00847-4. [DOI] [PubMed] [Google Scholar]
- Varga EV, Yamamura HI, Rubenzik MK, Stropova D, Navratilova E, Roeske WR. Molecular mechanisms of excitatory signaling upon chronic opioid agonist treatment. Life Sci. 2003b;74:299–311. doi: 10.1016/j.lfs.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Vidi P, Przybyla J, Hu C, Watts V. Visualization of G protein-coupled receptor (GPCR) interactions in living cells using bimolecular fluorescence complementation (BiFC) Curr Protoc Neurosci Chapter. 2010;5 doi: 10.1002/0471142301.ns0529s51. Unit 5.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vortherms TA, Nguyen CH, Bastepe M, Juppner H, Watts VJ. D2 dopamine receptor-induced sensitization of adenylyl cyclase type 1 is G alpha(s) independent. Neuropharmacology. 2006;50:576–584. doi: 10.1016/j.neuropharm.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Vortherms TA, Nguyen CH, Berlot CH, Watts VJ. Using molecular tools to dissect the role of Galphas in sensitization of AC1. Mol Pharmacol. 2004;66:1617–1624. doi: 10.1124/mol.104.000166. [DOI] [PubMed] [Google Scholar]
- Wang C, Buck DC, Yang R, Macey TA, Neve KA. Dopamine D2 receptor stimulation of mitogen-activated protein kinases mediated by cell type-dependent transactivation of receptor tyrosine kinases. J Neurochem. 2005;93:899–909. doi: 10.1111/j.1471-4159.2005.03055.x. [DOI] [PubMed] [Google Scholar]
- Wang SC, Lin JT, Chern Y. Novel regulation of adenylyl cyclases by direct protein-protein interactions: insights from snapin and ric8a. Neurosignals. 2009;17:169–180. doi: 10.1159/000200076. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ho G, Zhang JJ, Nieuwenhuijsen B, Edris W, Chanda PK, Young KH. Regulator of G protein signaling Z1 (RGSZ1) interacts with Galpha i subunits and regulates Galpha i-mediated cell signaling. J Biol Chem. 2002;277:48325–48332. doi: 10.1074/jbc.M206116200. [DOI] [PubMed] [Google Scholar]
- Watts V. Molecular mechanisms for heterologous sensitization of adenylate cyclase. J Pharmacol Exp Ther. 2002;302:1–7. doi: 10.1124/jpet.302.1.1. [DOI] [PubMed] [Google Scholar]
- Watts V, Neve K. Sensitization of adenylate cyclase by Galphai/o-coupled receptors. Pharmacol Ther. 2005;106(3):405–421. doi: 10.1016/j.pharmthera.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Watts V, Taussig R, Neve R, Neve K. Dopamine D2 receptor-induced heterologous sensitization of adenylyl cyclase requires Galphas: characterization of Galphas-insensitive mutants of adenylyl cyclase V. Mol Pharmacol. 2001;60:1168–1172. doi: 10.1124/mol.60.6.1168. [DOI] [PubMed] [Google Scholar]
- Watts VJ, Neve KA. Sensitization of endogenous and recombinant adenylate cyclase by activation of D2 dopamine receptors. Mol Pharmacol. 1996;50:966–976. [PubMed] [Google Scholar]
- Watts VJ, Neve KA. Activation of type II adenylate cyclase by D2 and D4 but not D3 dopamine receptors. Mol Pharmacol. 1997;52:181–186. doi: 10.1124/mol.52.2.181. [DOI] [PubMed] [Google Scholar]
- Watts VJ, Wiens BL, Cumbay MG, Vu MN, Neve RL, Neve KA. Selective activation of Galphao by D2L dopamine receptors in NS20Y neuroblastoma cells. J Neurosci. 1998;18:8692–8699. doi: 10.1523/JNEUROSCI.18-21-08692.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman GA, Wei J, Wong S, Storm DR. Regulation of type I adenylyl cyclase by calmodulin kinase IV in vivo. Molecular and cellular biology. 1996;16:6075–6082. doi: 10.1128/mcb.16.11.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Wayman G, Storm DR. Phosphorylation and inhibition of type III adenylyl cyclase by calmodulin-dependent protein kinase II in vivo. J Biol Chem. 1996;271:24231–24235. doi: 10.1074/jbc.271.39.24231. [DOI] [PubMed] [Google Scholar]
- Weitmann S, Schultz G, Kleuss C. Adenylyl cyclase type II domains involved in Gbetagamma stimulation. Biochemistry. 2001;40:10853–10858. doi: 10.1021/bi011176w. [DOI] [PubMed] [Google Scholar]
- Willoughby D, Cooper DM. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev. 2007;87:965–1010. doi: 10.1152/physrev.00049.2006. [DOI] [PubMed] [Google Scholar]
- Wong YH, Conklin BR, Bourne HR. Gz-mediated hormonal inhibition of cyclic AMP accumulation. Science. 1992;255:339–342. doi: 10.1126/science.1347957. [DOI] [PubMed] [Google Scholar]
- Xie K, Masuho I, Brand C, Dessauer CW, Martemyanov KA. The complex of G protein regulator RGS9-2 and Gbeta(5) controls sensitization and signaling kinetics of type 5 adenylyl cyclase in the striatum. Sci Signal. 2012;5(239) doi: 10.1126/scisignal.2002922. ra63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi I, Harmon SK, Todd RD, O’Malley KL. The rat D4 dopamine receptor couples to cone transducin (Galphat2) to inhibit forskolin-stimulated cAMP accumulation. J Biol Chem. 1997;272:16599–16602. doi: 10.1074/jbc.272.26.16599. [DOI] [PubMed] [Google Scholar]
- Yoon S, Choi MH, Chang MS, Baik JH. Wnt5a–dopamine D2 receptor interactions regulate dopamine neuron development via extracellular signal-regulated kinase (ERK) activation. J Biol Chem. 2011;286:15641–15651. doi: 10.1074/jbc.M110.188078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue X, Tumati S, Navratilova E, Strop D, St John PA, Vanderah TW, Roeske WR, Yamamura HI, Varga EV. Sustained morphine treatment augments basal CGRP release from cultured primary sensory neurons in a Raf-1 dependent manner. Eur J Pharmacol. 2008;584:272–277. doi: 10.1016/j.ejphar.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue X, Varga EV, Stropova D, Vanderah TW, Yamamura HI, Roeske WR. Chronic morphine-mediated adenylyl cyclase superactivation is attenuated by the Raf-1 inhibitor, GW5074. Eur J Pharmacol. 2006;540:57–59. doi: 10.1016/j.ejphar.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Zachariou V, Georgescu D, Sanchez N, Rahman Z, DiLeone R, Berton O, Neve RL, Sim-Selley LJ, Selley DE, Gold SJ, Nestler EJ. Essential role for RGS9 in opiate action. Proc Natl Acad Sci U S A. 2003;100:13656–13661. doi: 10.1073/pnas.2232594100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Loh HH, Law PY. A novel noncanonical signaling pathway for the mu-opioid receptor. Mol Pharmacol. 2013;84:844–853. doi: 10.1124/mol.113.088278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Tetrault J, Wang W, Loh HH, Law PY. Short- and long-term regulation of adenylyl cyclase activity by delta-opioid receptor are mediated by Galphai2 in neuroblastoma N2A cells. Mol Pharmacol. 2006;69:1810–1819. doi: 10.1124/mol.105.021352. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhao H, Qiu Y, Loh HH, Law PY. Src phosphorylation of micro-receptor is responsible for the receptor switching from an inhibitory to a stimulatory signal. J Biol Chem. 2009;284:1990–2000. doi: 10.1074/jbc.M807971200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann G, Zhou D, Taussig R. Genetic selection of mammalian adenylyl cyclases insensitive to stimulation by Gsalpha. J Biol Chem. 1998;273:6968–6975. doi: 10.1074/jbc.273.12.6968. [DOI] [PubMed] [Google Scholar]