Abstract
Common variable immune deficiency (CVID) is the most common symptomatic primary immune deficiency, affecting ∼1 in 25,000 persons. These patients suffer from impaired antibody responses, autoimmunity, and susceptibility to lymphoid cancers. To explore the cellular basis for these clinical phenotypes, we conducted high-throughput DNA sequencing of immunoglobulin heavy chain gene rearrangements from 93 CVID patients and 105 control subjects and sorted naïve and memory B cells from 13 of the CVID patients and 10 of the control subjects. CVID patients showed abnormal VDJ rearrangement and abnormal formation of complementarity determining region 3 (CDR3). We observed decreased selection against antibodies with long CDR3 regions in memory repertoires and decreased V gene replacement, offering possible mechanisms for increased patient autoreactivity. Our data indicate that patient immunodeficiency might derive both from decreased diversity of the naïve B cell pool and decreased somatic hypermutation in memory repertoires. CVID patients also exhibited abnormal clonal expansion of unmutated B cells relative to controls. Although impaired B cell germinal center activation is commonly viewed as causative in CVID, these data indicate that CVID B cells diverge from controls as early as the pro-B cell stage and suggest possible explanations for the increased incidence of autoimmunity, immunodeficiency, and lymphoma CVID patients.
Introduction
Common variable immune deficiency (CVID) is a primary immune deficiency that affects ∼1 in 25,000 Caucasians and is characterized by a marked reduction in serum IgG and IgA (<0.05 g/L), with serum IgM being low in about half of cases (1-4). Antibody deficiency leads to recurrent bacterial infections. Pathological features of CVID can also include autoimmunity, lymphoid hyperplasia, splenomegaly, gastrointestinal diseases, and increased risk of lymphoma (5-7). Since CVID was first recognized about six decades ago, investigators have attempted to discover the basis for the disorder (8). Various studies suggest that rare mutations in autosomal genes, including inducible T-cell costimulator (ICOS)(9), CD19 (10, 11) B-cell activating factor (BAFF) receptor (12), CD20 (13), and CD81(14) might lead to the CVID syndrome. Mutation of the gene encoding the transmembrane activator and calcium-modulating cyclophilin ligand interactor (TACI) are more common in CVID (found in 8 to 10% of subjects) (15-17), but some of the same mutations are also found in healthy controls (18), suggesting that this set of mutations is not sufficient to confer the disease phenotype (19). CVID genome wide association studies have emphasized the disease's unusual genetic characteristics, showing genomic regions of disease association, possible DNA gene repair variations, and excess copy number loss and gain (20, 21).
It remains unclear whether antibody deficiencies in CVID patients arise from intrinsic defects in B cell development and function, broader impairments resulting from defects in T or dendritic cells, or a combination of these possibilities. Most patients with CVID have normal total B cell counts (7), but about half have markedly decreased isotype switched memory B cells (22–26). Memory (CD27+) B cells, in contrast to naïve (CD27-) B cells, have typically undergone somatic hypermutation of immunoglobulin (Ig) variable (V)–region genes and isotype switching from IgM to other specialized effector isotypes. The differentiation of naïve B cells into memory B and plasma cells generally occurs within the germinal centers (GC) (27, 28). CVID patients with extremely low numbers of class-switched memory B cells (<0.55% of peripheral blood B cells) have been categorized as Group I and are at increased risk for autoimmunity, granulomatous disease, and other complications (26, 29) relative to patients with greater numbers of these cells, categorized as Group II.
Studies of in vitro immunoglobulin synthesis by CVID B cells in response to a variety of stimuli have identified patient subgroups: those with approximately normal Ig production in vitro, those with impaired but still detectable Ig responses, and those whose B cells do not produce Ig in culture in response to any stimulus (30-33). Previous studies of the molecular features of rearranged antibody genes in CVID B cells ex vivo have focused on somatic hypermutation (SHM), the specialized mutational process necessary for antibody affinity maturation, with conflicting results. Three studies of small numbers of CVID patients in which relatively few Ig chains were sequenced revealed some CVID subjects to have decreased (but sometimes inconsistently reduced) SHM compared to healthy controls (34-37). In contrast, Duvvuri et al. found no decrease in SHM in the IGHV3-23 gene segment in CVID compared to controls (38). Driessen et al. also found globally similar mutation rates for isotype-switched memory B cells in all patients and for marginal zone-like B cells in two thirds of patients (39).
We have carried out deep sequencing of the immunoglobulin heavy chain (IgH) V(D)J repertoires of 93 subjects with CVID compared to 105 control subjects and evaluated more than 800,000 IgH gene rearrangements derived from genomic DNA (gDNA). In addition, we analyzed IgH transcripts from sorted memory and naïve B cells from 13 of the CVID subjects and 10 of the control subjects, evaluating 7,148,144 sequences derived from mRNA. Our data indicate that CVID patients have defects in both B cell repertoire generation and selection, which result in an altered IgH complementarity determining region 3 (CDR3)—the region that often forms the primary interaction surface of an antibody with its cognate antigen (40). In addition, CVID B cells show decreased diversity in the naïve repertoire and display unusual expanded B-cell clones that express non-mutated IgH, indicating that B cell clonal proliferation in CVID is uncoupled from SHM. The implications of these B cell repertoire defects for immunodeficiency, autoimmunity, and lymphomagenesis in CVID are discussed below.
Results
B cell repertoire formation is altered in CVID
To evaluate possible CVID-specific differences in VDJ rearrangement, independent of the effects of negative or positive selection, we sequenced IgH genes from peripheral blood B cell genomic DNA. Nonproductive rearrangements, which do not give rise to expressed protein, are not subject to the same selection processes that shape in-frame rearrangements. Prior studies indicate that selection of developing B cells in the bone marrow decreases the proportion of expressed antibodies with long CDR3 regions, possibly because such antibodies tend to be autoreactive (41, 42). B cells expressing somatically mutated IGHV genes demonstrate even shorter CDR3 regions in their heavy
Our data indicate that CVID subjects generate shorter CDR3s during the initial VDJ recombination in the bone marrow (Fig. 1A, left panel). The decreased CDR3 length is due to the addition of fewer non-templated (N1 and N2) bases in both CVID Group I and II patients, and increased exonuclease activity in Group II CVID patients (Fig. 1B). In contrast, productive non-mutated IgH CDR3s in CVID patients and controls have similar lengths, indicating that expressed antibodies in CVID patients are subject to relatively less selection for shorter CDR3 regions than in healthy controls (Fig. 1A, middle panel). This trend increased further for in-frame mutated sequences likely derived from antigen-experienced B cells, indicating that selection for shorter CDR3 antibodies in these cells is also impaired in CVID Group I patients (Fig. 1A, right panel).
Fig. 1. CVID B cell repertoires show altered formation and selection of IgH.
(A, left) Out-of-frame IgH sequences of CVID patients have shorter CDR3s than healthy controls (P=0.0128 for CVID Group I, P=0.00021 for CVID Group II). These sequences provide a measure of pre-selection VDJ gene rearrangement features. (A, middle) Productive but non-mutated IgH CDR3s are shorter than those of out-of-frame sequences. The difference between nonproductive and productive sequence CDR3 lengths in CVID patients is less than in controls, resulting in a productive unmutated repertoire with similar CDR3 lengths. (A, right) Productive IgH sequences that have undergone SHM have even shorter CDR3s on average but the selection for shorter sequences is significantly weaker in CVID Group I than in CVID Group II or healthy controls (P=0.0005). (B) Shorter CDR3s of out-of-frame sequences in CVID have fewer non-templated (N1 and N2) bases compared to healthy controls (P=0.0099 for CVID Group I, P<0.00001 for CVID Group II) and increased exonuclease digestion of segment ends compared to healthy controls (P=0.003 for CVID Group II). (C) Weakened selection for shorter CDR3s in CVID Group I than in healthy controls is also observed in naïve B cell IgM and IgD (P=0.0270 for IgM, P=0.0120 for IgD). (D) This weakened selection is also seen in mRNA sequences from sorted memory B cells in switched and unswitched isotypes (IgM, P=0.0020 for CVID Group I, P=0.0066 for CVID Group II; IgD, P=0.0020 for CVID Group I, P=0.0140 for CVID Group II; IgG1, P=0.0182 for CVID Group I; IgG2, P=0.0039 for CVID Group II; IgG3, P=0.0357 for CVID Group I, P=0.0406 for CVID Group II). All P-values are from the Wilcoxon-Mann-Whitney test.
To further study the effects of successive selection events on the antibody heavy chain CDR3s expressed by naïve B cells and memory B cells in CVID and healthy controls, we analyzed isotype-specific IgH Illumina libraries prepared from strictly sorted naïve and memory B cells of 13 CVID patients and 10 healthy controls. Unmutated IgM and IgD from sorted naïve cells showed significantly longer CDR3s in CVID Group I patients compared to controls (Fig. 1C). Mutated sequences from memory B cells showed more marked differences, with CVID Group I patients demonstrating longer CDR3s than control subjects for all expressed isotypes, with the difference achieving statistical significance for IgG1, IgG3, IgM and IgD (Fig. 1D). CVID Group II patients showed intermediate CDR3 lengths in memory B cells. In both healthy control and CVID patient sequence libraries, average CDR3 lengths in cDNA Illumina data from sorted B cells were approximately one amino acid shorter than those from total B cell genomic DNA sequenced with the 454 platform, possibly as a result of the higher efficiency of cluster generation by shorter amplicons in the Illumina platform (46). The antibodies with longer CDR3 regions in the mutated B cell repertoire of Group I CVID patients may represent candidate antibodies of autoreactive B cells responsible for the clinical autoimmunity frequently observed in these subjects. Decreased selection for shorter CDR3s could arise as a consequence of fewer rounds of antigen-driven B cell division and selection, but would also be consistent with impaired elimination of autoreactive B cells.
CVID subjects show impaired V gene replacement
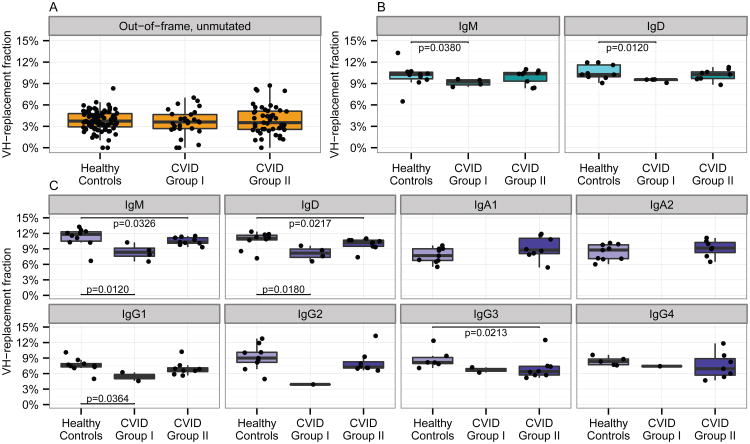
Secondary rearrangement of genomic DNA at the IGH locus can remove autoreactive B cells from the repertoire or rescue initially nonproductive rearrangements by using a cryptic RSS site in the initially rearranged V segment to enable V gene replacement. Evidence of such editing events can be detected in the form of nucleotide sequences in the V-D junction that share homology with the 3′ end of an alternative V segment. Hypothesizing that autoimmunity in CVID patients might be due in part to deficiencies in such secondary rearrangement processes, we attempted to infer the levels of V gene replacement in the B cell repertoires of CVID and control subjects using a Naïve Bayes model for detecting enrichment of nucleotide sequences matching the 3′ ends of known IGHV segments, in the V-D junctions of IgH rearrangements. Non-functional rearrangements from genomic DNA showed low levels of V gene replacement (Fig. 2A) and did not differ between CVID and control subjects. In contrast, naïve, unmutated IgM and IgD from Group I CVID subjects showed less VH-replacement than healthy controls, while Group II CVID subjects were not significantly different from controls (Fig. 2B). Greater decreases in VH replacement frequencies in CVID were seen in mutated antibodies from memory B cells, with Group I patients consistently showing the lowest replacement frequencies (Fig. 2C). These findings suggest that the less severe clinical phenotype of Group II CVID patients could be due, in part, to more active VH replacement reshaping the expressed B cell repertoire to remove autoreactive B cells.
Fig. 2. CVID subjects show decreased VH-replacement.
(A) Bayesian modeling of the sequence characteristics present after VH-replacement shows low levels of VH-replacement in non-functional IgH sequences in both CVID patients and controls. (B) Naïve B cells show less evidence of VH-replacement in Group I CVID compared to healthy controls (IgM, P=0.0380; IgD, P=0.0120), while Group II CVID was comparable to healthy controls. (C) Mutated memory B cells show less VH-replacement in CVID than healthy controls for several isotypes and consistently decreased VH-replacement in CVID Group I over CVID Group II and healthy controls (IgM, P=0.0120 for CVID Group I, P=0.0326 for CVID Group II; IgD, P=0.0180 for CVID Group I, P=0.0127 for CVID Group II; IgG1, P=0.0364 for CVID Group I; IgG3, P=0.0213 for CVID Group II). All P-values are from the Wilcoxon-Mann-Whitney test.
Richness of the unmutated B cell repertoire is decreased in CVID
Estimation of the “richness,” or the number of distinct receptors in a lymphocyte population, is challenging, because the richness can be strongly dependent on the number of rare species. We applied the ‘Chao2’ estimator of the lower limit of repertoire richness to our replicate sampling data of genomic IgH sequences from CVID and healthy individuals (47, 48). The estimate is affected by the total number of DNA sequence reads per sample, therefore we analyzed richness estimates as a function of read counts (Fig. 3). The richness of unmutated IgH sequences in CVID is significantly decreased (by approximately five to tenfold) compared to controls, whereas the richness of the mutated repertoire is comparable in CVID patients and controls. Three quarters of CVID subjects had normal or elevated B cell counts compared to the normal range, thus the reduced diversity of unmutated sequences is not likely to be due to differences in the numbers of B cells analyzed. The decreased naïve B cell repertoire richness of CVID subjects could lead to fewer antibodies able to bind to any given antigen, a deficiency that could contribute to impaired humoral responses.
Fig. 3. Naïve B cell repertoire richness is decreased in CVID.
(A) The estimated lower bound of the unmutated IgH repertoire richness plotted against sequence read numbers per sample, both in log-scale. A linear regression model is shown for CVID Group I and II and healthy controls with the 95% confidence band. Both CVID groups show decreased richness compared to healthy controls. (B) Mutated IgH sequences show comparable richness in CVID and healthy controls. Repertoire richness estimates were calculated with the Chao2 estimator (see Methods).
Somatic hypermutation is decreased in CVID
Prior literature reports of mutation status in CVID antibody genes have not been in agreement about whether there is a decrease in somatic mutation levels (34-39). We find that CVID patient B cells show decreased SHM compared to healthy controls. This decrease is most pronounced in CVID Group I patients, who have fewer mutated B cells (Fig. 4A) and lower mutation levels in their mutated B cells (Fig. 4B). Data derived from sorted memory B cells, evaluating each antibody isotype separately, reinforce both these results (Fig. 4, C and D). Among the larger group of 87 patients from whom gDNA was sequenced, 95% of Group I and 71% of Group II patients had SHM levels below the fifth percentile of the healthy controls. Mutational hotspots for activation-induced cytidine deaminase (AID) remained the most highly mutated regions in both CVID IgH sequences and controls. A greater proportion of sequence variants at non-hotspot sites was seen in the CVID samples, but the frequency of these mutations was comparable to the expected levels of PCR errors or sequencing errors.
Fig. 4. Somatic hypermutation levels are markedly decreased in CVID.
(A) Productive IgH sequences from CVID subjects have fewer sequences with SHM compared to healthy controls (P<0.00001 for CVID Group I, P=0.00089 for CVID Group II). (B) The average percentage of mutated IGHV-gene nucleotides in B cells with productive IgH sequences showing evidence of mutation is significantly reduced in both CVID Group I and II compared to healthy controls (P<0.00001 for CVID Group I, P<0.00001 for CVID Group II). (C) A smaller fraction of CVID memory B cells show IgH SHM compared to healthy controls (IgM, P=0.0281 for CVID Group I, P=0.0471 for CVID Group II; IgG1, P=0.0226 for CVID Group I, P=0.0375 for CVID Group II; IgG2 P=0.0242 for CVID Group II; IgG3, P=0.0334 for CVID Group I). (D) Sorted memory B cells have consistently reduced levels of SHM in both groups of CVID compared to healthy controls across isotypes (IgM, P=0.0040 for CVID Group I, P=0.0021 for CVID Group II; IgD, P=0.0010 for CVID Group I, P=0.0028 for CVID Group II; IgA1 P=0.0076 for CVID Group II; IgA2 P=0.0209 for CVID Group II; IgG1, P=0.0182 for CVID Group I; IgG2 P=0.0296 for CVID Group II; IgG3, P=0.0357 for CVID Group I). All P-values are from the Wilcoxon-Mann-Whitney test.
CVID patients show abnormal clonal expansion of non-mutated B cells
The B cell repertoire in the blood is composed of a mixture of B cell clones that have undergone different levels of clonal expansion in development and in subsequent responses to antigen, with variable degrees of contraction and preservation in the memory compartment. Using the data obtained by sequencing multiple replicate libraries of gDNA IgH rearrangements, we calculated a summary metric of the contributions of expanded B cell clones to the repertoire. This “clonality score” is independent of sequencing depth (see Methods), and can be considered to be the probability that two B cells selected at random from different replicate libraries will be members of the same clone. The presence of a few large clones or many smaller clones will both elevate the clonality score. Surprisingly, CVID patients had normal levels of clonality compared to controls (Fig. 5A). There was, however, a striking increase in the proportion of clones without somatic mutation in CVID patients (Fig. 5B). Unlike in healthy controls, where large clonally expanded B cell populations are typically mutated memory B cells, CVID patients appear to uncouple clonal expansion from somatic hypermutation. These clones may represent naïve cells that have expanded in the absence of antigenic stimulation, or alternatively they could be descendants of the normally short-lived pre-germinal center responding cells that produce antibody during primary infections, among other possibilities.
Fig. 5. CVID subjects show altered B cell clonal expansions.
(A) Contributions of clonally-expanded B cells to the repertoires of CVID and healthy controls are similar, as gauged by a ‘clonality score’ metric that is independent of sequencing depth. (B) CVID patients show a striking increase in the proportion of clonally-expanded B cells with germline IgH sequences, compared to healthy controls (P<0.00001 for CVID Group I, P<0.00001 for CVID Group II). P-values are from the Wilcoxon-Mann-Whitney test.
CVID peripheral blood B cell clonality shows limited correlation with lymphoma history
Given the high incidence of B cell lymphoma in CVID patients, a method for early detection of lymphoma, or of tracking residual disease following treatment, could be useful for patient management. We evaluated whether peripheral blood B cell populations showed abnormally high levels of clonality in CVID patients with either a current or historic lymphoma diagnosis, compared to patients with no such history. In general, in our data set, subjects with previously treated, or highly localized lymphoma could not reliably be differentiated from either CVID subjects without lymphoma or from healthy controls, although some CVID Group II with known active disease did show high clonality (Fig. 6, Table 1).
Fig. 6. Only a subset of CVID patients with lymphoma show elevated peripheral blood B cell clonality.
(A) CVID patients without a history of lymphoma show similar clonality compared to healthy controls. (B) Some CVID Group I patients with a history of lymphoma show increased clonality of peripheral blood B cells.
Table 1. Clinical data for CVID subjects with a lymphoma diagnosis.
| Subject | Age (years) | Sex | Lymphoma status | Notes | B cella (%) | IgGb (mg/dl) | IgAc (mg/dl) | IgMd (mg/dl) |
|---|---|---|---|---|---|---|---|---|
| a | 46 | F | B cell lymphoma in chest (MALT) | No treatment; stable | 0.22 | 150 | 3 | 19 |
| b | 39 | M | B cell marginal zone lymphoma | Died; no treatment | 57.29 | 42 | 8 | 10 |
| c | 44 | M | Diffuse Large B Cell Lymphoma in the jejunum | DNA sample presurgery; resected; CHOP rituximab; stable | 21.84 | 39 | 0 | 10 |
| d | 41 | M | Diffuse Large B Cell Lymphoma | Sample pre CHOP+ rituximab; in remission | 3.43 | 267 | 49 | 20 |
| e | 41 | F | Low grade marginal zone lymphoma | Sample pre Rituximab; given 5 years later | 20.24 | 380 | 5 | 3 |
| f | 77 | F | Large Cell Lymphoma of small intestine | Resected 25 years previously; no disease | 6.03 | 105 | 34 | 5 |
| g | 69 | M | Monoclonal B cell lymphocytosis | No treatment | 13.07 | 114 | 14 | 10 |
| h | 46 | F | NHL-B Cell, mass above kidney | Mo treatment | 5.1 | 9 | 7 | 7 |
| i | 67 | F | NHL-T cell rich diffuse large cell (EBV+) | Sample post 9 years; CHOP, rituximab; in remission | 0.69 | 33 | 44 | |
| j | 87 | F | NHL, Poorly differentiated, Stage IV | Sample 20 years after CHOP; no disease | 20.25 | 175 | 10 | 15 |
| k | 46 | M | Plasmablastic lymphoma of jejunum | Sample 3 years pre-lymphoma; intensive therapy; died | 0.77 | 6 | 21 | 16 |
Subject letters correspond to those in Fig. 6.
Normal ranges: B cells= 5 to 15%,
IgG=700 to 1600,
IgA=70 to 400,
IgM=40 to 230.
Discussion
We have used high-throughput DNA sequencing of antibody heavy chain rearrangements from CVID patients and healthy controls to evaluate the formation of B cell repertoires and their selection in naïve and memory B cells. B cells of CVID patients showed significant alterations in each of these developmental or selection steps, suggesting mechanisms for the immunodeficiency, autoimmunity, and lymphoid malignancies that are characteristic of this disease.
Our current understanding of the pathogenesis of CVID has emphasized defects of peripheral B cell activation and maturation. A recent study describing B cell subpopulation patterns using flow cytometric immunophenotyping and κ-deleting recombination excision circle (KREC) assays, used to evaluate the number of cell divisions a B cell clone has undergone following kappa light chain rearrangement in development, implicated defective post–bone marrow developmental stages for the majority of CVID, with only one third of subjects showing evidence of defects in B cell production or survival (39). Our findings extend these prior results by demonstrating that the IgH repertoires from subjects with CVID show consistent differences in the VDJ rearrangements taking place in B cell precursors. From analysis of nonproductive IgH rearrangements, we find evidence that B cell precursors expressing CDR3 regions with decreased length prior to selection in the bone marrow are a common feature of CVID, suggesting a defect in lymphocyte progenitors or stem cells. As an alternative, these changes could arise as a consequence of an altered bone marrow environment in which aberrant B cell development occurs. If this phenotypic feature is the result of a stem cell defect, it could provide a mechanistic link underlying both the B and the various T and myeloid cell aberrations previously associated with CVID (49-55).
Selection accompanying central B cell development was also abnormal in CVID, as evidenced by decreased selection against long CDR3 sequences in unmutated IgM and IgD sequences from naïve B cells of Group I patients. In healthy controls, antibodies expressed by B cells that have undergone SHM have even shorter IgH CDR3 segments than those in naïve B cells (43-45). We found that this step of selection was only minimally active in CVID Group I subjects, who show longer CDR3 regions in mutated memory B cell sequences compared to controls and to CVID Group II subjects. Group II subjects show an intermediate phenotype. The persistence of B cells with these longer CDR3 sequences could represent a correlate of the increased autoimmunity in CVID. CVID patients, particularly in the clinically severe Group I category, also show decreased evidence of post-rearrangement VH replacement, a finding that could indicate impaired receptor editing of autoreactive antibodies.
Previous studies of somatic hypermutation in CVID that examined limited numbers of rearranged Ig genes in small patient cohorts have yielded inconsistent results (34-39). We found that SHM is defective in CVID, with both a reduced number of mutated sequences as well as many fewer mutations per sequence, in particular for Group I subjects. A lower proportion of class-switched memory B cells has previously been correlated with increased risk for autoimmunity, granulomatous disease, and other complications in CVID subjects (29). A prior study identified reduced levels of SHM as markers of increased risk of severe respiratory infections (36), indicting that SHM measurement might have broad clinical significance.
Expanded B cell clones that express unmutated IgH were prominent in CVID patients compared to healthy controls. These clones could indicate altered homeostasis of B cells prior to antigen exposure, or could arise from increased expansion and survival of unmutated IgM-expressing antigen-specific cells. Such cells could alternatively arise through escape from the GC after initiation of proliferation at the centroblast state prior to the initiation of SHM. Although far from definitive evidence exists for this latter possibility, a small study of biopsied lymph nodes from three subjects with CVID found evidence of centroblasts but a lack of centrocytes, consistent with successful expansion of pre-mutated B cell populations in CVID (56). Increased B cell clonality is expected in patients who develop B cell lymphomas. However, in this study, only a subset of subjects with a history of lymphoma showed increased clonality. These results were not entirely unexpected, given that the lymphomas of most of the patients in this study were either tissue-restricted or in remission, but further investigation is warranted.
A key question in CVID biology is whether patients have decreased B cell repertoire diversity or “richness” (the number of unique rearrangements present in the patient) compared to healthy controls, given that most CVID patients do not have decreased total B cell numbers compared to controls. The estimated minimum diversity of unmutated B cells was significantly lower in CVID patients compared to controls in our data, whereas the richness of the repertoire of mutated sequences did not differ. A decreased repertoire of naïve B cells might be less able to respond to diverse foreign antigens, contributing to immunodeficiency. The total B cell numbers in the CVID patients we studied were not significantly different from those of controls, indicating that the lower richness estimates do not arise simply from fewer cells being sampled (table S1).
Although we have identified IgH sequence features of CVID patient repertoires that could contribute to autoreactivity, we were unable to discern consistent differences between the B cell repertoires of CVID subjects with and without confirmed clinical autoimmunity. This observation might either reflect some innate characteristics of ITP and AIHA autoantibodies or could merely reflect the fact that the clinically significant autoreactive sequences are relatively rare. As an alternative, although patients with CVID may have higher frequencies of autoreactive B cells, these B cells might be less likely to secrete antibodies in vivo, as is the case for patients with myeloid differentiation primary response gene 88 (MyD88) or interleukin-1 receptor-associated kinase 4 (IRAK4) deficiency (57). Further study of nonimmune-deficient individuals with hemolytic cytopenias might help to clarify which of these explanations is more likely.
The identification of aberrations of central B cell development in our study help explain some CVID features that are puzzling in the context of the peripheral B cell defects in this syndrome. If the early B cell defects in CVID patients are attributable to a more general hematopoietic stem-cell defect, then the increasing incidence for T cell defects (50, 53, 58) and of various defects in cytokine production (52, 58-61) becomes more intelligible. If supported by additional data, such reclassification of CVID as a stem-cell defect could improve patient care, because understanding that CVID has aspects of a combined immunodeficiency could lead to increased clinical vigilance for noninfectious complications and consequent improved clinical outcomes.
Materials and Methods
Study design
This observational study was conducted to evaluate immunoglobulin molecular features including somatic mutation status, heavy chain CDR3 features, B cell clonality, isotype usage and B cell population richness in CVID subjects compared to healthy controls. These variables have not been previously measured in CVID subjects using high-throughput DNA sequencing methods, therefore, formal power calculations to determine sample size were not carried out prior to the study. The largest available cohort of CVID patients was studied, and experimental results for all subjects were obtained and evaluated together. CVID subjects were diagnosed on the basis of: (i) reduced serum IgG, IgA, and/or IgM two or more confidence intervals below the normal ranges for age, and (ii) documented antibody deficiency (62, 63). DNA samples of 87 CVID patients, age 7 to 87 years, including 41 males and 46 females, were examined. Thirty-five patients had a history of or ongoing autoimmunity, including 19 with one or more episodes of idiopathic thrombocytopenic purpura (ITP), 3 with autoimmune hemolytic anemia (AHA), and 7 subjects who had had both. One other subject had Felty's syndrome and another had ulcerative colitis. Eleven subjects had either a history of current, but not yet treated tissue-restricted lymphoma or of previously treated B cell lymphoma. Features of these subjects can be found in table S1.
In addition, RNA derived from sorted naïve and memory B cells from 13 CVID subjects, age 23 to 77 years, including five males and eight females, and 10 healthy control subjects was converted to cDNA and sequenced. Features of these subjects can be found in table S2. CVID subjects were subdivided into those with very reduced isotype switched memory B cells (<0.55% of peripheral B cells) Group I (35 patients gDNA, 4 cDNA), and those with greater numbers of these cells Group II, (51 patients gDNA, 9 cDNA), with 3 subjects (gDNA) not having data needed for Group designation (29). All subjects were receiving ongoing replacement immunoglobulin at intervals of 3 to 4 weeks (intravenous) or weekly (subcutaneous) at time of study participation, and none were on immunomodulatory or immunosuppressive medications at the time of sample collection. Data from all subjects studied are reported; there was no identification or exclusion of outliers. The replicate structure of the sequencing experiments is described in the Supplementary Methods. Of the CVID subjects, 89 were recruited at Mount Sinai Medical Center and 5 were recruited at Stanford University. Ninety-five healthy adult volunteers, aged 21 to 88, were used as controls for gDNA based sequencing. These controls were recruited at Stanford University as healthy controls in an ongoing influenza vaccine study, or were recruited at the Oklahoma Medical Research Foundation and determined to be negative for systemic autoimmune or immune deficiency disease. For cDNA based sequencing, ten healthy adult controls, ages 24 to 53, were similarly screened at the Mount Sinai Medical Center. This study was approved by the Institutional Review Boards of Mount Sinai Hospital, Oklahoma Medical Research Foundation and Stanford University, with written informed consent obtained from all participants.
Isolation of genomic DNA from peripheral blood mononuclear cells
Heparinized whole blood samples (5 to 8 ml) were collected and PBMCs isolated by centrifugation of diluted blood over Hypaque 1077 (Sigma-Aldrich) or Ficoll-Paque (GE Healthcare, Uppsala, Sweden). Genomic DNA was isolated via column purification (Qiagen, Valencia, CA), magnetic bead-based isolation (Magnapure, Roche Diagnostics Corportation, Indianapolis, IN), or centrifugation-based purification (ArchivePure, 5Prime, Hamburg).
Isolation of RNA from flow cytometric sorted naïve and memory B cells
PBMCs were stained with antibodies specific for CD19 (clone HIB19, eBiosciences, San Diego, CA), IgM (clone MHM-88), IgD (clone IA6-2), and CD27 (clone O323, all from BioLegend, San Diego, CA). B cell populations were sorted using an Aria II (BD Biosciences) into naive (CD19+CD27- and IgM+ and/or IgD+) or memory (CD19+CD27+) cells. Purity of sorted populations was tested and confirmed to have <2% contamination by the other B cell subset. Cells were lysed using TRIzol Reagent (Life Technologies, Grand Island, NY) and stored at -80°C until RNA extraction.
PCR amplification of IgH libraries for high-throughput DNA sequencing
Protocols for PCR amplification of IgH rearrangements from PBMC genomic DNA template and cDNA template for Illumina MiSeq and Roche 454 DNA sequencing are described in the Supplementary Methods. Primer sequences for amplifications are listed in Table S3.
IgH sequence filtering and analysis
Sequences from the 454 platform with exact matches to V and J barcodes were assigned to the corresponding samples and replicate libraries. Barcodes and IGHV primer sequences were trimmed. V, D, and J gene segments and junctional bases were assigned using the iHMMune-align program (26, 27). CDR3 amino acid sequences were identified based on the conserved cysteine-104 and tryptophan-118 residues, using the IMGT numbering scheme (28). Non-IgH artifactual sequences were removed from the data as well as sequences with V-gene insertion or deletions, and chimeric sequences. Samples with fewer than 100 sequences were excluded from further analysis. In total, 286,391 sequences from CVID patients (mean = 3,330 per individual) and 531,562 sequences from healthy controls (mean = 5,595 per individual) were obtained and analyzed from the 454 sequencing data set. The positions and distribution of somatic mutations along the V-region were obtained by alignment with gapped IMGT germline sequences. To avoid over-interpreting PCR or sequencing error as somatic hypermutation, a threshold of 1% mutation was used to distinguish between mutated and non-mutated sequences. We have previously measured the per-base error rate of 454 sequencing of IgH molecules prepared with this experimental protocol to be approximately 0.3% (64).
Filtering and analysis of Illumina platform sequences derived from cDNA were analyzed by barcode and IGHV and constant region primer sequence trimming, followed by gene segment and junctional base assignment using the IgBLAST program (65). Determination of antibody isotype and subtype was accomplished by selecting the best Smith-Watermen alignment of known constant regions from the IMGT database to the constant region sequence in each amplicon after primer trimming (66). In total, 3,719,320 sequences from the 13 CVID patients (mean = 268,318 per individual) and 1,646,618 sequences from the 10 healthy controls (mean = 164,662 per individual) were analyzed.
Inference of clonal lineages in RNA data
To control for variability in amplification and abundance of RNA derived sequences and to follow a clonal lineage across different isotype and maturation states, sequences were clustered into putative clonal lineages using single-linkage clustering. Briefly, the process starts with all sequences in their own lineages and, iteratively, two lineages are merged if any two reads, one from each lineage, satisfies the following four criteria: (1) come from the same individual, (2) share the same V and J annotations (not including allele call), (3) have equal CDR3 length, and (4) CDR3 nucleotide sequences match with at least 90% identity. The process ends when there are no lineages satisfying the criteria. This clustering protocol produced 2,163,253 clones from 3,906,943 unique (per-person) sequences. The mean number of reads per lineage was 2.58, and the median was one.
V gene replacement analysis
The amount of VH-replacement observed in a participant is estimated using a Naive Bayes Classifier. Briefly, the categorical distribution of nucleotide 1-mers to 8-mers observed in the 40 bases at 3′-end of known V-segments is generated. For a given antibody sequence, the likelihoods under the above distributions of m-mers of length 1 through 8 from the junctional sequence are compared to a uniform distribution of m-mers using the Naive Bayes assumption of m-mer independence. The resulting likelihood ratio is then calibrated to give a ∼8% frequency of VH-replacement in in-frame, unmutated sequences from healthy controls, between the 5% and 12% reported in control individuals from repertoire sequencing data (67). Upon manual inspection, sequences with a high likelihood ratio show evidence of VH-replacement, but our comparison of likelihood ratios is used to assess the relative frequency of VH-replacement in an individual and not to definitively identify specific products of VH-replacement with a particular likelihood ratio cut-off.
Repertoire richness estimates
To estimate IgH species richness, we used multiple replicate libraries amplified from separate genomic DNA template aliquots from each sample, and applied the Chao2 non-parametric estimator of unseen species (47). The data are represented as whether a particular clone was seen, or not seen, in a particular replicate library, thus circumventing the challenges related to amplification and abundance quantification of single-replicate experimental designs. The Chao2 estimator underestimates the total number of species, or true richness, but it is not subject to the wide variation in species richness estimates that can result from the choice of a particular parametric model to describe the abundance of clones in a population (68, 69). Sequencing error and PCR error in library generation can generate apparently varying species that originate from a common template molecule. We examined each putative clone S that appeared in only one replicate, and decided whether it could be a sequencing error with respect to another clone. We rejected S as a distinct species if another sequence satisfying the following four criteria was identified: (1) one sharing the same V and J annotations; (2) having equal CDR3 length; (3) differing by Hamming distance of up to two in the CDR3 nucleotides; and (4) with greater abundance.
Clonality analysis
Genomic DNA derived IgH sequences were considered to belong to the same clone if they shared V and J gene usage (not taking into account the allele calls) and identical CDR3 amino acid sequence. To calculate a summary measure of the contribution of clonally-expanded B cells to the repertoire of each individual, normalizing for sequencing depth in each subject, we calculated a ‘clonality score’ as:
where Nij and Nik are the copy numbers of clone i observed in independent replicate PCR libraries j and k generated from independent aliquots of template DNA; Tj and Tk are total read numbers in the corresponding replicate libraries (70). The log base 10 of the clonality score is shown in Figs. 5 and 6.
Statistical analysis
For Figs. 1, 2, 4, and 5, P values were calculated by one-sided Wilcoxon-Mann-Whitney. Box-whisker plots show median (horizontal line), interquartile range (box), 1.5 times the interquartile range (whiskers).
Supplementary Material
Table S1. Clinical Features of CVID Patients, gDNA Sequencing.
Table S2. Clinical Features of CVID Patients, cDNA Sequencing.
Table S3. Sequence of first PCR primer sets and second PCR primers.
Footnotes
This manuscript has been accepted for publication in Science Translational Medicine. This version has not undergone final editing. Please refer to the complete version of record at www.sciencetranslationalmedicine.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
Author contributions: KR, NS, KN, CC and SB planned the study; KR, NS, YL, JP DF, CD, MD, JJ, KN, CC and SB provided samples, clinical data, reagents or analytical tools; NS, JP and DF prepared cell samples or performed flow cytometry; JL, KS, RH, TP and SB isolated nucleic acids, amplified libraries and performed DNA sequencing; KR, NS, and YL wrote and ran scripts for analysis; KR, NS, YL, CC and SB planned data analysis approaches, and reviewed all analyzed data for CVID subjects and controls; KR, NS, YL, JL, KS, RH, TP, JP, DF, CD, MD, JJ, KN, CC and SB wrote, edited and approved the manuscript; MD, JJ, KN, CC and SB provided resources or facilities supporting the study.
Competing interests: The authors declare that they have no competing financial interests.
Data and materials availability: The data for this study have been deposited in dbGaP (http://www.ncbi.nlm.nih.gov/gap) with accession number phs000934.v1.p1.
References
- 1.Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies) Clin Immunol. 1999;93:190–197. doi: 10.1006/clim.1999.4799. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham-Rundles C. Common variable immunodeficiency. Curr Allergy Asthma Rep. 2001;1:421–429. doi: 10.1007/s11882-001-0027-1. [DOI] [PubMed] [Google Scholar]
- 3.Chapel H, Cunningham-Rundles C. Update in understanding common variable immunodeficiency disorders (CVIDs) and the management of patients with these conditions. Br J Haematol. 2009;145:709–727. doi: 10.1111/j.1365-2141.2009.07669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Herz W, Bousfiha A, Casanova JL, Chatila T, Conley ME, Cunningham-Rundles C, Etzioni A, Franco JL, Gaspar HB, Holland SM, Klein C, Nonoyama S, Ochs HD, Oksenhendler E, Picard C, Puck JM, Sullivan K, Tang ML. Primary immunodeficiency diseases: an update on the classification from the international union of immunological societies expert committee for primary immunodeficiency. Frontiers Immunol. 2014;5:162. doi: 10.3389/fimmu.2014.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapel H, Lucas M, Lee M, Bjorkander J, Webster D, Grimbacher B, Fieschi C, Thon V, Abedi MR, Hammarstrom L. Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood. 2008;112:277–286. doi: 10.1182/blood-2007-11-124545. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal S, Cunningham-Rundles C. Autoimmunity in common variable immunodeficiency. Current allergy and asthma reports. 2009;9:347–352. doi: 10.1007/s11882-009-0051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Resnick ES, Moshier EL, Godbold JH, Cunningham-Rundles C. Morbidity and mortality in common variable immune deficiency over 4 decades. Blood. 2012;119:1650–1657. doi: 10.1182/blood-2011-09-377945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanford JP, Favour CB, Tribeman MS. Absence of serum gamma globulins in an adult. N Engl J Med. 1954;250:1027–1029. doi: 10.1056/NEJM195406172502403. [DOI] [PubMed] [Google Scholar]
- 9.Grimbacher B, Hutloff A, Schlesier M, Glocker E, Warnatz K, Drager R, Eibel H, Fischer B, Schaffer AA, Mages HW, Kroczek RA, Peter HH. Homozygous loss of ICOS is associated with adult-onset common variable immunodeficiency. Nat Immunol. 2003;4:261–268. doi: 10.1038/ni902. [DOI] [PubMed] [Google Scholar]
- 10.van Zelm MC, Reisli I, van der Burg M, Castano D, van Noesel CJ, van Tol MJ, Woellner C, Grimbacher B, Patino PJ, van Dongen JJ, Franco JL. An antibody-deficiency syndrome due to mutations in the CD19 gene. N Engl J Med. 2006;354:1901–1912. doi: 10.1056/NEJMoa051568. [DOI] [PubMed] [Google Scholar]
- 11.Kanegane H, Agematsu K, Futatani T, Sira MM, Suga K, Sekiguchi T, van Zelm MC, Miyawaki T. Novel mutations in a Japanese patient with CD19 deficiency. Genes Immunity. 2007;8:663–670. doi: 10.1038/sj.gene.6364431. [DOI] [PubMed] [Google Scholar]
- 12.Warnatz K, Salzer U, Rizzi M, Fischer B, Gutenberger S, Bohm J, Kienzler AK, Pan-Hammarstrom Q, Hammarstrom L, Rakhmanov M, Schlesier M, Grimbacher B, Peter HH, Eibel H. B-cell activating factor receptor deficiency is associated with an adult-onset antibody deficiency syndrome in humans. Proc Natl Acad Sci U S A. 2009;106:13945–13950. doi: 10.1073/pnas.0903543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuijpers TW, Bende RJ, Baars PA, Grummels A, Derks IA, Dolman KM, Beaumont T, Tedder TF, van Noesel CJ, Eldering E, van Lier RA. CD20 deficiency in humans results in impaired T cell-independent antibody responses. J Clin Invest. 2010;120:214–222. doi: 10.1172/JCI40231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Zelm MC, Smet J, Adams B, Mascart F, Schandene L, Janssen F, Ferster A, Kuo CC, Levy S, van Dongen JJ, van der Burg M. CD81 gene defect in humans disrupts CD19 complex formation and leads to antibody deficiency. J Clin Invest. 2010;120:1265–1274. doi: 10.1172/JCI39748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salzer U, Chapel HM, Webster AD, Pan-Hammarstrom Q, Schmitt-Graeff A, Schlesier M, Peter HH, Rockstroh JK, Schneider P, Schaffer AA, Hammarstrom L, Grimbacher B. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nat Genet. 2005;37:820–828. doi: 10.1038/ng1600. [DOI] [PubMed] [Google Scholar]
- 16.Castigli E, Wilson SA, Garibyan L, Rachid R, Bonilla F, Schneider L, Geha RS. TACI is mutant in common variable immunodeficiency and IgA deficiency. Nat Genet. 2005;37:829–834. doi: 10.1038/ng1601. [DOI] [PubMed] [Google Scholar]
- 17.Pan-Hammarstrom Q, Salzer U, Du L, Bjorkander J, Cunningham-Rundles C, Nelson DL, Bacchelli C, Gaspar HB, Offer S, Behrens TW, Grimbacher B, Hammarstrom L. Reexamining the role of TACI coding variants in common variable immunodeficiency and selective IgA deficiency. Nat Genet. 2007;39:429–430. doi: 10.1038/ng0407-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L, Radigan L, Salzer U, Behrens TW, Grimbacher B, Diaz G, Bussel J, Cunningham-Rundles C. Transmembrane activator and calcium-modulating cyclophilin ligand interactor mutations in common variable immunodeficiency: clinical and immunologic outcomes in heterozygotes. J Allergy Clin Immunol. 2007;120:1178–1185. doi: 10.1016/j.jaci.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conley ME, Dobbs AK, Farmer DM, Kilic S, Paris K, Grigoriadou S, Coustan-Smith E, Howard V, Campana D. Primary B cell immunodeficiencies: comparisons and contrasts. Annu Rev Immunol. 2009;27:199–227. doi: 10.1146/annurev.immunol.021908.132649. [DOI] [PubMed] [Google Scholar]
- 20.Orange JS, Glessner JT, Resnick E, Sullivan KE, Lucas M, Ferry B, Kim CE, Hou C, Wang F, Chiavacci R, Kugathasan S, Sleasman JW, Baldassano R, Perez EE, Chapel H, Cunningham-Rundles C, Hakonarson H. Genome-wide association identifies diverse causes of common variable immunodeficiency. J Allergy Clin, Immunol. 2011;127:1360–1367. doi: 10.1016/j.jaci.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Offer SM, Pan-Hammarstrom Q, Hammarstrom L, Harris RS. Unique DNA repair gene variations and potential associations with the primary antibody deficiency syndromes IgAD and CVID. PLoS One. 2010;5:e12260. doi: 10.1371/journal.pone.0012260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brouet JC, Chedeville A, Fermand JP, Royer B. Study of the B cell memory compartment in common variable immunodeficiency. Eur J Immunol. 2000;30:2516–2520. doi: 10.1002/1521-4141(200009)30:9<2516::AID-IMMU2516>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 23.Agematsu K, Futatani T, Hokibara S, Kobayashi N, Takamoto M, Tsukada S, Suzuki H, Koyasu S, Miyawaki T, Sugane K, Komiyama A, Ochs HD. Absence of memory B cells in patients with common variable immunodeficiency. Clin Immunol. 2002;103:34–42. doi: 10.1006/clim.2001.5197. [DOI] [PubMed] [Google Scholar]
- 24.Warnatz K, Denz A, Drager R, Braun M, Groth C, Wolff-Vorbeck G, Eibel H, Schlesier M, Peter HH. Severe deficiency of switched memory B cells (CD27(+)IgM(-)IgD(-)) in subgroups of patients with common variable immunodeficiency: a new approach to classify a heterogeneous disease. Blood. 2002;99:1544–1551. doi: 10.1182/blood.v99.5.1544. [DOI] [PubMed] [Google Scholar]
- 25.Sánchez-Ramón S, Radigan L, Yu JE, Bard S, Cunningham-Rundles C. Memory B cells in common variable immunodeficiency: clinical associations and sex differences. Clin Immunol. 2008;128:314–321. doi: 10.1016/j.clim.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wehr C, Kivioja T, Schmitt C, Ferry B, Witte T, Eren E, Vlkova M, Hernandez M, Detkova D, Bos PR, Poerksen G, von Bernuth H, Baumann U, Goldacker S, Gutenberger S, Schlesier M, Bergeron-van der Cruyssen F, Le Garff M, Debre P, Jacobs R, Jones J, Bateman E, Litzman J, van Hagen PM, Plebani A, Schmidt RE, Thon V, Quinti I, Espanol T, Webster AD, Chapel H, Vihinen M, Oksenhendler E, Peter HH, Warnatz K. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 2008;111:77–85. doi: 10.1182/blood-2007-06-091744. [DOI] [PubMed] [Google Scholar]
- 27.Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188:1679–1689. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maruyama M, Lam KP, Rajewsky K. Memory B-cell persistence is independent of persisting immunizing antigen. Nature. 2000;407:636–642. doi: 10.1038/35036600. [DOI] [PubMed] [Google Scholar]
- 29.Sanchez-Ramon S, Radigan L, Yu JE, Bard S, Cunningham-Rundles C. Memory B cells in common variable immunodeficiency: clinical associations and sex differences. Clin Immunol. 2008;128:314–321. doi: 10.1016/j.clim.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eisenstein EM, Chua K, Strober W. B cell differentiation defects in common variable immunodeficiency are ameliorated after stimulation with anti-CD40 antibody and IL-10. J Immunol. 1994;152:5957–5968. [PubMed] [Google Scholar]
- 31.Borte S, Pan-Hammarstrom Q, Liu C, Sack U, Borte M, Wagner U, Graf D, Hammarstrom L. Interleukin-21 restores immunoglobulin production ex vivo in patients with common variable immunodeficiency and selective IgA deficiency. Blood. 2009;114:4089–4098. doi: 10.1182/blood-2009-02-207423. [DOI] [PubMed] [Google Scholar]
- 32.Bryant A, Calver NC, Toubi E, Webster AD, Farrant J. Classification of patients with common variable immunodeficiency by B cell secretion of IgM and IgG in response to anti-IgM and interleukin-2. Clin Immunol Immunopathol. 1990;56:239–248. doi: 10.1016/0090-1229(90)90145-g. [DOI] [PubMed] [Google Scholar]
- 33.Saiki O, Ralph P, Cunningham-Rundles C, Good RA. Three distinct stages of B-cell defects in common varied immunodeficiency. Proc Natl Acad Sci U S A. 1982;79:6008–6012. doi: 10.1073/pnas.79.19.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levy Y, Gupta N, Le Deist F, Garcia C, Fischer A, Weill JC, Reynaud CA. Defect in IgV gene somatic hypermutation in common variable immuno-deficiency syndrome. Proc Natl Acad Sci U S A. 1998;95:13135–13140. doi: 10.1073/pnas.95.22.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonhomme D, Hammarstrom L, Webster D, Chapel H, Hermine O, Le Deist F, Lepage E, Romeo PH, Levy Y. Impaired antibody affinity maturation process characterizes a subset of patients with common variable immunodeficiency. J Immunol. 2000;165:4725–4730. doi: 10.4049/jimmunol.165.8.4725. [DOI] [PubMed] [Google Scholar]
- 36.Andersen P, Permin H, Andersen V, Schejbel L, Garred P, Svejgaard A, Barington T. Deficiency of somatic hypermutation of the antibody light chain is associated with increased frequency of severe respiratory tract infection in common variable immunodeficiency. Blood. 2005;105:511–517. doi: 10.1182/blood-2003-12-4359. [DOI] [PubMed] [Google Scholar]
- 37.Ballegaard V, Permin H, Katzenstein TL, Marquart HV, Schejbel L. Long-term follow-up on affinity maturation and memory B-cell generation in patients with common variable immunodeficiency. J Clin Immunol. 2013;33:1067–1077. doi: 10.1007/s10875-013-9893-2. [DOI] [PubMed] [Google Scholar]
- 38.Duvvuri B, Duvvuri VR, Grigull J, Martin A, Pan-Hammarström Q, Wu GE, Larijani M. Altered spectrum of somatic hypermutation in common variable immunodeficiency disease characteristic of defective repair of mutations. Immunogenetics. 2011;63:1–11. doi: 10.1007/s00251-010-0483-7. [DOI] [PubMed] [Google Scholar]
- 39.Driessen GJ, van Zelm MC, van Hagen PM, Hartwig NG, Trip M, Warris A, de Vries E, Barendregt BH, Pico I, Hop W, van Dongen JJ, van der Burg M. B-cell replication history and somatic hypermutation status identify distinct pathophysiologic backgrounds in common variable immunodeficiency. Blood. 2011;118:6814–6823. doi: 10.1182/blood-2011-06-361881. [DOI] [PubMed] [Google Scholar]
- 40.Xu JL, Davis MM. Diversity in the CDR3 region of V(H) is sufficient for most antibody specificities. Immunity. 2000;13:37–45. doi: 10.1016/s1074-7613(00)00006-6. [DOI] [PubMed] [Google Scholar]
- 41.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 42.Larimore K, McCormick MW, Robins HS, Greenberg PD. Shaping of Human Germline IgH Repertoires Revealed by Deep Sequencing. J Immunol. 2012;189:3221–3230. doi: 10.4049/jimmunol.1201303. [DOI] [PubMed] [Google Scholar]
- 43.McHeyzer-Williams MG, McLean MJ, Lalor PA, Nossal GJ. Antigen-driven B cell differentiation in vivo. J Exp Med. 1993;178:295–307. doi: 10.1084/jem.178.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brezinschek HP, Foster SJ, Brezinschek RI, Dörner T, Domiati-Saad R, Lipsky PE. Analysis of the human VH gene repertoire. Differential effects of selection and somatic hypermutation on human peripheral CD5(+)/IgM+ and CD5(-)/IgM+ B cells. J Clin Invest. 1997;99:2488–2501. doi: 10.1172/JCI119433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brezinschek HP, Foster SJ, Dörner T, Brezinschek RI, Lipsky PE. Pairing of variable heavy and variable kappa chains in individual naive and memory B cells. J Immunol. 1998;160:4762–4767. [PubMed] [Google Scholar]
- 46.Quail MA, Swerdlow H, Turner DJ. Improved protocols for the illumina genome analyzer sequencing system. Curr Protocols Human Genet. 2009;62:18.2.1–18.2.27. doi: 10.1002/0471142905.hg1802s62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chao A. Estimating the population size for capture-recapture data with unequal catchability. Biometrics. 1987;43:783–791. [PubMed] [Google Scholar]
- 48.Chao A. Nonparametric Estimation of the Number of Classes in a Population. Scandinavian J Statistics. 1984;11:265–270. [Google Scholar]
- 49.Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol. 1999;92:34–48. doi: 10.1006/clim.1999.4725. [DOI] [PubMed] [Google Scholar]
- 50.Kondratenko I, Amlot PL, Webster AD, Farrant J. Lack of specific antibody response in common variable immunodeficiency (CVID) associated with failure in production of antigen-specific memory T cells. MRC Immunodeficiency Group. Clin Exp Immunol. 1997;108:9–13. doi: 10.1046/j.1365-2249.1997.d01-993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mechanic LJ, Dikman S, Cunningham-Rundles C. Granulomatous disease in common variable immunodeficiency. Annals of internal medicine. 1997;127:613–617. doi: 10.7326/0003-4819-127-8_part_1-199710150-00005. [DOI] [PubMed] [Google Scholar]
- 52.Stagg AJ, Funauchi M, Knight SC, Webster AD, Farrant J. Failure in antigen responses by T cells from patients with common variable immunodeficiency (CVID) Clin Exp Immunol. 1994;96:48–53. doi: 10.1111/j.1365-2249.1994.tb06228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salzer U, Warnatz K, Peter HH. Common variable immunodeficiency - an update. Arthritis Res Ther. 2012;14:223. doi: 10.1186/ar4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cunningham-Rundles C, Radigan L, Knight AK, Zhang L, Bauer L, Nakazawa A. TLR9 activation is defective in common variable immune deficiency. J Immunol. 2006;176:1978–1987. doi: 10.4049/jimmunol.176.3.1978. [DOI] [PubMed] [Google Scholar]
- 55.Yu JE, Knight AK, Radigan L, Marron TU, Zhang L, Sanchez-Ramón S, Cunningham-Rundles C. Toll-like receptor 7 and 9 defects in common variable immunodeficiency. J Allergy Clin Immunol. 2009;124:349–356. doi: 10.1016/j.jaci.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taubenheim N, von Hornung M, Durandy A, Warnatz K, Corcoran L, Peter HH, Eibel H. Defined blocks in terminal plasma cell differentiation of common variable immunodeficiency patients. J Immunol. 2005;175:5498–5503. doi: 10.4049/jimmunol.175.8.5498. [DOI] [PubMed] [Google Scholar]
- 57.Isnardi I, Ng YS, Srdanovic I, Motaghedi R, Rudchenko S, von Bernuth H, Zhang SY, Puel A, Jouanguy E, Picard C, Garty BZ, Camcioglu Y, Doffinger R, Kumararatne D, Davies G, Gallin JI, Haraguchi S, Day NK, Casanova JL, Meffre E. IRAK-4- and MyD88- dependent pathways are essential for the removal of developing autoreactive B cells in humans. Immunity. 2008;29:746–757. doi: 10.1016/j.immuni.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sneller MC. Common variable immunodeficiency. Am J Med Sci. 2001;321:42–48. doi: 10.1097/00000441-200101000-00007. [DOI] [PubMed] [Google Scholar]
- 59.Kruger G, Welte K, Ciobanu N, Cunningham-Rundles C, Ralph P, Venuta S, Feldman S, Koziner B, Wang CY, Moore MA, et al. Interleukin-2 correction of defective in vitro T-cell mitogenesis in patients with common varied immunodeficiency. J Clin Immunol. 1984;4:295–303. doi: 10.1007/BF00915297. [DOI] [PubMed] [Google Scholar]
- 60.Eisenstein EM, Jaffe JS, Strober W. Reduced interleukin-2 (IL-2) production in common variable immunodeficiency is due to a primary abnormality of CD4+ T cell differentiation. J J Clin Immunol. 1993;13:247–258. doi: 10.1007/BF00919383. [DOI] [PubMed] [Google Scholar]
- 61.Zhou Z, Huang R, Danon M, Mayer L, Cunningham-Rundles C. IL-10 production in common variable immunodeficiency. Clin Immunol Immunopathol. 1998;86:298–304. doi: 10.1006/clin.1997.4483. [DOI] [PubMed] [Google Scholar]
- 62.Notarangelo LD, Fischer A, Geha RS, Casanova JL, Chapel H, Conley ME, Cunningham-Rundles C, Etzioni A, Hammartrom L, Nonoyama S, Ochs HD, Puck J, Roifman C, Seger R, Wedgwood J. Primary immunodeficiencies: 2009 update. J Allergy Clin Immunol. 2009;124:1161–1178. doi: 10.1016/j.jaci.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Al-Herz W, Bousfiha A, Casanova JL, Chapel H, Conley ME, Cunningham-Rundles C, Etzioni A, Fischer A, Franco JL, Geha RS, Hammarstrom L, Nonoyama S, Notarangelo LD, Ochs HD, Puck JM, Roifman CM, Seger R, Tang ML. Primary immunodeficiency diseases: an update on the classification from the international union of immunological societies expert committee for primary immunodeficiency. Front Immunol. 2011;2:54. doi: 10.3389/fimmu.2011.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boyd SD, Marshall EL, Merker JD, Maniar JM, Zhang LN, Sahaf B, Jones CD, Simen BB, Hanczaruk B, Nguyen KD, Nadeau KC, Egholm M, Miklos DB, Zehnder JL, Fire AZ. Measurement and clinical monitoring of human lymphocyte clonality by massively parallel VDJ pyrosequencing. Sci Transl Med. 2009;1:12ra23. doi: 10.1126/scitranslmed.3000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ye J, Ma N, Madden TL, Ostell JM. IgBLAST: an immunoglobulin variable domain sequence analysis tool. Nucleic Acids Res. 2013;41:W34–40. doi: 10.1093/nar/gkt382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lefranc MP. IMGT, the international ImMunoGeneTics database. Nucleic Acids Res. 2001;29:207–209. doi: 10.1093/nar/29.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Z, Zemlin M, Wang YH, Munfus D, Huye LE, Findley HW, Bridges SL, Roth DB, Burrows PD, Cooper MD. Contribution of Vh gene replacement to the primary B cell repertoire. Immunity. 2003;19:21–31. doi: 10.1016/s1074-7613(03)00170-5. [DOI] [PubMed] [Google Scholar]
- 68.Bunge J, Fitzpatrick M. Estimating the Number of Species: A Review. J Am Statistical Assn. 1993;88:364–373. [Google Scholar]
- 69.Chao A, Chiu CH, Hsieh TC. Proposing a resolution to debates on diversity partitioning. Ecology. 2012;93:2037–2051. doi: 10.1890/11-1817.1. [DOI] [PubMed] [Google Scholar]
- 70.Parameswaran P, Liu Y, Roskin KM, Jackson KK, Dixit VP, Lee JY, Artiles KL, Zompi S, Vargas MJ, Simen BB, Hanczaruk B, McGowan KR, Tariq MA, Pourmand N, Koller D, Balmaseda A, Boyd SD, Harris E, Fire AZ. Convergent antibody signatures in human dengue. Cell Host Microbe. 2013;13:691–700. doi: 10.1016/j.chom.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Clinical Features of CVID Patients, gDNA Sequencing.
Table S2. Clinical Features of CVID Patients, cDNA Sequencing.
Table S3. Sequence of first PCR primer sets and second PCR primers.