Abstract
We evaluated the in vitro potency of cefepime combined with AAI101, a novel extended-spectrum β-lactamase inhibitor, against a population of clinical Escherichia coli and Klebsiella pneumoniae collected from USA hospitals. Of the 223 cefepime non-susceptible isolates, 95% were ceftazidime non-susceptible, 49% ertapenem non-susceptible, 57% piperacillin/tazobactam non-susceptible, 90% were multidrug-resistant (resistant to ≥3 drug classes), 22% produced carbapenemases, and 67% produced ESBLs. Addition of AAI101 restored the activity of cefepime such that the MIC50 was reduced from >64 mg/L for cefepime to 0.13 mg/L for cefepime/AAI101, supporting its continued development treatment for infections caused by these organisms.
Keywords: Escherichia coli, Klebsiella pneumonia, ESBL, carbapenemase, β-lactamase inhibitor
1. Introduction
The increasing prevalence of resistance among Gram-negative pathogens has resulted in tremendous challenges for clinicians across the globe. Secondary to the pathogenicity of Enterobacteriaceae across diseases states, considerable attention has been given to these once highly susceptible organisms. This is particularly true for those organisms that are resistant to our most potent β-lactams, such as late-generation cephalosporins and carbapenems [1]. While non-enzymatic resistance mechanisms exist (i.e., porin mutations, efflux pump overexpression), the most common cause of β-lactam resistance in Enterobacteriaceae is production of β-lactamases [2]. Capitalizing on this common mechanism of resistance, a combination of cefepime, a widely-used cephalosporin, and AAI101, a novel β-lactamase inhibitor with activity against extended-spectrum β-lactamases (ESBL), as well as, some class A and class D carbapenemases, is in early clinical development [3,4]. While previous studies have focused on smaller collections of genotypically characterized isolates, many of which were derived in the laboratory, this study was designed to understand the potency of this new combination against a larger, more clinically focused, yet challenging distribution [3,4]. Namely, we evaluated the in vitro activity of cefepime/AAI101 against a panel of highly resistant clinical isolates of Escherichia coli and Klebsiella pneumoniae collected during 2013–2014 from hospitals across the USA, and compared its activity with those of currently available therapies.
2. Results
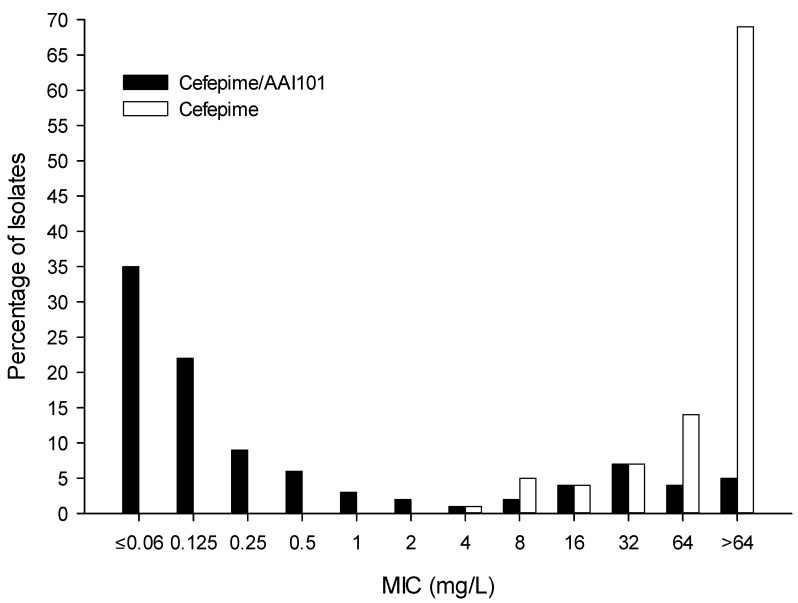
Of the 223 strains evaluated, 165 (74%) were collected from patients residing in the general ward, while the remaining 58 (26%) were from ICU patients. The majority of strains were isolated from the blood (42%), followed by wounds (21%) and the lower respiratory tract (17%). 200 (90%) strains were multidrug-resistant, 150 (67%) were confirmed ESBL producers, and 50 (22.4%) produced carbapenemases. Overall, all agents had greater activity against E. coli than against K. pneumoniae. The percent susceptibility, MIC50, MIC90, and ranges of MIC values for cefepime/AAI101 and comparators are displayed in Table 1. The cefepime/AAI101 activity against specific resistance phenotypes, including ESBL and carbapenemase producers are shown in Table 2. To highlight the ability of AAI101 to restore the potency of cefepime, Figure 1 shows the MIC distributions of cefepime and cefepime/AAI101 against the full complement of isolates.
Table 1.
MIC profile of cefepime/AAI101 and comparator agents against the selected Enterobacteriaceae isolates (n = 223).
| Antimicrobial Agent | % Susceptible | MIC50 (mg/L) | MIC90 (mg/L) | Range (mg/L) |
|---|---|---|---|---|
| Cefepime/AAI101 | ND | 0.125 | 64 | ≤0.06 to >64 |
| Cefepime | 0 * | >64 | >64 | 4 to >64 |
| Ceftazidime | 5 | >64 | >64 | 0.5 to >64 |
| Ciprofloxacin | 9 | >16 | >16 | ≤0.015 to >16 |
| Ertapenem | 51 | 0.5 | 64 | ≤0.015 to >16 |
| Meropenem | 70 | 0.125 | 64 | ≤0.06 to >64 |
| Piperacillin/tazobactam | 43 | 32 | >256 | 1 to >256 |
| Tobramycin | 40 | 16 | 64 | ≤0.06 to >64 |
ND, not defined; * 7% susceptible dose-dependent.
Table 2.
MIC profile of cefepime/AAI101 against Enterobacteriaceae isolates exhibiting various resistance phenotypes.
| Antimicrobial Agent | MIC50 (mg/L) | MIC90 (mg/L) | Range (mg/L) |
|---|---|---|---|
| Cefepime Resistant (n = 208) | 0.125 | 64 | ≤0.06 to >64 |
| Ciprofloxacin Resistant (n = 210) | 0.125 | 64 | ≤0.06 to >64 |
| Ertapenem Resistant (n = 110) | 1 | >64 | ≤0.06 to >64 |
| Piperacillin/tazobactam Resistant (n = 127) | 1 | >64 | ≤0.06 to >64 |
| Multi-drug Resistant (n = 200) | 0.125 | 64 | ≤0.06 to >64 |
| ESBL Producers (n = 150) | 0.125 | 0.5 | ≤0.06 to >64 |
| Carbapenemase Producers (n = 50) | 32 | >256 | ≤0.06 to >64 |
Figure 1.
MIC distributions for cefepime and cefepime/AAI101 against 223 recent Enterobacteriaceae clinical isolates.
3. Discussion
Against this challenging population of Enterobacteriaceae, cefepime/AAI101 was quite potent based on MIC50 and MIC90 values. Although a breakpoint has not yet been established for cefepime/AAI101, if one considers its MIC distribution in the context of current cefepime breakpoints, susceptibility in this population of isolates would be greater than that of all other agents evaluated. Namely, application of the susceptible-dose dependent (SDD) breakpoint for a 2 g q8h dose of cefepime, as recommended for patients with serious infections, (i.e., ≤8 mg/L) [5] to cefepime/AAI101, would result in 80% susceptibility of the strains examined in this study. While clinical pharmacokinetic and pharmacodynamic data are required to validate this assumption, experience with other β-lactam/β-lactamase inhibitor combinations suggests that when the β-lactamase inhibitor content is sufficient to restore activity, the pharmacodynamics of the β-lactam antibiotic partner prevail [6,7,8,9].
Given the number of isolates evaluated, we were unable to genetically verify β-lactamase content of the studied strains. However, through the use of phenotypic methodologies we could identify those strains that produced ESBLs or carbapenemases. As noted in Table 2, cefepime/AAI101 was quite potent against ESBL producing strains and retained activity against a proportion of carbapenemase producers. These findings are similar to previous studies of cefepime/AAI101 against small numbers of genotypically described strains that highlighted AAI101’s ability to protect cefepime against hydrolysis by ESBLs, such as TEM-type, SHV-type, CTX-M-type, and AmpC, as well as some carbapenemases, including OXA-48s and KPCs [3,4]. Of course, further studies evaluating specific genotypes are warranted.
It is worth noting that only cefepime-resistant strains were selected to study the benefit of protecting cefepime by AAI101. Although this selection facilitated direct comparison of potency of various antibiotics, the reported susceptibilities do not represent those expected to be encountered routinely in the clinical setting. Although data for cefepime/AAI101 against a normal distribution are unavailable, consideration of cefepime alone against this population can provide valuable insights. Namely, against the full collection of >2500 strains from which the studied isolates were selected, the percentages of E. coli and K. pneumoniae isolates susceptible to cefepime were 85% and 87%, respectively [10]. Similarly, global data from the 2009 to 2012 SENTRY database reported cefepime percent susceptibility rates of 78%–90% for respiratory E. coli and K. pneumoniae collected from the USA, Europe, and the Mediterranean [11]. Taken collectively, the isolates studied herein conservatively represent the upper 22% of the E. coli and K. pneumoniae MIC distribution that would likely be encountered during daily clinical practice and consequently underestimate the activity of cefepime/AAI101 against the total population.
4. Experimental Section
Non-urine Enterobacteriaceae (118 E. coli and 105 K. pneumoniae) were selected from the Center for Anti-Infective Research and Development’s (Hartford, CT) isolate inventory, which contains organisms collected from 43 US hospitals during 2013–2014 [10]. Isolates were selected to capture the upper end of the MIC distribution for cefepime, focusing on those outside the susceptibility range (i.e., >2 mg/L).
MICs were determined using broth microdilution as described by the Clinical and Laboratory Standards Institute (CLSI) [5]. Standard powders of cefepime, ceftazidime, ciprofloxacin, ertapenem, meropenem, piperacillin/tazobactam, and tobramycin were obtained from Sigma-Aldrich (St Louis, MO, USA), and AAI101 was provided by Allecra Therapeutics SAS (St-Louis, France). For cefepime/AAI101, doubling dilutions of cefepime were utilized in combination with a fixed concentration of 8 mg/L of AAI101 [12].
Isolates were characterized using current CLSI susceptibility breakpoints as follows: cefepime, MIC ≤ 2 mg/L; ceftazidime, MIC ≤ 4 mg/L; ciprofloxacin, MIC ≤ 1 mg/L; ertapenem, MIC ≤ 0.5 mg/L; meropenem, MIC ≤ 1 mg/L; piperacillin/tazobactam, MIC ≤ 16 mg/L; tobramycin, MIC ≤ 4 mg/L [5]. Multidrug resistance (MDR) was defined as resistance to 3 or more classes of antimicrobials (i.e., carbapenems, cephalosporins, monobactams, aminoglycosides, penicillins or fluoroquinolones). All isolates were evaluated phenotypically for production of ESBLs using methods described by the CLSI [5]. Briefly, ceftazidime and cefotaxime MICs were determined with and without clavulanate; those isolates that exhibited MIC shifts of ≥8-fold in the presence of clavulanate were classified as ESBL producers. Isolates non-susceptible to meropenem (≥4 mg/L), regardless of results of ESBL evaluations, were examined for carbapenemase production using the CarbaNP test [13].
5. Conclusions
The addition of AAI101 restores the activity of cefepime against a highly resistant population of E. coli and K. pneumoniae. In an era of increasing resistance and limited therapeutic options, cefepime/AAI101 represents a new potential treatment option warranting continued development for difficult-to-treat Gram-negative pathogens.
Acknowledgments
The authors thank Mary Anne Banevicius, Henry Christenson, Kim Greenwood, Jennifer Hull, Cindy Lamb, Sara Robinson, Deb Santini, Christina Sutherland, and Pam Tessier (Center for Anti-Infective Research and Development) for their assistance in these experiments.
This project was supported by funding from Allecra Therapeutics SAS (St-Louis, France). The founding sponsor had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Author Contributions
Jared L. Crandon and David P. Nicolau conceived and designed the experiments, performed the experiments, analyzed the data and wrote the paper.
Conflicts of Interest
David P. Nicolau is a grant investigator for Allecra Therapeutics SAS. Jared L. Crandon has nothing to disclose. These data, in part, were presenting in abstract form at the 54th Interscience Conference on Antimicrobial Agents and Chemotherapy, 5–9 September 2015, Washington, DC, USA.
References
- 1.Gupta N., Limbago B.M., Patel J.B., Kallen A.J. Carbapenem-resistant Enterobacteriaceae: Epidemiology and prevention. Clin. Infect. Dis. 2011;53:60–67. doi: 10.1093/cid/cir202. [DOI] [PubMed] [Google Scholar]
- 2.Lynch J.P., III, Clark N.M., Zhanel G.G. Evolution of antimicrobial resistance among Enterobacteriaceae (focus on extended spectrum beta-lactamases and carbapenemases) Expert Opin. Pharmacother. 2013;14:199–210. doi: 10.1517/14656566.2013.763030. [DOI] [PubMed] [Google Scholar]
- 3.Mushtaq S., Chaudhry A., Adkin R., Woodford N., Benedict N., Pypstra R., Shapiro S. In-vitro activity of diverse β-lactam/aai101 combinations vs. Multidrug-resistant gram-negative clinical strains; Proceedings of the 24th European Congress of Clinical Microbiology and Infectious Diseases; Barcelona, Spain. 10–13 May 2014; Abstract eP452. [Google Scholar]
- 4.Nordmann P., Girlich D., Benedict N., Pypstra R., Shapiro S. Characterization of b-lactamase inhibition by aai101; Proceedings of the 24th European Congress of Clinical Microbiology and Infectious Diseases; Barcelona, Spain. 10–13 May 2014; Abstract eP451. [Google Scholar]
- 5.Clinical Laboratory Standard Institute . Performance Standards for Antimicrobial Suceptibility Testing; Twenty-Fourth Informational Supplement. CLSI publication; Wayne, PA, USA: 2014. M100-S24. [Google Scholar]
- 6.Crandon J.L., Nicolau D.P. Human simulated studies of aztreonam and aztreonam-avibactam to evaluate activity against challenging gram-negative organisms, including metallo-beta-lactamase producers. Antimicrob. Agents Chemother. 2014;57:3299–3306. doi: 10.1128/AAC.01989-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crandon J.L., Schuck V.J., Banevicius M.A., Beaudoin M.E., Nichols W.W., Tanudra M.A., Nicolau D.P. Comparative in vitro and in vivo efficacies of human simulated doses of ceftazidime and ceftazidime-avibactam against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2013;56:6137–6146. doi: 10.1128/AAC.00851-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.VanScoy B., Mendes R.E., Nicasio A.M., Castanheira M., Bulik C.C., Okusanya O.O., Bhavnani S.M., Forrest A., Jones R.N., Friedrich L.V., et al. Pharmacokinetics-pharmacodynamics of tazobactam in combination with ceftolozane in an in vitro infection model. Antimicrob. Agents Chemother. 2013;57:2809–2814. doi: 10.1128/AAC.02513-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dudley M.N. Combination beta-lactam and beta-lactamase-inhibitor therapy: Pharmacokinetic and pharmacodynamic considerations. Am. J. Health Syst. Pharm. 1995;52:S23–S28. doi: 10.1093/ajhp/52.6_Suppl_2.S23. [DOI] [PubMed] [Google Scholar]
- 10.Sutherland C., Nicolau D. Susceptibility profile of commonly utilized parenteral antimicrobials against E. coli, K. pneumoniae and P. aeruginosa from us hospitals; Proceedings of the 54th Interscience Conference on Antimicrobial Agents and Chemotherapy; Washington, DC, USA. 5–9 September 2014; Abstract C119. [Google Scholar]
- 11.Sader H.S., Farrell D.J., Flamm R.K., Jones R.N. Antimicrobial susceptibility of gram-negative organisms isolated from patients hospitalised with pneumonia in us and european hospitals: Results from the sentry antimicrobial surveillance program, 2009–2012. Int. J. Antimicrob. Agents. 2014;43:328–334. doi: 10.1016/j.ijantimicag.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Crandon J.L., Nicolau D.P. In vivo activities of simulated human doses of cefepime and cefepime-aai101 against multidrug-resistant gram-negative Enterobacteriaceae. Antimicrob. Agents Chemother. 2015;59:2688–2694. doi: 10.1128/AAC.00033-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dortet L., Poirel L., Nordmann P. Rapid identification of carbapenemase types in Enterobacteriaceae and Pseudomonas spp. By using a biochemical test. Antimicrob. Agents Chemother. 2012;56:6437–6440. doi: 10.1128/AAC.01395-12. [DOI] [PMC free article] [PubMed] [Google Scholar]