Abstract
Pramipexole is a D3 dopamine receptor-preferring agonist indicated for the treatment of Parkinson disease. Studies associate pramipexole with pathological gambling and impulse control disorders suggesting a role for D3 receptors in reinforcement processes. Clinical studies showed pramipexole decreased cocaine craving and reversed central deficits in individuals with cocaine use disorder. Preclinical studies have shown acute administration of pramipexole increases cocaine’s reinforcing effects whereas other reports suggest chronic pramipexole produces tolerance to cocaine. In a randomized, double-blind, placebo-controlled study we examined the impact of pramipexole treatment on the subjective effects produced by cocaine in volunteers with cocaine use disorder. Volunteers received pramipexole titrated up to 3.0 mg/d or placebo over 15 days. Participants then received intravenous cocaine (0, 20 and 40 mg) on day 15. Cardiovascular and subjective effects were obtained with visual analog scales at time points across the session. Pramipexole alone increased peak heart rate following saline and diastolic blood pressure following cocaine. Pramipexole produced upwards of two-fold increases in positive subjective effects ratings following cocaine. These results indicate that chronic D3 receptor activation increases the subjective effects of cocaine in humans. Caution should be used when prescribing pramipexole to patients that may also use cocaine.
Keywords: Drug reinforcement, Stimulants, Substance use disorder, Cocaine use disorder, Drug reward, Peak effects
1. Introduction
A large body of evidence suggests that the reinforcing effects of cocaine are mediated, in part, by cocaine’s ability to increase synaptic dopamine (DA) levels within mesocorticolimbic brain areas (Haile et al., 2012a). Increased DA levels lead to activation of pre and post-synaptic DA receptor subtypes (D1–D5) that differ in their function and neuroanatomical distribution. The contribution of specific DA receptor subtypes in mediating the reinforcing effects of cocaine is not fully understood though data suggest dopamine D3 receptors may be important.
Pramipexole is a D3 receptor-preferring agonist indicated for the treatment of Parkinson disease and restless legs syndrome (Sokoloff et al., 1990; Mierau et al., 1995). Reports associate pramipexole with pathological gambling and impulse control disorders suggesting the medication may in some way contribute toward aberrant reward processing (Basu et al., 2011; Kelley et al., 2012). D3 receptor distribution is highly localized within the nucleus accumbens and prefrontal cortex, structures critical in the expression of the reinforcing effects of drugs (Sokoloff et al., 1990; Parsons et al., 1996). D3 receptor mRNA and receptor numbers are significantly increased in the nucleus accumbens (NAc) of cocaine overdose victims (Staley and Mash, 1996; Segal et al., 1997). and in volunteers dependent on another stimulant, methamphetamine (Boileau et al., 2012). Taken together, these data suggest that D3 receptors may be a viable therapeutic target. Indeed, one report described successful reduction of cocaine craving with 1.5 mg/day of pramipexole (Rosenbaum and Fredman, 1999), though an 8 week outpatient clinical trial assessing the impact of treatment with 0.5 mg pramipexole three times per day found no effect on cocaine use (Ciraulo et al., 2005).
Rodent studies assessing pramipexole on behavior elicited by cocaine are contradictory. For example, in rodents, acute administration of pramipexole decreases self-administration of a high dose of cocaine, presumably by shifting the dose response curve leftward (Caine et al., 1997). Acute pramipexole has also been shown to increases the reinforcing efficacy of conditioned stimuli previously paired with cocaine (Collins et al., 2012). Yet, the D3-preferring partial agonist, BP 897, blocked cue-induced cocaine-seeking in a rodent model of relapse (Pilla et al., 1999)., In contrast to acute administration of pramipexole, Ellinwood and colleagues reported that chronic treatment with pramipexole produced tolerance to cocaine-induced locomotor activation without producing stereotypy (Ellinwood et al., 2002). Based on this finding, we hypothesized that chronic treatment with pramipexole would produce tolerance to the positive subjective effects of cocaine in volunteers with cocaine use disorder. Therefore, we conducted the present study to determine if chronic treatment with pramipexole would attenuate the positive subjective effects produced by cocaine in non-treatment-seeking volunteers with cocaine-use disorder.
2. Methods
2.1. Participants and setting
Ten non-treatment-seeking volunteers with cocaine-use disorder completed the study which was conducted at the Baylor College of Medicine (BCM) and the Michael E. DeBakey Veteran’s Administration Medical Center (MEDVAMC). Volunteers were recruited using advertisements and paid $50/day for their participation. Participants also had the opportunity to earn additional money as part of choice procedures, as described below.
All participants met DSM-IV-TR criteria for cocaine dependence determined using the MINI neuropsychiatric interview. Additional inclusion criteria included age between 18 and 55 years, a history of cocaine use by smoking or intravenous routes, being in good health as confirmed by standard evaluations. Females tested negative for pregnancy by urinary human chorionic gonadotropin. Participants were excluded if they met criteria for dependence on drugs other than cocaine or nicotine. Additional exclusion criteria included a history of seizures, head trauma, experiencing an adverse effect associated with cocaine use, or the presence of current axis I psychiatric disorders other than those noted above. Serious medical conditions such as symptomatic HIV disease, asthma, heart and neurological disease were also exclusionary. Use of centrally acting concomitant medications was not allowed. The BCM/VA institutional review board approved this study and all participants gave informed consent after being fully informed of potential risks of participating in the study.
2.2. Medications
Sterile cocaine HCl for human use was provided by a contractor for NIDA’s medication supply program. Cocaine and saline solutions were prepared by the hospital’s Research Pharmacy. Pramipexole extended-release (ER) was purchased commercially (Greenpark Pharmacy, Houston, TX). To ensure that a clinically relevant dose was used, we chose a maximum dose of pramipexole of 3.0 mg/day, a dose used for the treatment of Parkinson’s disease (Bennett and Piercey, 1999).
2.3. Study design
We used a randomized, double-blind, placebo-controlled, parallel groups design. Following screening, those that met inclusion/exclusion criteria were housed on the Research Commons of the MEDVAMC for the duration of the study. Daily urine drug screens were employed to ensure that participants did not take any drugs not administered as part of this study. Pramipexole ER treatment was started at 0.375 mg/d. On the fourth day the dose was increased to 0.75 mg/d. The dose was then increased every fourth day by 0.75 mg/d until the final dose of 3.0 mg/d was reached. Participants randomized to placebo received identical numbers of placebo capsules. Cocaine was administered on day 15 of medication treatment, corresponding to treatment with 3.0 mg/d pramipexole. Non-contingent doses of cocaine or placebo saline (0, 20, 40 mg, IV) were administered at hourly intervals in the morning in pseudorandom order such that the 40 mg dose always followed the 20 mg dose. A physician was available during all infusion sessions and participants were monitored for 4 additional hours after the last infusion to ensure safety. Following completion of all study procedures, study medication was reduced to 1.5 mg/d for one day and then discontinued. The participant was then discharged. Participants were contacted during the two weeks following study completion to inquire about any late-occurring side effects.
2.4. Outcome measures
Cardiovascular measures including heart rate (HR), systolic (SBP) and diastolic (DBP) blood pressure, and cardiac rate and rhythm (electrocardiogram, ECG) were assessed at the beginning and at numerous time points throughout the experimental sessions (−5, 15, 30, 45, 60, 75, 90, 105, 120, 135 min following IV dosing). Criteria for participants to receive cocaine included a resting HR < 90 bpm and BP < 150 mmHg systolic and < 90 mmHg diastolic. Other stopping criteria were in place but were not met during the course of the study.
Subjective effects ratings were collected prior to and at frequent intervals (−15, 5, 10, 15, 20 and 30 min) following non-contingent cocaine dosing. Participants rated “HIGH”, “GOOD DRUG EFFECT”, “ANY DRUG EFFECT”, “LIKE DRUG”, “STIMULATED”, cocaine if available, “DESIRE” cocaine, “BAD EFFECT”, and “ANXIOUS”,”DEPRESSED”, “LIKELY TO USE” and willing to “PAY” on visual-analog scales (VAS) anchored at 0 (not at all) and 100 (most ever).
2.5. Data analysis
All data were first subjected to Shapiro–Wilk normality test. If data did not pass normality the appropriate non-parametric statistical analysis was used. Potential differences between groups on participant distribution, demographic and drug use characteristics were assessed using Fisher’s Exact Test, independent t-tests or Mann–Whitney Rank Sum test. Peak cardiovascular measures and subjective ratings at each time point (5, 10, 15, 20 and 30 min) following cocaine were analyzed using 2 × 3 analysis of variance (ANOVA) with pramipexole treatment (0 mg and 3 mg) and cocaine dose (0 mg, 20 mg and 40 mg) as factors. significant main effects were subjected to post-hoc pairwise multiple comparison procedures using Student–Newman–Keuls Method. Data sets for peak subjective ratings that did not meet normality assumptions were analyzed using Kruskal–Wallis ANOVA on Ranks. significant main effects were then further analyzed using Dunn’s Method. Significance was set at p < 0.05.
3. Results
3.1. Demographics
Participant distribution, demographics and drug use are presented in Table 1. The majority of the participants were about 40 years of age, mostly African American males. Most used alcohol but did not meet criteria for alcohol use disorder. A majority of participants also smoked cigarettes and met criteria for nicotine use disorder. Demographic measures and drug use histories did not significantly differ between groups (p’s > 0.05). Participant enrollment, allocation to study group and data that underwent final analysis is presented in Fig. 1.
Table 1.
Demographics and drug use histories.
| PLACEBO | PRAMIPEXOLE | p-value | |
|---|---|---|---|
| Gender | |||
| Male | 7 | 4 | 1.00 |
| Female | 5 | 2 | |
| Age (years) | 39.75 ± 1.96 | 43.83 ± 2.16 | 0.20 |
| Education (years) | 12.41 ± 0.41 | 11.72 ± 0.60 | 0.35 |
| Cocaine | |||
| Years used | 10.70 ± 2.14 | 16.67 ± 2.60 | 0.12 |
| Grams per day | 2.27 ± 1.39 | 6.42 ± 3.03 | 0.15 |
| Use in last 30 daysa | 18.16 ± 2.07 | 12.60 ± 2.97 | 0.14 |
| Preferred route of administration | |||
| Smoke | 11 | 6 | |
| Intranasal | – | – | |
| Intravenous | 1 | – | |
| Nicotine | |||
| Years used | 13.41 ± 2.77 | 19.67 ± 4.40 | 0.23 |
| Use per day | 9.25 ± 2.93 | 10.50 ± 4.46 | 0.81 |
| Use in last 30 days | 20.04 ± 3.68 | 21.33 ± 5.57 | 0.82 |
| Alcohol | |||
| Years used | 13.81 ± 2.65 | 19.83 ± 4.02 | 0.37 |
| Use per day | 1.59 ± 0.56 | 3.00 ± 1.13 | 0.23 |
| Use in last 30 days | 4.54 ± 2.07 | 7.50 ± 3.29 | 0.43 |
| Cannabis | |||
| Years used | 10.83 ± 3.45 | 11.83 ± 5.24 | 0.23 |
| Use per day | 2.83 ± 2.14 | 0.75 ± 0.75 | 0.51 |
| Use in last 30 days | 3.00 ± 1.64 | 4.16 ± 4.16 | 0.76 |
| Weight (kg) | 75.87 ± 3.80 | 88.03 ± 10.78 | 0.20 |
Use in last 30 days indicates number of days of drug use in the 30 days preceding entry into this study.
Fig. 1.
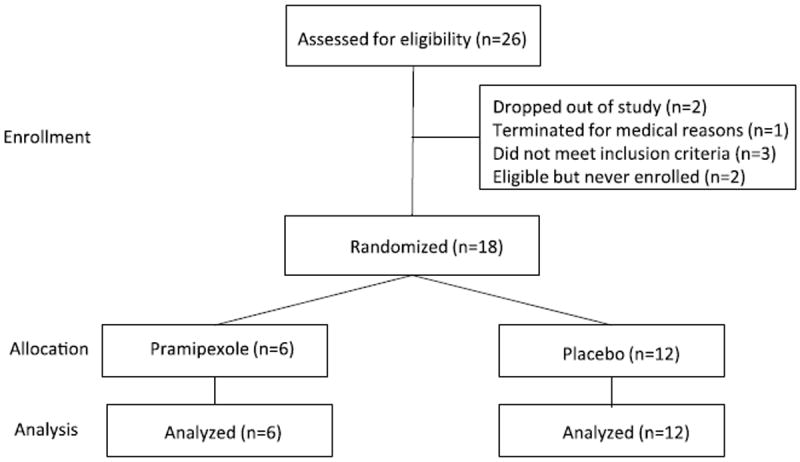
CONSORT flow diagram.
3.2. Cardiovascular effects
Peak HR showed significant main effects for pramipexole (F1,53 = 6.308; p=0.015) and cocaine dose (F2,53 = 6.338; p=0.004) and no interaction (F2,53 = 1.001; p=0.375). Post-hoc comparisons following a main effect for cocaine dose revealed both cocaine doses significantly increased peak HR compared to saline (0 mg vs. 20 mg p < 0.010; 0 mg vs. 40 mg p=0.007) whereas peak HR did not differ between cocaine doses (p=0.807). Compared to placebo (0 mg), pramipexole (3 mg) treatment was associated with greater peak HR following saline (0 mg, p=0.028) but not cocaine (p’s > 0.05). Peak SBP showed a significant main effect for cocaine dose (F2,53 = 4.55; p=0.015) but not for pramipexole (F1,53 = 2.45; p=0.124) and no interaction (F2,53 = 0.249; p=0.781). Post-hoc analysis showed peak SBP was significantly higher following cocaine (40 mg) compared to saline (p=0.015). DBP showed a main effect for pramipexole (F1,53 = 6.26; p=0.016) but not cocaine dose (F2,53 = 1.79; p=0.178) and no interaction (F2,53 = 0.897; p=0.415). Post-hoc analysis showed pramipexole (3 mg) was associated with higher peak DBP following the highest cocaine dose (40 mg) compared to placebo (0 mg, p=0.018) (Fig. 2).
Fig. 2.
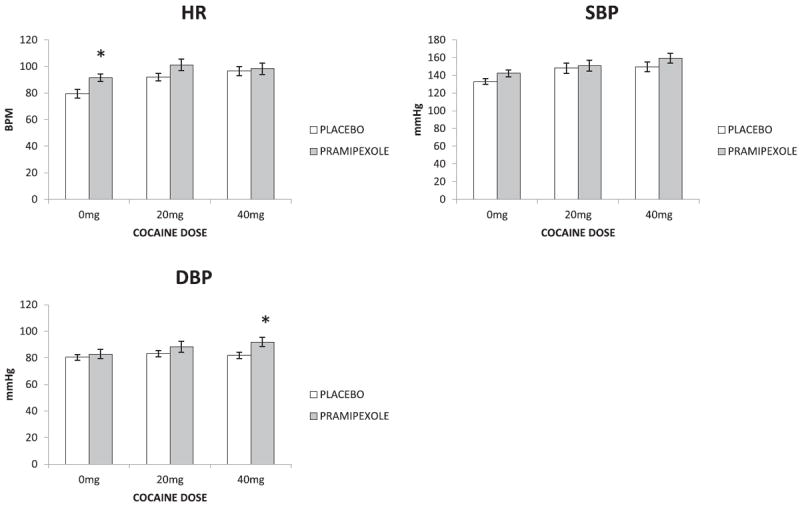
Mean (+SEM) subjective effects ratings for “HIGH” (A), “ANY DRUG EFFECT” (B), “GOOD DRUG EFFECT” (C) and “LIKE DRUG” (D) following 0, 20 and 40 mg cocaine from participants treated with placebo (pramipexole 0 mg) and pramipexole (3 mg). *p < 0.05, ** <0.01.
3.3. Subjective effects
Subjective effects ratings following non-contingent IV cocaine (0 mg, 20 mg and 40 mg) in participants receiving pramipexole (0 mg and 3 mg) are presented in Fig. 3A–D. “HIGH” ratings showed significant main effects for pramipexole treatment (F1,53 = 12.687; p < 0.001) and cocaine dose (F2,53 = 19.437; p < 0.001) but no interaction (F2,53 = 2.690; p=0.078). Post-hoc comparisons revealed significantly greater ratings for “HIGH” by participants treated with pramipexole (3 mg) compared to placebo (pramipexole 0 mg) following 20 mg cocaine (p < 0.001; Fig. 3A). For “ANY DRUG EFFECT” (Fig. 3B) ratings analysis revealed a significant main effect for pramipexole treatment (F1,53 = 4.138; p=0.047) and cocaine dose (F2,29 = 18.15; p=0.002) and no interaction (F2,53 = 0.906; p=0.411). Post-hoc analysis showed greater ratings for “ANY DRUG EFFECT” by participants that received pramipexole compared to placebo (p=0.028) following 20 mg cocaine. Similarly, analysis of “GOOD DRUG EFFECTS” ratings revealed a significant main effect for pramipexole treatment (F1,53 = 8.295; p=0.006) and cocaine dose (F2,53 = 19.869; p < 0.001) and no interaction (F2,53 = 1.549; p=0.223). Post-hoc comparisons showed treatment with pramipexole was associated with higher ratings for “GOOD DRUG EFFECTS” compared to placebo (p=0.004) following 20 mg cocaine (Fig. 3C). For “LIKE DRUG” ratings ANOVA revealed a significant main effect for pramipexole treatment (F1,53 = 9.310; p=0.004) and cocaine dose (F2,29 = 18.600; p < 0.001) and no interaction (F2,53 = 1.156; p=0.323). Post-hoc comparisons revealed that treatment with pramipexole was associated with higher ratings for “LIKE DRUG” compared to placebo following 20 mg cocaine only (p=0.005; Fig. 3D). For “STIMULATED” ratings ANOVA revealed a significant main effect for cocaine dose (F2,53 = 7.968; p=0.001) but not pramipexole treatment (F1,53 = 3.457; p=0.069) and no interaction (F2,53 = 0.002; p=0.998). Post-hoc comparisons revealed overall that both doses of cocaine (20 and 40 mg) were associated with higher ratings for “STIMULATED” compared to saline (0 mg cocaine; p’s < 0.01; data not shown). No significant main effects were found for “BAD”, “DESIRE”, “DEPRESSED”, “ANXIOUS”, “LIKELY TO USE” and “WILLING TO PAY” (p’s > 0.05) ratings.
Fig. 3.
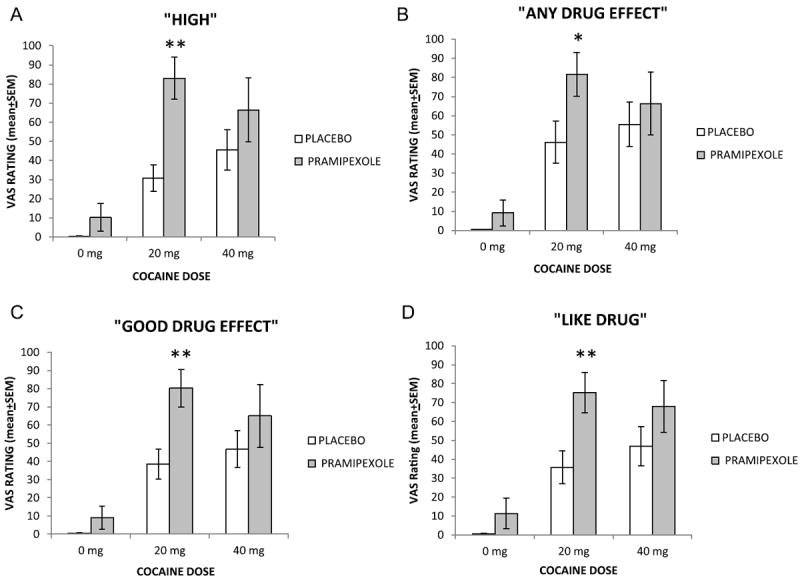
Mean (+SEM) peak VAS ratings obtained within the 30 min test session between placebo and pramipexole treated groups following different doses of cocaine (0 mg, 20 mg and 40 mg). Asterisks represent significant difference between groups (*p < 0.05, **p < 0.01).
4. Discussion
We found that chronic pramipexole treatment enhanced the positive subjective effects produced by cocaine. Our results are consistent with preclinical studies showing pramipexole enhances the effects of low doses of cocaine yet discordant with an earlier finding in rodents demonstrating that chronic pramipexole treatment produced tolerance to cocaine’s locomotor activating effects (Ellinwood et al., 2002).
We used a target dose of pramipexole 3.0 mg/d in the present study which is a dose indicated for the treatment of Parkinson disease. Depending on the dose conversion calculation method, the dose used by Ellinwood and colleagues in their rodent study was 4 mg/d for a 150 g rat which corresponds to between 45 and 60 mg/d (for a 70 kg individual), a dose too high to administer to humans (West et al., 1997; Reagan-Shaw et al., 2008). Further, it is unlikely that increasing the pramipexole dose beyond 3.0 mg/d would lead to attenuation of cocaine’s effects without producing significant side effects.
It is possible that tolerance to the motor effects of cocaine measured by Ellinwood does not parallel tolerance to positive subjective effects or reinforcing effects. This has been found with disulfiram. Disulfiram attenuates the reinforcing effects of cocaine in humans and blocks drug-induced reinstatement of cocaine-seeking in a rodent model of relapse yet enhances cocaine-induced locomotor activation (Haile et al., 2003; Schroeder et al., 2010; Haile et al., 2012b). Another explanation is that the dose of pramipexole Ellinwood and colleagues used may have produced stereotypy that interfered with locomotion, though they used a scale specifically designed to measure stereotypy (Ellinwood and Balster, 1974). Other factors including species differences may explain divergent findings.
The mechanism by which pramipexole enhances cocaine’s effects presumably involves enhanced activation of D3 receptors, which are localized in the nucleus accumbens and pre-frontal cortex, brain regions known to be important in mediating the reinforcing effects of cocaine. However, Chernoloz and colleagues have recently shown that chronic pramipexole treatment also produces desensitization of presynaptic D2/3 and 5-HT1A auto-receptors and decreased sensitivity of presynaptic NE α2 receptors, leading to enhanced DA, 5-HT and possibly, NE release (Chernoloz et al., 2009, 2012). Desensitization of presynaptic D2/3 and NE α2 autoreceptors would likely lead to enhanced DA and NE signaling in response to cocaine or other reinforcing stimuli as well. Indeed, studies have reported patients with Parkinson’s disease receiving pramipexole and other D3 receptor-preferring agonists are prone to develop behaviors such as pathological gambling, that may reflect enhanced reward sensitivity (Weintraub et al., 2010). Another mechanism that may be involved in pramipexole’s ability to enhance cocaine’s subjective effects may be due to increases in D3 receptor expression. For example, although counter-intuitive, administration of pramipexole (1 mg/kg twice daily for 14 days), but not the D2-preferring agonist bro-mocriptine, leads to increases in D3 receptor mRNA and protein within the striatum of rodents (Tokunaga et al., 2012). Evidence suggests that increased D3 receptor numbers or activity may signal predisposition to developing a substance use disorder. Indeed, post-mortem studies have shown D3 receptor numbers are increased in the NAc in cocaine overdose victims (Staley and Mash, 1996). Recent imaging studies in cocaine users confirmed previous studies indicating increases in D3 receptor numbers correlate with impulsivity (Payer et al., 2013). Increases in D3 receptor numbers has also been found in individuals with methamphetamine use disorder (Boileau et al., 2012).
The present findings have several clinical implications. Given treatment with a D3 receptor-preferring agonist intensified the effects of cocaine, selective D3 receptor antagonists would be expected to attenuate the effects of cocaine (Newman et al., 2012; Heidbreder, 2013). This has been observed with several different D3 receptor antagonists in preclinical studies (Vorel et al., 2002; Xi et al., 2005; Song et al., 2012) and a metabolite of buspirone has been identified that appears to have D3 receptor antagonist effects (Newman et al., 2012). It remains to be seen whether reductions in reinforcing effects produced by D3 antagonists can be sufficiently dissociated from motor effects, however.
Our results provide evidence against using pramipexole or other D3-preferring agonists as a replacement therapy for cocaine dependence, as had been suggested earlier (Caine et al., 1997). In general, D3 receptor-preferring agonists should be avoided for patients with histories of cocaine use, and possibly for those with other substance use disorders or impulse control disorders, such as gambling (Kolla et al., 2010). Recent studies have also suggested pramipexole may be useful as an augmentation therapy for depression in individuals who do not respond to conventional anti-depressant and antipsychotic medication (Kelleher et al., 2012; Cusin et al., 2013). Substance use disorders and psychiatric disorders commonly co-occur presenting a challenge for proper medical management if pramipexole is being considered as an augmentation therapy for patients with depression. For those at risk who need treatment with a DA agonist, treatment with a D2 receptor-preferring agonist without activity at D3 receptors may be preferable, though this has not yet been demonstrated.
The most obvious limitation of this study is the small sample size however the magnitude of the observed impact of pramipexole treatment on the effects produced by cocaine suggests that the results are likely to be valid in spite of the small sample size and lack of statistical power.
In summary, results from this study underscore the role played by D3 receptor stimulation in mediating the positive subjective effects of cocaine. These observations can inform subsequent research aimed at developing medication treatments for stimulant-use disorders.
Acknowledgments
This study was funded by the National Institute of Health (NIH), NIDA grant: P50 DA018197-10 (Clinicaltrials.gov identifier: NCT01651377). NIH played no role in the collection, analysis or interpretation of data described in the study. The authors declare no potential conflicts of interest related to this study. We would like to acknowledge the expert assistance of the BCM General Clinical Research nursing staff. This work was conducted at, and supported by, the Michael E. DeBakey VA Medical Center, Houston, TX.
Footnotes
Contributors
Authors Thomas Newton and Richard De La Garza designed the study. Authors Thomas Newton, Colin Haile, James Mahoney, Ravi Shah, and Richard De La Garza conducted the experimental protocol. Thomas Kosten directed the study. Colin Haile undertook the statistical analysis. Thomas Newton and Colin Haile wrote the initial manuscript. Richard De La Garza, Christopher Verrico and Thomas Kosten significantly contributed to the final draft of the manuscript.
Conflicts of interest
The authors declare no relevant conflict of interest or financial interests to declare relating to this study.
References
- Basu S, Kwee TC, Surti S, Akin EA, Yoo D, Alavi A. Fundamentals of PET and PET/CT imaging. Ann N Y Acad Sci. 2011;1228:1–18. doi: 10.1111/j.1749-6632.2011.06077.x. [DOI] [PubMed] [Google Scholar]
- Bennett JP, Jr, Piercey MF. Pramipexole—a new dopamine agonist for the treatment of Parkinson’s disease. J Neurol Sci. 1999;163:25–31. doi: 10.1016/s0022-510x(98)00307-4. [DOI] [PubMed] [Google Scholar]
- Boileau I, Payer D, Houle S, Behzadi A, Rusjan PM, Tong J, Wilkins D, Selby P, George TP, Zack M, Furukawa Y, McCluskey T, Wilson AA, Kish SJ. Higher binding of the dopamine D3 receptor-preferring ligand [11C]-(+)-pro-pyl-hexahydro-naphtho-oxazin in methamphetamine polydrug users: a positron emission tomography study. J Neurosci. 2012;32:1353–1359. doi: 10.1523/JNEUROSCI.4371-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Koob GF, Parsons LH, Everitt BJ, Schwartz JC, Sokoloff P. D3 receptor test in vitro predicts decreased cocaine self-administration in rats. NeuroReport. 1997;8:2373–2377. doi: 10.1097/00001756-199707070-00054. [DOI] [PubMed] [Google Scholar]
- Chernoloz O, El Mansari M, Blier P. Sustained administration of pramipexole modifies the spontaneous 3ring of dopamine, norepinephrine, and serotonin neurons in the rat brain. Neuropsychopharmacology. 2009;34:651–661. doi: 10.1038/npp.2008.114. [DOI] [PubMed] [Google Scholar]
- Chernoloz O, El Mansari M, Blier P. Long-term administration of the dopamine D3/2 receptor agonist pramipexole increases dopamine and serotonin neurotransmission in the male rat forebrain. J Psychiatry Neurosci: JPN. 2012;37:113–121. doi: 10.1503/jpn.110038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciraulo DA, Sarid-Segal O, Knapp CM, Ciraulo AM, LoCastro J, Bloch DA, Montgomery MA, Leiderman DB, Elkashef A. Efficacy screening trials of paroxetine, pentoxifylline, riluzole, pramipexole and venlafaxine in cocaine dependence. Addiction. 2005;100(Suppl 1):12–22. doi: 10.1111/j.1360-0443.2005.00985.x. [DOI] [PubMed] [Google Scholar]
- Collins GT, Cunningham AR, Chen J, Wang S, Newman AH, Woods JH. Effects of pramipexole on the reinforcing effectiveness of stimuli that were previously paired with cocaine reinforcement in rats. Psychopharmacology. 2012;219:123–135. doi: 10.1007/s00213-011-2382-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusin C, Iovieno N, Iosifescu DV, Nierenberg AA, Fava M, Rush AJ, Perlis RH. A randomized, double-blind, placebo-controlled trial of pramipexole augmentation in treatment-resistant major depressive disorder. J Clin Psychiatry. 2013;74:e636–e641. doi: 10.4088/JCP.12m08093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinwood EH, Balster RL. Rating the behavioral effects of amphetamine. Eur J Pharmacol. 1974;28:35–41. doi: 10.1016/0014-2999(74)90109-5. [DOI] [PubMed] [Google Scholar]
- Ellinwood EH, Davidson C, Yu GZ, King GR, Lee TH. Effect of daily dosing duration of direct and indirect dopamine receptor agonists: cocaine cross-tolerance following chronic regimens. Eur Neuropsychopharmacol. 2002;12:407–415. doi: 10.1016/s0924-977x(02)00055-x. [DOI] [PubMed] [Google Scholar]
- Haile CN, Mahoney JJ, 3rd, Newton TF, De La Garza R., 2nd Pharma-cotherapeutics directed at deficiencies associated with cocaine dependence: focus on dopamine, norepinephrine and glutamate. Pharmacol Ther. 2012a;134:260–277. doi: 10.1016/j.pharmthera.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile CN, De La Garza R, 2nd, Mahoney JJ, 3rd, Nielsen DA, Kosten TR, Newton TF. The impact of disulfiram treatment on the reinforcing effects of cocaine: a randomized clinical trial. PLoS One. 2012b;7:e47702. doi: 10.1371/journal.pone.0047702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile CN, During MJ, Jatlow PI, Kosten TR, Kosten TA. Disulfiram facilitates the development and expression of locomotor sensitization to cocaine in rats. Biol Psychiatry. 2003;54:915–921. doi: 10.1016/s0006-3223(03)00241-5. [DOI] [PubMed] [Google Scholar]
- Heidbreder C. Rationale in support of the use of selective dopamine D (3) receptor antagonists for the pharmacotherapeutic management of substance use disorders. Naunyn-Schmiedeberg’s Arch Pharmacol. 2013;386:167–176. doi: 10.1007/s00210-012-0803-6. [DOI] [PubMed] [Google Scholar]
- Kelleher JP, Centorrino F, Huxley NA, Bates JA, Drake JK, Egli S, Baldessarini RJ. Pilot randomized, controlled trial of pramipexole to augment anti-psychotic treatment. Eur Neuropsychopharmacol J Eur Coll Neuropsycho-pharmacol. 2012;22:415–418. doi: 10.1016/j.euroneuro.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Kelley BJ, Duker AP, Chiu P. Dopamine agonists and pathologic behaviors. Parkinson’s Dis. 2012;2012 doi: 10.1155/2012/603631. 603631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolla BP, Mansukhani MP, Barraza R, Bostwick JM. Impact of dopamine agonists on compulsive behaviors: a case series of pramipexole-induced pathological gambling. Psychosomatics. 2010;51:271–273. doi: 10.1176/appi.psy.51.3.271. [DOI] [PubMed] [Google Scholar]
- Mierau J, Schneider FJ, Ensinger HA, Chio CL, Lajiness ME, Huff RM. Pramipexole binding and activation of cloned and expressed dopamine D2, D3 and D4 receptors. Eur J Pharmacol. 1995;290:29–36. doi: 10.1016/0922-4106(95)90013-6. [DOI] [PubMed] [Google Scholar]
- Newman AH, Blaylock BL, Nader MA, Bergman J, Sibley DR, Skolnick P. Medication discovery for addiction: translating the dopamine D3 receptor hypothesis. Biochem Pharmacol. 2012;84:882–890. doi: 10.1016/j.bcp.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons LH, Caine SB, Sokoloff P, Schwartz JC, Koob GF, Weiss F. Neurochemical evidence that postsynaptic nucleus accumbens D3 receptor stimulation enhances cocaine reinforcement. J Neurochem. 1996;67:1078–1089. doi: 10.1046/j.1471-4159.1996.67031078.x. [DOI] [PubMed] [Google Scholar]
- Payer DE, Behzadi A, Kish SJ, Houle S, Wilson AA, Rusjan PM, Tong J, Selby P, George TP, McCluskey T, Boileau I. Heightened D dopamine receptor levels in cocaine dependence and contributions to the addiction behavioral phenotype: a positron emission tomography study with [C]-(+)-PHNO. Neuropsychopharmacology. 2013;39:311–318. doi: 10.1038/npp.2013.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, Schwartz JC, Everitt BJ, Sokoloff P. Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature. 1999;400:371–375. doi: 10.1038/22560. [DOI] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. Fed Am Soc Exp Biol. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JF, Fredman SJ. Pramipexole treatment for cocaine cravings. Am J Psychiatry. 1999;156:1834. doi: 10.1176/ajp.156.11.1834. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Cooper DA, Schank JR, Lyle MA, Gaval-Cruz M, Ogbonmwan YE, Pozdeyev N, Freeman KG, Iuvone PM, Edwards GL, Holmes PV, Weinshenker D. Disulfiram attenuates drug-primed reinstatement of cocaine seeking via inhibition of dopamine beta-hydroxylase. Neuropsychopharmacology. 2010;35:2440–2449. doi: 10.1038/npp.2010.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal DM, Moraes CT, Mash DC. Up-regulation of D3 dopamine receptor mRNA in the nucleus accumbens of human cocaine fatalities. Brain Research Mol Brain Res. 1997;45:335–339. doi: 10.1016/s0169-328x(97)00025-9. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Song R, Yang RF, Wu N, Su RB, Li J, Peng XQ, Li X, Gaal J, Xi ZX, Gardner EL. YQA14: a novel dopamine D3 receptor antagonist that inhibits cocaine self-administration in rats and mice, but not in D3 receptor-knockout mice. Addict Biol. 2012;17:259–273. doi: 10.1111/j.1369-1600.2011.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley JK, Mash DC. Adaptive increase in D3 dopamine receptors in the brain reward circuits of human cocaine fatalities. J Neurosci. 1996;16:6100–6106. doi: 10.1523/JNEUROSCI.16-19-06100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga N, Choudhury ME, Nishikawa N, Nagai M, Tujii T, Iwaki H, Kaneta M, Nomoto M. Pramipexole upregulates dopamine receptor D2 and D3 expression in rat striatum. J Pharmacol Sci. 2012;120:133–137. doi: 10.1254/jphs.12096sc. [DOI] [PubMed] [Google Scholar]
- Vorel SR, Ashby CR, Jr, Paul M, Liu X, Hayes R, Hagan JJ, Middlemiss DN, Stemp G, Gardner EL. Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. J Neurosci. 2002;22:9595–9603. doi: 10.1523/JNEUROSCI.22-21-09595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub D, Koester J, Potenza MN, Siderowf AD, Stacy M, Voon V, Whetteckey J, Wunderlich GR, Lang AE. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67:589–595. doi: 10.1001/archneurol.2010.65. [DOI] [PubMed] [Google Scholar]
- West GB, Brown JH, Enquist BJ. A general model for the origin of allo-metric scaling laws in biology. Science. 1997;276:122–126. doi: 10.1126/science.276.5309.122. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Pak AC, Ashby CR, Jr, Heidbreder CA, Gardner EL. Selective dopamine D3 receptor antagonism by SB-277011A attenuates cocaine reinforcement as assessed by progressive-ratio and variable-cost-variable-payoff fixed-ratio cocaine self-administration in rats. Eur J Neurosci. 2005;21:3427–3438. doi: 10.1111/j.1460-9568.2005.04159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


