Abstract
Evidence from human histopathology and experimental studies with rodents and zebrafish has shown that hepatocytes and cholangiocytes may function as facultative stem cells for each other in conditions of impaired regeneration. The interpretation of the findings derived from these studies has generated considerable discussion and some controversies. This review examines the evidence obtained from the different experimental models and considers implications that these studies may have for human liver disease.
Few topics of liver tissue biology have attracted as much attention as the existence of liver-specific tissue stem cells. Routine liver histology reveals two types of epithelial cells, hepatocytes and cholangiocytes (also known as biliary epithelial cells). Endothelial cells line the hepatic capillaries (sinusoids), with macrophages (Kupffer cells) interspersed along the sinusoid lumen. Stellate cells exist under the sinusoids and in close proximity to hepatocytes. None of these cells appears to have functions of a fully committed tissue specific stem cell, analogous to the cells of the intestinal crypts, the basal layer of the epidermis, bone marrow stem cells, etc.
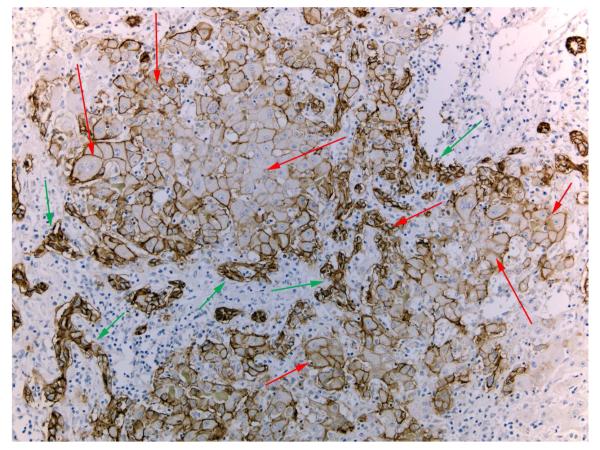
Hepatocytes and cholangiocytes can be easily identified based on their morphology and cell-specific biomarkers. Hepatocytes and cholangiocytes, however, often have mutually mixed expression of biomarkers in pathologic conditions. In patients with fulminant hepatic failure (FHF), there is rampant proliferation of cholangiocytes organized in ductular structures (“ductular reaction”1, 2). Many of these cholangiocytes (known as ductular hepatocytes) express biomarkers associated with hepatocytes, (HNF4, albumin, HEPPAR3, etc.). They are seen surrounding cells ranging in size from small to typical hepatocytes, and with a gradient of expression of cholangiocyte-associated biomarkers (e.g. EpCAM) decreasing from the periphery to the center (Regenerative Clusters: see Figure 1). It is not clear in FHF whether cholangiocytes give rise to hepatocytes or vice versa. Most cells in liver tissues from patients with FHF, however, are typical cholangiocytes, so it is likely that these are the source of hepatocytes detected in the (more rarely seen) regenerative clusters.
The term “progenitor” cells (used in tissue biology to describe the immediate progeny of stem cells) is most often used to collectively cover these proliferating cells with mixed hepatobiliary biomarkers in rats, mice, humans and fish. This may be inappropriate because it implies that such cells are generated by tissue-specific stem cells, even though such stem cells are not identifiable in the liver. Though the term “progenitor cells” does not fulfill criteria used in other tissues, it does imply a transition from one type of cell differentiation to another. Thus, the term has persisted in hepatic biology, even though it is not entirely appropriate. However, in most of the scenarios below, hepatocytes and cholangiocytes appear to function as “facultative stem cells” for each other. Thus the term “progenitor” is not entirely inappropriate. (The term “facultative stem cell”, better defined in the intestine4, implies that normal cells function as stem cells when necessary).
We will use the term “liver progenitor cells” (LPC) to be consistent with the majority of existing literature. We should caution, however, that not all cells named LPC are necessarily the same. Cells transitioning from hepatocytes to cholangiocytes appear different than cells transitioning in the opposite direction, and the latter appear different in details from rats (oval cells) and humans (ductular hepatocytes) and zebrafish 5, 6. The term LPC, used in a generic sense, is currently a useful term to employ until such time as the peculiarities of these cells in the different situations are better understood.
Possible Origins of LPC
LPC might derive from preexisting hepatic cells with mixed hepatobiliary differentiation. Cholangiocytes at the end of the canals of Hering, in sites with immediate proximity to hepatocytes, have mixed expression of transcription factors7. LPC might also derive from pre-existing hepatic tissue-specific stem cells. As mentioned above, such cells are simply not seen under sophisticated microscopy or complete tissue dissociation. If they exist, they must be present in exceedingly small numbers. There has been no evidence provided for their existence as a standard histologic element of liver lobules.
Much discussion in the literature has focused on the possibility that LPC may derive from hepatocytes or cholangiocytes undergoing trans-differentiation. During this process, cholangiocytes and hepatocytes function as “facultative stem cells,”4 and undergo transdifferentiation, or reprogramming, from one cell type to the other to rescue failed regeneration of hepatocytes or cholangiocytes. Several models have been designed to test these possibilities.
Cholangiocytes might become LPC to rescue hepatocytes. This model is controversial, because, until very recently8, different results have been obtained from studies of rats and mice. In rats fed a diet containing acetylaminofluorene (AAF), which is carcinogenic in rats and causes DNA damage, levels of p21 increase, leading to hepatocyte cell cycle arrest9. When rats given AAF undergo partial hepatectomy, liver growth does not occur for 5–6 days. Then, rapidly proliferating cells (LPC) appear in periportal areas, resembling cholangiocytes, with markers of biliary cells and hepatocytes 10, 11. Rats given AAF-containing diets followed by CCl4-induced centrilobular necrosis have similar periportal responses 12. A pulse of H3-thymidine, administered at the time of the LPC proliferation, labeled most of the nuclei. LPC that retained sufficient H3-thymidine migrated towards all zones of the lobule, expanded in size, and became small hepatocytes. These hepatocytes, retaining the H3-thymidine label of LPC, increased in size and eventually appeared throughout the lobule 10, 13, 14. The “tagging” of the LPC with H3-Thymidine did not depend on permanent genomic alterations, as in “lineage” tagging” done in mice. Critical analysis of the experimental results, however, demonstrates that the tagging was reliable and the follow-up was thorough and conclusive.
The main question in these studies was the origin of the LPC. Most of the evidence indicated that LPC derived from cholangiocytes. Prior to the emergence of LPC, expression of hepatocyte biomarkers including albumin, alpha-fetoprotein15 and HNF4α16 was seen in biliary ductules. Soon after their emergence as a distinct cell population, LPC expressed most biliary biomarkers (HNF1β, CK19, etc.17) and the above hepatocyte biomarkers. The biliary biomarkers are gradually lost as LPC expanded within the hepatic lobule and transitioned to mature hepatocytes. It should be noted that Farber et a. 18 in one publication reported that cholangiocytes did not differentiate into LPC in rats fed AAF or subjected to partial hepatectomy. However, studies from several other groups with multiple publications reported that cholangiocytes did differentiate into LPC in this model11, 19, 20. In other studies, administration of DAPM (a biliary-specific toxin) prior to AAF and partial hepatectomy prevented the appearance of LPC21. The LPC expressed EGFR, MET, FGFR1 and FGFR2.17, 22-24. TWEAK, a member of the tumor necrosis factor family, is a mitogen for these cells 25. When all evidence is taken together, it appears that in rats, in response to a strong regenerative stimulus and with severely inhibited hepatocyte proliferation, some cholangiocytes express hepatocyte-associated transcription factors, proliferate as LPC, and become hepatocytes. This model satisfies the criteria for Ockham’s razor (among competing hypotheses that predict equally well, the one with the fewest assumptions should be selected). There were no genetically defined lineage tags in this system; however, such an approach was pursued in the studies with mice.
Mice cannot activate AAF to carcinogenic electrophiles due to lack of a sulfotransferase and thus AAF does not generate DNA adducts that block hepatocyte proliferation26. AAF therefore cannot be used in such studies of mice. A porphyria-inducing diet, containing diethyl 1,4-dihydro-2,4,6-trimethyl-pyridine-3,5-dicarboxylate (DDC)27 causes appearance of cells resembling the oval cells seen in rats. Such cells also emerge with diets causing hepatic toxicity, hepatocyte death and rapid cell turnover (choline deficient (CD) diet with ethionine (CDE), high dose galactosamine with LPS, etc.28). Cholangiocytes and hepatocytes can be tagged specifically and irreversibly in mice by expression of markers under control of a cell-specific promoter. Using this approach, Rodrigo-Torres et al29 demonstrated that cells in a typical ductular reaction of human FHF expressed the cholangiocyte-specific transcription factor HNF1β. They applied this observation in mice and generated strains with hepatic cell lineages tagged based on HNF1β. In these mice there was no evidence of generation of hepatocytes from cholangiocytes, except in mice fed CDE diet, in which 1.83% of hepatocytes were “tagged”. Espanol-Suner et al labeled cells using the osteopontin promoter30 and found 2.45% of hepatocytes to derive from LPC or biliary cells. Both studies found no evidence for generation of hepatocytes from cholangiocytes (or LPC) following partial hepatectomy or CCl4 injury. Schaub et al31 tagged hepatocytes using AAV vectors that expressed Cre recombinase under the transthyretin promoter and did not observe generation of hepatocytes from cells other than hepatocytes in mice on choline-deficient diets. Yanger et al tagged cholangiocytes by expressing a marker under the keratin 19, type I promoter, in mice placed under various conditions (including those on a DDC-containing diet); they did not observe generation of hepatocytes from tagged cholangiocytes under any of the conditions32. Similar results were obtained by Jors et al who tagged cholangiocytes by expressing a marker from the Hnf1b promoter 33.
Tarlow et al tagged SOX9-positive cells in mice, analyzed formation of organoids in culture, monitored responses of cells in mice on CDE diet or diets containing DDC, and also tracked cells transferred into Fah-/- mice34. (These mice suffer from tyrosinemia and are kept alive by administering the drug (2-[2-nitro-4-trifluoromethylbenzoyl]-1,3-cyclohexanedione (NTCB). Removal of the drug results in acute tyrosinemia, hepatocyte death and liver failure. Hepatocytes from normal, immune-compatible donors, can be transplanted and recolonize the liver of these mice. It is an excellent model to test the capacity of transplanted cells to give rise to hepatocytes.) They found less than 1% of hepatocytes derive from SOX9-positive precursors. In a subsequent study38, these authors found a DDC-containing diet to induce generation of cholangiocytes from hepatocytes. Upon termination of the diet, these cells reverted to hepatocytes. The authors concluded that this pathway allows hepatocytes to undergo ductal metaplasia and then revert back to hepatocytes, as a mechanism of survival following severe chronic injury. The hepatocyte-derived cholangiocytes continued to express some hepatocyte-specific genes (such as HNF4, low expression of EPCAM35). It is not clear whether ductal cells derived from hepatocytes in this study are similar to those seen in rats with DPP4 chimeric livers36. Furthermore, in previous studies by the same group with Fah-/- mice on CDE diets, hepatocytes did not appear to be the originators of LPC, because FAH was not expressed in the LPC generated in the study37.
In a very recent publication, Lu et al presented convincing evidence of conversion of cholangiocytes to hepatocytes when hepatocyte Mdm2 was removed8. Mdm2flox/flox mice were crossed with mice expressing AhCre and beta-napthoflavone was used to activate Cre and induce removal of Mdm2 from hepatocytes. That resulted in over-expression of p53 and p21 causing widespread hepatocyte death by apoptosis. There was a massive ductular reaction and in several months hepatic histology was restored primarily by cells that had retained Mdm2. The authors used several lineage tagging approaches as well as enhancement of the response by the LPC mitogen TWEAK to demonstrate that the source of tissue restoration was from LPC. In separate experiments they tagged cholangiocyte lineage and demonstrated that the bipotential clonal fraction of LPC derived from cholangiocytes. It is of interest that this phenomenon was finally demonstrated in mice when p21 was induced and blocked hepatocyte proliferation, similar to the rat model in which cholangiocyte conversion to LPC and hepatocytes was also seen when the AAF diet induced an upregulation of p21 in hepatocytes9. It is conceivable that the cholangiocyte conversion to hepatocytes via LPC is uniquely dependent on inhibition of hepatocyte proliferation due to induction of p21.
Studies with biliary organoids derived from human liver provide evidence that cholangiocytes can differentiate into hepatocytes. Huch et al isolated cholangiocytes from human liver based on expression of EPCAM38. The cells were grown into organoids, induced to transdifferentiate in culture, and expressed hepatocyte-specific genes. When the human biliary organoids were transplanted into mice given retrorsine (a DNA cross-linking agent that is activated in only hepatocytes) and subjected to hepatectomy, the cholangiocyte-derived organoids colonized the livers with human hepatocytes. Cholangiocytes isolated from liver biopsies from patients with liver diseases also differentiated into hepatocytes in the organoid cultures, but still carried markers of the patients’ diseases, such as globules that contain the abnormally folded ATz mutant form of α-1 antitrypsin38. It is important to note, however, that the transdifferentiation of cholangiocytes to hepatocytes occurred in culture—the hepatocyte phenotype detected after transplantation of the cells into mice was observed before the cells were transplanted.
There is also evidence from zebrafish studies that cholangiocytes can generate hepatocytes after the latter are severely damaged15, 6, 39. When hepatocytes were destroyed with metronidazole, cholangiocytes trans-differentiated to hepatocytes and rescued the liver. In one of the studies39, similar to observations in rats21, administration of the biliary toxin DAPM inhibited the rescue of destroyed hepatocytes by cholangiocytes.
It seems therefore fair to conclude that under most conditions of chronic toxic injury or normal liver regeneration, hepatocytes and cholangiocytes proliferate and retain their phenotype. This is strongly supported by both the rat and the mouse studies31, 32, 34. In extreme conditions of complete elimination of hepatocyte replication (AAF/Partial hepatectomy in rats10, 11, 13-16, 19, 22-24, 40,metronidazole in zebrafish6, 39, expression of p21 in mice8) the evidence that cholangiocyte-derived LPC give rise to cells which eventually become hepatocytes is also very strong. It is not clear, however, whether all cholangiocytes only a fraction do so 41. Because of the findings with regenerative clusters from human liver with FHF (Figure 1), pathways by which cholangiocytes differentiate into LPC and hepatocytes should continue to be explored. They might lead to new approaches to treating human liver disease. FHF most commonly leads to death or liver transplantation. Some FHF cases however recover spontaneously42. LPC have been frequently detected in liver tissues from patients with FHF 43, but their significance in recovery was not assessed until recently 43. There is also evidence that many of the hepatocyte nodules observed in patients with micro-nodular cirrhosis are derived from cholangiocytes, providing a new angle of research for liver cell biologists 44.
Figure 1. Regenerative clusters of mixed phenotypes in fulminant hepatic failure.
Regenerative clusters composed of cholangiocytes (green arrows) organized in tortuous ductules surrounding and connecting with hepatocytes (red arrows). Hepatocytes express EPCAM, a marker of cholangiocytes, to various degrees. EPCAM expression decreases towards the center of the regenerative cluster. These features are frequently observed in histologic analyses of liver tissues from patients with fulminant hepatic failure with massive hepatocyte necrosis and ductular reaction.
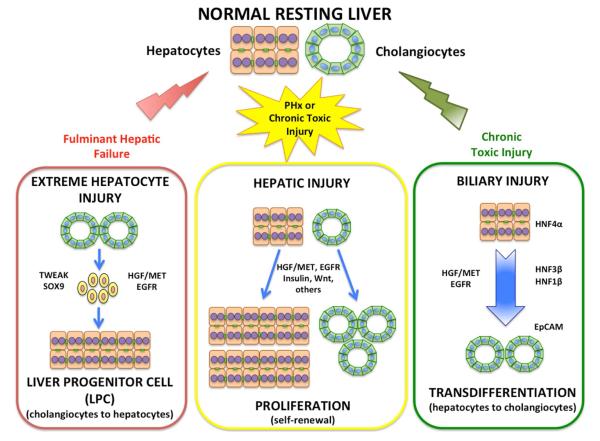
There is evidence from animal and human studies that hepatocytes can transdifferentiate to cholangiocytes. DPP4-negative rats were given retrorsine, a DNA crosslinking agent blocking hepatocyte proliferation45. When these rats received partial hepatectomies followed by injection of DPP4-positive hepatocytes, the transplanted hepatocytes colonized the host liver, resulting in a chimeric liver with predominance of donor DPP4-positive hepatocytes. Cholangiocytes are not affected by retrorsine, proliferate and remain DPP4-negative. Bile duct ligation generated biliary ductules of which 1%–2% expressed the donor hepatocyte marker (DPP4). However, when the cholangiocyte specific toxin DAPM was injected before bile duct ligation (to impair the capacity of cholangiocytes to undergo self-repair), approximately 50% of bile ductules expressed DPP4, indicating that when cholangiocytes were damaged and lost the capacity to proliferate and restore their lineage, more cholangiocytes were derived from donor hepatocytes36. It is interesting that there was no evidence of formation of LPC. The immediate periportal hepatocytes transdifferentiated directly into ductal structures lined by ductular hepatocytes, and became biliary ductules36. This indicates that the immediate periportal hepatocytes have increased capacity to transdifferentiate to cholangiocytes, consistent with findings by Carpentier et al, that immediate periportal hepatocytes derive from remnants of the ductal plate 46. In humans, expression of cholangiocyte-specific transcription factor HFN3β was widespread in patients with biliary obstruction or primary biliary cirrhosis47. In mice, Yanger et al showed that lineage-tagged hepatocytes from all areas of the lobule could transdifferentiate into cholangiocytes and implicated activation of Notch signaling48 as part of this process. Yimlamai et al showed that acute inactivation of the Hippo pathway (and consequent elevation of nuclear YAP) induced dedifferentiation and activation of a ductal phenotype in hepatocytes49. In hepatic organoids in culture, only signals transmitted via the EGF receptor or MET (the receptor for HGF) activated transdifferentiation of hepatocytes into cholangiocytes; PI3K activation was an essential element in this process and induced only by activation of EGFR and MET50. Fan et al51 and Sekiya et al52 also provided evidence that cholangiocarcinomas can arise from hepatocytes. All these studies demonstrate that hepatocytes participate in repair and salvage of critically injured biliary epithelium in rodents and humans, and that Notch, Hippo/Yap, EGFR, HGF/MET and PI3K signaling (all of them also important for liver regeneration53-57) are involved in this process. A schematic illustrating the transdifferentiation relationships between cholangiocytes and hepatocytes is shown in Figure 2.
Figure 2. Potential mechanisms of liver regeneration in relation to parenchymal cell injury.
Following partial hepatectomy (PHx), most liver cells are capable of regeneration, with hepatocytes and cholangiocytes respectively contributing to self-replication (center panel). Under extreme hepatocyte loss, such as in cases of fulminant hepatic failure or AAF exposure, cholangiocytes become progenitor cells that become hepatocytes (left panel). With biliary injury (following administration of DAPM), hepatocytes undergo transdifferentiation directly into cholangiocytes (right panel). These types of reprograming events result in phenotypic inter-conversion to replace the injured cell type, and involve a number of regenerative pathways including HGF signaling via MET, and EGFR signaling.
There have been reports that stellate cells can give rise to hepatocytes. Recent studies from Kordes et al58 demonstrated that transplantation of lineage-tagged stellate cells in rats subjected to the AAF/partial hepatectomy protocol resulted in hepatocytes and cholangiocytes bearing the stellate lineage marker. On the other hand, Schwabe and coworkers using carefully controlled stellate cell specific fate-tracing could find no evidence whatsoever that epithelial cells of the liver could be traced back to stellate cells59.
Liver tissue maintenance in steady state
Strong evidence shown above indicates that during standard liver regeneration after hepatectomy or in conditions of selective hepatocyte or cholangiocyte damage, active proliferation of hepatic cells is involved in tissue restoration. Not much is understood however related to maintenance of hepatic tissue in steady state. BRDU or Thymidine labeling of hepatocytes in adult rodents is generally less than 0.1% and randomly distributed in the lobule. Detailed studies in mice using long term H3-thymidine label immediately after birth showed extensive labeling of hepatocytes in all zones60. Loss of H3-thymidine grains was slightly different in different lobule zones, with a slight edge in the perivenous region, suggesting a higher rate of proliferation. Other studies had shown that the immediate pericentral, glutamine-synthetase (GS) positive hepatocytes have a low rate of proliferation compared to hepatocytes in the other zones61, 62. Surprisingly, however, Wang et al in a recent study63 demonstrated that lineage tagging of some pericentral GS-positive hepatocytes generated progeny of tagged hepatocytes that eventually encompassed on average 30% of the lobular area. The tagging was made based on Axin2, part of the ubiquitin ligase complex associated with destruction of beta-catenin. Although Furuyama et al showed progressive migration of Sox9 lineage-tagged cells being the source for steady state replacement of most of the hepatocytes in all lobular zones64, follow-up studies by Lemaigre et al did not confirm this finding 65, and it is believed that lineage tracing by Furuyama et al. was not sufficiently specific. Several studies using labeling of hepatocyte DNA in resting liver had appeared in the past, suggesting a continuous “streaming” of hepatocytes through the liver lobule66, 67. Other studies, however, looking into local progeny of single labeled cells did not support the concept68. More studies should be performed to resolve these controversies and it will be crucial to be able to reproduce any of the above findings using more than one type of methodology. Liver has many options to restore tissue damage, not all of which are understandable to us. Whatever the pathway chosen, Nature knows best.
Conclusions
Studies of transdifferentiation between hepatic cell types have generated significant divergence of views and some controversies. At this stage, the potential of hepatocytes to rescue cholangiocytes is accepted by most groups in the field. Most of the controversy relates to the possibility of cholangiocytes giving rise to LPC and hepatocytes. On this issue, studies with mice suggest that this occurs very rarely or not at all. Recent studies, however, seem to indicate that this transdifferentiation is possible in mice and depends on inhibition of hepatocyte proliferation due to elevation of p218. Findings with rats, humans and zebrafish provide evidence that in situations of severe hepatocyte depletion or near absolute inhibition of hepatocyte proliferation, cholangiocytes can generate LPC and hepatocytes. This should not be entirely surprising. Several studies from the 70s and 80s have shown the plasticity of the hepatic epithelial phenotypes. Hepatocytes in primary culture express biomarkers characteristic of cholangiocytes (gamma-glutamyl transpeptidase, alkaline phosphatase)69. Human cholangiocytes can differentiate into various endoderm-derived cell types, including intestinal mucosal cells, pancreatic acinar cells etc. 70-72. Pancreatic ductules can give rise to hepatocytes73-75. The etiologies eliciting catastrophic conditions in cholangiocytes and hepatocytes are quite different. From an evolutionary perspective, the ability of hepatocytes and cholangiocytes to function as facultative stem cells for each other perhaps is more effective in preventing organ failure than the use of tissue-specific stem cells. Further studies of LPC could lead to new strategies for treatment of tissue damage in liver disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Demetris AJ, Seaberg EC, Wennerberg A, et al. Ductular reaction after submassive necrosis in humans. Special emphasis on analysis of ductular hepatocytes. Am J Pathol. 1996;149:439–48. [PMC free article] [PubMed] [Google Scholar]
- 2.Desmet V, Roskams T, Van Eyken P. Ductular reaction in the liver. Pathol Res Pract. 1995;191:513–24. doi: 10.1016/s0344-0338(11)80870-8. [DOI] [PubMed] [Google Scholar]
- 3.Butler SL, Dong H, Cardona D, et al. The antigen for Hep Par 1 antibody is the urea cycle enzyme carbamoyl phosphate synthetase 1. Lab Invest. 2008;88:78–88. doi: 10.1038/labinvest.3700699. [DOI] [PubMed] [Google Scholar]
- 4.Gracz AD, Fuller MK, Wang F, et al. Brief report: CD24 and CD44 mark human intestinal epithelial cell populations with characteristics of active and facultative stem cells. Stem Cells. 2013;31:2024–30. doi: 10.1002/stem.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verfaillie CM. Biliary cells to the rescue of Prometheus. Gastroenterology. 2014;146:611–4. doi: 10.1053/j.gastro.2014.01.039. [DOI] [PubMed] [Google Scholar]
- 6.Choi TY, Ninov N, Stainier DY, et al. Extensive conversion of hepatic biliary epithelial cells to hepatocytes after near total loss of hepatocytes in zebrafish. Gastroenterology. 2014;146:776–88. doi: 10.1053/j.gastro.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isse K, Lesniak A, Grama K, et al. Preexisting epithelial diversity in normal human livers: a tissue-tethered cytometric analysis in portal/periportal epithelial cells. Hepatology. 2013;57:1632–43. doi: 10.1002/hep.26131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu WY, Bird TG, Boulter L, et al. Hepatic progenitor cells of biliary origin with liver repopulation capacity. Nat Cell Biol. 2015 doi: 10.1038/ncb3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trautwein C, Will M, Kubicka S, et al. 2-acetaminofluorene blocks cell cycle progression after hepatectomy by p21 induction and lack of cyclin E expression. Oncogene. 1999;18:6443–53. doi: 10.1038/sj.onc.1203045. [DOI] [PubMed] [Google Scholar]
- 10.Evarts RP, Nagy P, Nakatsukasa H, et al. In vivo differentiation of rat liver oval cells into hepatocytes. Cancer Res. 1989;49:1541–7. [PubMed] [Google Scholar]
- 11.Golding M, Sarraf CE, Lalani EN, et al. Oval cell differentiation into hepatocytes in the acetylaminofluorene- treated regenerating rat liver. Hepatology. 1995;22:1243–53. doi: 10.1016/0270-9139(95)90635-5. [DOI] [PubMed] [Google Scholar]
- 12.Petersen BE, Zajac VF, Michalopoulos GK. Hepatic oval cell activation in response to injury following chemically induced periportal or pericentral damage in rats. Hepatology. 1998;27:1030–8. doi: 10.1002/hep.510270419. [DOI] [PubMed] [Google Scholar]
- 13.Evarts RP, Nagy P, Marsden E, et al. A precursor-product relationship exists between oval cells and hepatocytes in rat liver. Carcinogenesis. 1987;8:1737–40. doi: 10.1093/carcin/8.11.1737. [DOI] [PubMed] [Google Scholar]
- 14.Paku S, Nagy P, Kopper L, et al. 2-acetylaminofluorene dose-dependent differentiation of rat oval cells into hepatocytes: confocal and electron microscopic studies. Hepatology. 2004;39:1353–61. doi: 10.1002/hep.20178. [DOI] [PubMed] [Google Scholar]
- 15.Evarts RP, Nagy P, Marsden E, et al. In situ hybridization studies on expression of albumin and alpha- fetoprotein during the early stage of neoplastic transformation in rat liver. Cancer Res. 1987;47:5469–75. [PubMed] [Google Scholar]
- 16.Nagy P, Bisgaard HC, Thorgeirsson SS. Expression of hepatic transcription factors during liver development and oval cell differentiation. J Cell Biol. 1994;126:223–33. doi: 10.1083/jcb.126.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alison MR, Islam S, Lim S. Stem cells in liver regeneration, fibrosis and cancer: the good, the bad and the ugly. The Journal of pathology. 2009;217:282–98. doi: 10.1002/path.2453. [DOI] [PubMed] [Google Scholar]
- 18.Tatematsu M, Ho RH, Kaku T, et al. Studies on the proliferation and fate of oval cells in the liver of rats treated with 2-acetylaminofluorene and partial hepatectomy. The American journal of pathology. 1984;114:418–30. [PMC free article] [PubMed] [Google Scholar]
- 19.Alison M, Golding M, Lalani EN, et al. Wholesale hepatocytic differentiation in the rat from ductular oval cells, the progeny of biliary stem cells. J Hepatol. 1997;26:343–52. doi: 10.1016/s0168-8278(97)80051-7. [DOI] [PubMed] [Google Scholar]
- 20.Alison MR, Poulsom R, Jeffery R, et al. Expression of hepatocyte growth factor mRNA during oval cell activation in the rat liver. J Pathol. 1993;171:291–9. doi: 10.1002/path.1711710410. [DOI] [PubMed] [Google Scholar]
- 21.Petersen BE, Zajac VF, Michalopoulos GK. Bile ductular damage induced by methylene dianiline inhibits oval cell activation. Am J Pathol. 1997;151:905–9. [PMC free article] [PubMed] [Google Scholar]
- 22.Evarts RP, Hu Z, Fujio K, et al. Activation of hepatic stem cell compartment in the rat: role of transforming growth factor alpha, hepatocyte growth factor, and acidic fibroblast growth factor in early proliferation. Cell Growth Differ. 1993;4:555–61. [PubMed] [Google Scholar]
- 23.Fujio K, Evarts RP, Hu Z, et al. Expression of stem cell factor and its receptor, ckit, during liver regeneration from putative stem cells in adult rat. Lab Invest. 1994;70:511–6. [PubMed] [Google Scholar]
- 24.Hu Z, Evarts RP, Fujio K, et al. Expression of fibroblast growth factor receptors flg and bek during hepatic ontogenesis and regeneration in the rat. Cell Growth Differ. 1995;6:1019–25. [PubMed] [Google Scholar]
- 25.Jakubowski A, Ambrose C, Parr M, et al. TWEAK induces liver progenitor cell proliferation. The Journal of clinical investigation. 2005;115:2330–40. doi: 10.1172/JCI23486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeBaun JR, Rowley JY, Miller EC, et al. Sulfotransferase activation of N-hydroxy-2-acetylaminofluorene in rodent livers susceptible and resistant to this carcinogen. Proc Soc Exp Biol Med. 1968;129:268–73. doi: 10.3181/00379727-129-33301. [DOI] [PubMed] [Google Scholar]
- 27.Magnus IA, Roe DA, Bhutani LK. Factors affecting the induction of porphyria in the laboratory rat. Biochemical and photobiological studies using diethyl 1,4-dihydro-2,4,6-trimethyl-pyridine-3,5-dicarboxylate (DDC) as a porphyrogenic agent. J Invest Dermatol. 1969;53:400–13. doi: 10.1038/jid.1969.167. [DOI] [PubMed] [Google Scholar]
- 28.Guest I, Ilic Z, Sell S. Age dependence of oval cell responses and bile duct carcinomas in male fischer 344 rats fed a cyclic choline-deficient, ethionine-supplemented diet. Hepatology. 2010;52:1750–7. doi: 10.1002/hep.23880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodrigo-Torres D, Affo S, Coll M, et al. The biliary epithelium gives rise to liver progenitor cells. Hepatology. 2014;60:1367–77. doi: 10.1002/hep.27078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Espanol-Suner R, Carpentier R, Van Hul N, et al. Liver progenitor cells yield functional hepatocytes in response to chronic liver injury in mice. Gastroenterology. 2012;143:1564–1575. e7. doi: 10.1053/j.gastro.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 31.Schaub JR, Malato Y, Gormond C, et al. Evidence against a stem cell origin of new hepatocytes in a common mouse model of chronic liver injury. Cell Rep. 2014;8:933–9. doi: 10.1016/j.celrep.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yanger K, Knigin D, Zong Y, et al. Adult hepatocytes are generated by self-duplication rather than stem cell differentiation. Cell Stem Cell. 2014;15:340–9. doi: 10.1016/j.stem.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jors S, Jeliazkova P, Ringelhan M, et al. Lineage fate of ductular reactions in liver injury and carcinogenesis. J Clin Invest. 2015;125:2445–57. doi: 10.1172/JCI78585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarlow BD, Finegold MJ, Grompe M. Clonal tracing of Sox9+ liver progenitors in mouse oval cell injury. Hepatology. 2014;60:278–89. doi: 10.1002/hep.27084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tarlow BD, Pelz C, Naugler WE, et al. Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell Stem Cell. 2014;15:605–18. doi: 10.1016/j.stem.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michalopoulos GK, Barua L, Bowen WC. Transdifferentiation of rat hepatocytes into biliary cells after bile duct ligation and toxic biliary injury. Hepatology. 2005;41:535–44. doi: 10.1002/hep.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Foster M, Al-Dhalimy M, et al. The origin and liver repopulating capacity of murine oval cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(Suppl 1):11881–8. doi: 10.1073/pnas.1734199100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huch M, Gehart H, van Boxtel R, et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 2015;160:299–312. doi: 10.1016/j.cell.2014.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He J, Lu H, Zou Q, et al. Regeneration of liver after extreme hepatocyte loss occurs mainly via biliary transdifferentiation in zebrafish. Gastroenterology. 2014;146:789–800. e8. doi: 10.1053/j.gastro.2013.11.045. [DOI] [PubMed] [Google Scholar]
- 40.Bisgaard HC, Nagy P, Santoni-Rugiu E, et al. Proliferation, apoptosis, and induction of hepatic transcription factors are characteristics of the early response of biliary epithelial (oval) cells to chemical carcinogens. Hepatology. 1996;23:62–70. doi: 10.1002/hep.510230110. [DOI] [PubMed] [Google Scholar]
- 41.Dorrell C, Erker L, Schug J, et al. Prospective isolation of a bipotential clonogenic liver progenitor cell in adult mice. Genes & development. 2011;25:1193–203. doi: 10.1101/gad.2029411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whitehouse T, Wendon J. Acute liver failure. Best Pract Res Clin Gastroenterol. 2013;27:757–69. doi: 10.1016/j.bpg.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 43.Weng HL, Cai X, Yuan X, et al. Two sides of one coin: massive hepatic necrosis and progenitor cell-mediated regeneration in acute liver failure. Front Physiol. 2015;6:178. doi: 10.3389/fphys.2015.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stueck AE, Wanless IR. Hepatocyte buds derived from progenitor cells repopulate regions of parenchymal extinction in human cirrhosis. Hepatology. 2015 doi: 10.1002/hep.27706. [DOI] [PubMed] [Google Scholar]
- 45.Michalopoulos GK, Bowen WC, Mule K, et al. Hepatocytes undergo phenotypic transformation to biliary epithelium in organoid cultures. Hepatology. 2002;36:278–83. doi: 10.1053/jhep.2002.34858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carpentier R, Suner RE, van Hul N, et al. Embryonic ductal plate cells give rise to cholangiocytes, periportal hepatocytes, and adult liver progenitor cells. Gastroenterology. 2011;141:1432–8. 1438, e1–4. doi: 10.1053/j.gastro.2011.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Limaye PB, Alarcon G, Walls AL, et al. Expression of specific hepatocyte and cholangiocyte transcription factors in human liver disease and embryonic development. Laboratory investigation; a journal of technical methods and pathology. 2008;88:865–72. doi: 10.1038/labinvest.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yanger K, Zong Y, Maggs LR, et al. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes Dev. 2013;27:719–24. doi: 10.1101/gad.207803.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yimlamai D, Christodoulou C, Galli GG, et al. Hippo pathway activity influences liver cell fate. Cell. 2014;157:1324–38. doi: 10.1016/j.cell.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Limaye PB, Bowen WC, Orr AV, et al. Mechanisms of hepatocyte growth factor-mediated and epidermal growth factor-mediated signaling in transdifferentiation of rat hepatocytes to biliary epithelium. Hepatology. 2008;47:1702–13. doi: 10.1002/hep.22221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fan B, Malato Y, Calvisi DF, et al. Cholangiocarcinomas can originate from hepatocytes in mice. J Clin Invest. 2012;122:2911–5. doi: 10.1172/JCI63212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sekiya S, Suzuki A. Intrahepatic cholangiocarcinoma can arise from Notch-mediated conversion of hepatocytes. J Clin Invest. 2012;122:3914–8. doi: 10.1172/JCI63065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Michalopoulos GK. Principles of liver regeneration and growth homeostasis. Compr Physiol. 2013;3:485–513. doi: 10.1002/cphy.c120014. [DOI] [PubMed] [Google Scholar]
- 55.Michalopoulos GK. Advances in liver regeneration. Expert Rev Gastroenterol Hepatol. 2014:1–11. doi: 10.1586/17474124.2014.934358. [DOI] [PubMed] [Google Scholar]
- 56.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–6. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 57.Kohler C, Bell AW, Bowen WC, et al. Expression of Notch-1 and its ligand Jagged-1 in rat liver during liver regeneration. Hepatology. 2004;39:1056–65. doi: 10.1002/hep.20156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kordes C, Sawitza I, Gotze S, et al. Hepatic stellate cells contribute to progenitor cells and liver regeneration. J Clin Invest. 2014;124:5503–15. doi: 10.1172/JCI74119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mederacke I, Hsu CC, Troeger JS, et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823. doi: 10.1038/ncomms3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Magami Y, Azuma T, Inokuchi H, et al. Cell proliferation and renewal of normal hepatocytes and bile duct cells in adult mouse liver. Liver. 2002;22:419–25. doi: 10.1034/j.1600-0676.2002.01702.x. [DOI] [PubMed] [Google Scholar]
- 61.Gebhardt R, Cruise J, Houck KA, et al. Differential effect of growth factors on growth stimulation and phenotypic stability of glutamine-synthetase-positive and - negative hepatocytes in primary culture. Differentiation. 1986;33:45–55. doi: 10.1111/j.1432-0436.1986.tb00409.x. [DOI] [PubMed] [Google Scholar]
- 62.Volk A, Michalopoulos G, Weidner M, et al. Different proliferative responses of periportal and pericentral rat hepatocytes to hepatocyte growth factor. Biochem Biophys Res Commun. 1995;207:578–84. doi: 10.1006/bbrc.1995.1227. [DOI] [PubMed] [Google Scholar]
- 63.Wang B, Zhao L, Fish M, et al. Self-renewing diploid Axin2+ cells fuel homeostatic renewal of the liver. Nature. 2015 doi: 10.1038/nature14863. doi:10.1038/nature 14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Furuyama K, Kawaguchi Y, Akiyama H, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nature genetics. 2011;43:34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- 65.Lemaigre FP. Determining the fate of hepatic cells by lineage tracing: facts and pitfalls. Hepatology. 2015;61:2100–3. doi: 10.1002/hep.27659. [DOI] [PubMed] [Google Scholar]
- 66.Lamers WH. The streaming liver: can the age of a hepatocyte be determined from its position on the portohepatic radius? Hepatology. 1990;12:372–4. doi: 10.1002/hep.1840120231. [DOI] [PubMed] [Google Scholar]
- 67.Zajicek G, Schwartz-Arad D. Streaming liver. VII: DNA turnover in acinus zone-3. Liver. 1990;10:137–40. doi: 10.1111/j.1600-0676.1990.tb00448.x. [DOI] [PubMed] [Google Scholar]
- 68.Bralet MP, Branchereau S, Brechot C, et al. Cell lineage study in the liver using retroviral mediated gene transfer. Evidence against the streaming of hepatocytes in normal liver. The American journal of pathology. 1994;144:896–905. [PMC free article] [PubMed] [Google Scholar]
- 69.Sirica AE, Richards W, Tsukada Y, et al. Fetal phenotypic expression by adult rat hepatocytes on collagen gel/nylon meshes. Proc Natl Acad Sci U S A. 1979;76:283–7. doi: 10.1073/pnas.76.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wolf HK, Burchette JL, Jr., Garcia JA, et al. Exocrine pancreatic tissue in human liver: a metaplastic process? The American journal of surgical pathology. 1990;14:590–5. doi: 10.1097/00000478-199006000-00011. [DOI] [PubMed] [Google Scholar]
- 71.Elmore LW, Sirica AE. Phenotypic characterization of metaplastic intestinal glands and ductular hepatocytes in cholangiofibrotic lesions rapidly induced in the caudate liver lobe of rats treated with furan. Cancer Res. 1991;51:5752–9. [PubMed] [Google Scholar]
- 72.Elmore LW, Sirica AE. Sequential appearance of intestinal mucosal cell types in the right and caudate liver lobes of furan-treated rats. Hepatology. 1992;16:1220–6. [PubMed] [Google Scholar]
- 73.Makino T, Usuda N, Rao S, et al. Transdifferentiation of ductular cells into hepatocytes in regenerating hamster pancreas. Lab Invest. 1990;62:552–61. [PubMed] [Google Scholar]
- 74.Rao MS, Dwivedi RS, Subbarao V, et al. Almost total conversion of pancreas to liver in the adult rat: a reliable model to study transdifferentiation. Biochem Biophys Res Commun. 1988;156:131–6. doi: 10.1016/s0006-291x(88)80814-3. [DOI] [PubMed] [Google Scholar]
- 75.Reddy JK, Rao MS, Qureshi SA, et al. Induction and origin of hepatocytes in rat pancreas. J Cell Biol. 1984;98:2082–90. doi: 10.1083/jcb.98.6.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]