Abstract
We conducted an open-label crossover trial to test whether proton pump inhibitors (PPIs) affect the gastrointestinal microbiome to facilitate Clostridium difficile infection (CDI). Twelve healthy volunteers each donated 2 baseline fecal samples, 4 weeks apart (at weeks 0 and 4). They then took PPIs for 4 weeks (40 mg omeprazole, twice daily) and fecal samples were collected at week 8. Six individuals took the PPIs for an additional 4 weeks (from week 8 to 12) and fecal samples were collected from all subjects at week 12. Samples were analyzed by 16S rRNA gene sequencing. We found no significant within-individual difference in microbiome diversity when we compared changes during baseline vs changes on PPIs. There were, however, significant changes during PPI use in taxa associated with CDI (increased Enterococcaceae and Streptococcaceae, decreased Clostridiales) and taxa associated with gastrointestinal bacterial overgrowth (increased Micrococcaceae and Staphylococcaceae). In a functional analysis, there were no changes in bile acids on PPIs, but there was an increase in genes involved in bacterial invasion. These alterations could provide a mechanism by which PPIs predispose to CDI. ClinicalTrials.gov:NCT01901276.
Keywords: Clostridium difficile infection, pharmacology, gastroesophageal reflux disease, acid suppression
Proton pump inhibitors (PPIs) have been associated with Clostridium difficile infection (CDI), but the mechanism linking PPIs and CDI is unknown. Broad-spectrum antibiotics are the most important risk factor for CDI, and cause loss of diversity within the gastrointestinal microbiome.1 There are also more specific changes within the microbiome that precede CDI. Increases in Enterococcaceae and decreases within key Clostridial taxa at the time of hospital admission are associated with increased risk for subsequent development of CDI.2
This study tested whether PPIs given in the absence of antibiotics alter the human colonic microbiome to predispose to CDI. Twelve healthy volunteers each donated a fecal sample at week 0 and week 4 of the study (see Supplementary Methods for complete description). They subsequently all took omeprazole 40 mg twice daily for 4 weeks and donated an additional sample (week 8). The subjects were then randomized 1:1 to stop PPIs or continue them for an additional 4 weeks, after which they donated a final sample (week 12) (Supplementary Figure 1). We excluded those who used antibiotics within one year or tested positive for the C. difficile toxin B gene at week 0 (Supplementary Table 1 & Figure 2). We used 16S rRNA gene sequencing to describe the fecal microbiome. Our a priori primary outcome was fecal microbial diversity, defined as the within-individual difference in Shannon’s index of diversity comparing change during the 4-week baseline period to change during the 4-week period on PPIs. To focus on taxa predisposing to CDI, we pre-specified taxa of interest referencing 1) studies of lower GI microbiome changes preceding CDI, and 2) studies of upper GI microbiome changes after PPIs (Supplementary Table 2).
Figure 2. Principal coordinate analyses.

Weighted (A) and unweighted (B) UniFrac analyses from immediately before and after 4 weeks of PPIs. Circles represent samples from before PPIs (green) or after PPIs (red); the corresponding subject’s number is adjacent to each circle.
We found no within-individual changes in diversity after 4 weeks of PPI treatment (Figure 1A). Two subjects received antibiotics between week 8 and week 12 for reasons unrelated to the study; for these subjects, the samples taken after antibiotics were excluded from the final analyses. In the remaining subjects that received 8 weeks of PPIs (n=5), there was no difference between 8 weeks of PPIs compared to baseline (p=.79). On principal coordinates analyses, there was no distinct clustering of samples from before versus after PPI treatment (Figure 2). In sum, overall fecal microbial composition remained stable during use of PPIs.
Figure 1. Changes in fecal microbiotal diversity and specific taxa throughout the study.
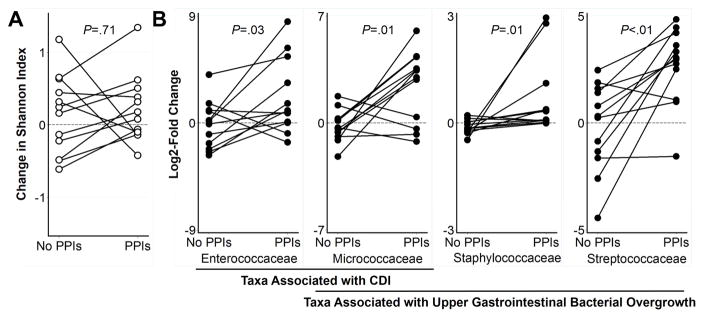
(A) There was no significant change in overall diversity after 4 weeks of PPIs compared to 4 weeks at baseline. (B) Four weeks of PPIs induced within-individual changes in the relative abundance of prespecified taxa associated with CDI and with upper gastrointestinal bacterial overgrowth. Lines connect individuals.
However, PPI treatment for 4 weeks did induce significant within-individual increases in Enterococcaceae and Streptococcaceae, taxa that have been associated with exposure to antibiotics and increased risk for CDI (Figure 1B).3–7 In a hospital-based study, patients who later developed CDI had low diversity and increased Enterococcaceae compared to control patients.3 Enterococci are present in low abundance in human stool but can rapidly expand after broad-spectrum antibiotics.4 In mice, treatment with clindamycin is followed by proliferation of Enterococci and CDI.5 Dynamic modeling suggests that an increase in Enterococci is a key step preceding C. difficile colonization.6 Streptococcaceae, which are predominantly upper GI tract organisms, were increased over 10-fold after PPIs. Gastric and small intestinal bacterial overgrowth with Streptococcus is an established consequence of PPIs, but the direct pH-raising effects of PPIs are attenuated by the distal duodenum.8–10 Streptococcaceae are disrupted by broad-spectrum antibiotics and have been associated with CDI.7 Our results are consistent with the hypothesis that PPI-induced hypochloryhydria causes increased gastric and fecal Streptococcus, leading to increased risk for CDI.
To identify additional changes caused by 4 weeks of PPIs, we looked across 97 bacterial families present in all samples and compared within-individual changes before and after PPIs. We found a 44% median decrease in Clostridiaceae (p=.03). There were no further alterations in pre-specified taxa in either arm of the study during the final 4 weeks of the study (Supplementary Figure 3). Stool PCR testing and culture for C. difficile was performed on all samples. One subject had an equivocal toxin B test following 8 weeks of PPIs and also growth of C. difficile in culture before and after PPIs.
Bacterial production of secondary bile salts may play an important role in CDI by inhibiting C. difficile spore germination. In cirrhotics given PPIs, there were decreased levels of urinary dimethylamine, which is produced by the bacterial metabolism of bile salts; subjects also had increased Streptococcaceae after PPIs, as was seen in our study.11 Cluster XIVa Clostridia including C. scindens actively produce secondary bile acids.12 In our study, there was a decrease in Clostridiaceae after PPIs, but we were unable to directly map C. scindens within our reference library. Instead, we performed RT-PCR for the baiCD gene, which encodes the rate-limiting enzyme in the production of secondary bile acids.12 We found no change in baiCD gene copy number after PPIs (p=.79) and, to further investigate bile acids, we then used LC-MS to directly assess bile acid levels in our samples. There was no change after PPIs in any of 10 dominant human primary and secondary bile acids (Supplementary Figure 4).
Next, we used PICRUSt to impute the metagenome from our 16S sequencing results.13 We found no changes in the KEGG pathways for bile acid biosynthesis after PPIs (Supplementary Figure 5A). We then performed an unbiased metagenomic analysis across all KEGG pathways, assessing for within-individual differences after PPIs compared to the baseline period. After 4 weeks of PPIs, there was a significant increase in the pathway corresponding to genes for S. aureus infection, which includes genes for antimicrobial lectins (Supplementary Figure 5B). After 8 weeks of PPIs, there were significant increases in the pathways corresponding to genes for bacterial invasion of epithelial cells and for the renin-angiotensin system (Supplementary Figure 5C); these pathways include genes for antibacterial peptides and maintenance of epithelial integrity. Together, these results imply that PPIs do not increase risk for CDI by altering fecal levels of secondary bile acids but rather that PPIs may be important after C. difficile sporulation, by lowering colonization resistance.
Antibiotics cause CDI and reduce the diversity and overall size of the microbiome.14 We did not find a reduction in fecal microbial diversity after PPIs, but loss of diversity may represent an epiphenomenon that often accompanies the key changes within specific taxa that are permissive for CDI. One prior human study found a small but statistically significant reduction in total bacterial operational taxonomic units (OTUs) after PPIs, but did not identify changes in Shannon diversity or within specific bacterial taxa.15 In contrast, we did not find changes in OTU counts after PPIs (p=.12). In conclusion, 4 weeks of high-dose PPIs did not change fecal microbial diversity beyond baseline variability but significantly affected certain taxa including Streptococcaceae and Enterococcaceae. PPIs may increase risk for CDI by altering crucial taxa involved in colonization resistance to C. difficile.
Supplementary Material
Acknowledgments
Grant support: DEF was funded by the National Center for Advancing Translational Sciences (NIH KL2 TR000081). HHW was funded by the NIH Director’s Early Independence Award (1DP5OD009172-02) and the National Science Foundation CAREER Award (MCB-1453219). SPC was funded by the Columbia University’s Medical Scientist Training Program.
The authors thank Justin Cross for assistance in measuring primary and secondary fecal bile acid levels.
Abbreviations
- CDI
Clostridium difficile infection
- GI
gastrointestinal
- PPIs
proton pump inhibitors
Footnotes
Author contributions: DEF and JAA were involved in all aspects of the study. NCT was involved in acquisition, analysis, and interpretation of data, and revision of the manuscript. SPC and SW were involved in acquisition of the data and revision of the manuscript. AJR was involved in acquisition and analysis of the data and revision of the manuscript. TCW was involved in study concept, interpretation of the data, and revision of the manuscript. HHW was involved in study concept, acquisition, analysis, and interpretation of data, and revision of the manuscript.
Disclosures: We have no relevant conflicts to disclose. The funding agencies had no role in the study design; the collection, analysis, or interpretation of the data; the writing of the article; or the decision to submit it for publication.
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or other funding organizations.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chang JY, et al. J Infect Dis. 2008;197:435–8. doi: 10.1086/525047. [DOI] [PubMed] [Google Scholar]
- 2.Manges AR, et al. J Infect Dis. 2010;202:1877–84. doi: 10.1086/657319. [DOI] [PubMed] [Google Scholar]
- 3.Vincent C, et al. Microbiome. 2013;1:18. doi: 10.1186/2049-2618-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donskey CJ, et al. N Engl J Med. 2000;343:1925–32. doi: 10.1056/NEJM200012283432604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawley TD, et al. Infect Immun. 2009;77:3661–9. doi: 10.1128/IAI.00558-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stein RR, Bucci V, et al. PLoS Comput Biol. 2013;9:e1003388. doi: 10.1371/journal.pcbi.1003388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez-Cobas AE, et al. Gut. 2013;62:1591–601. doi: 10.1136/gutjnl-2012-303184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosen R, et al. JAMA Pediatr. 2014 doi: 10.1001/jamapediatrics.2013.2911. [DOI] [PubMed] [Google Scholar]
- 9.Rosen R, et al. J Pediatr. 2015 [Google Scholar]
- 10.Lo WK, et al. Clin Gastroenterol Hepatol. 2013;11:483–90. doi: 10.1016/j.cgh.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Bajaj JS, et al. Am J Physiol Gastrointest Liver Physiol. 2014 [Google Scholar]
- 12.Buffie CG, et al. Nature. 2014 [Google Scholar]
- 13.Langille MG, et al. Nat Biotechnol. 2013;31:814–21. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antonopoulos DA, et al. Infect Immun. 2009;77:2367–75. doi: 10.1128/IAI.01520-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seto CT, et al. Microbiome. 2014;2:42. doi: 10.1186/2049-2618-2-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


