Abstract
Malignant pleural mesothelioma (MPM) is a cancer associated with exposure to asbestos fibers, which accumulate in the pleural space, damage tissue and stimulate regeneration. Hedgehog signaling is a pathway important during embryonic mesothelium development and is inactivated in adult mesothelium. The pathway is reactivated in some MPM patients with poor clinical outcome, mainly mediated by the expression of the ligands. Nevertheless, mutations in components of the pathway have been observed in a few cases. Data from different MPM animal models and primary culture suggest that both autocrine and paracrine Hedgehog signaling are important to maintain tumor growth. Drugs inhibiting the pathway at the level of the smoothened receptor (Smo) or glioma-associated protein transcription factors (Gli) have been used mostly in experimental models. For clinical development, biomarkers are necessary for the selection of patients who can benefit from Hedgehog signaling inhibition.
Keywords: Hedgehog signaling, malignant pleural mesothelioma, Gli-1, desert Hedgehog, HHip, TCGA, autocrine signaling, paracrine signaling
1. Introduction: Malignant Pleural Mesothelioma
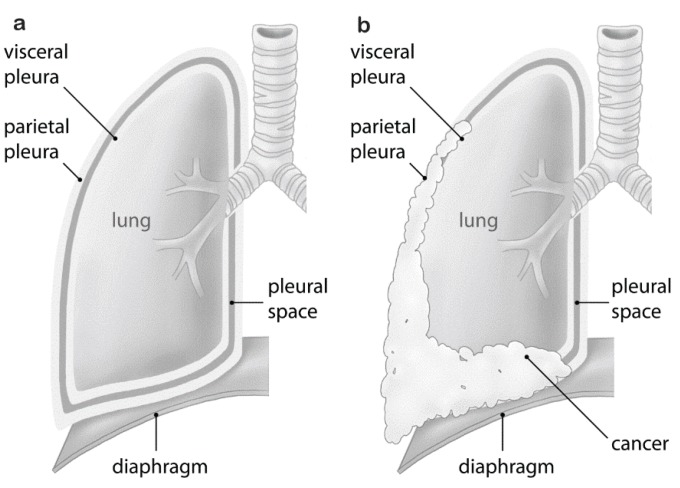
Malignant pleural mesothelioma (MPM) is an aggressive tumor arising from the mesothelial lining cells of the pleura (Figure 1). MPM is a rare cancer, difficult to treat and commonly associated with asbestos exposure (reviewed in [1]). Although Wagner had observed the association between asbestos and mesothelioma in 1960 [2], it took 30 years for the first regulatory measures to be implemented in developed countries, beginning in the United Kingdom (U.K.) and shortly thereafter the United States (U.S.). In many developing countries, asbestos is still being used. In Europe, the incidence of mesothelioma is about 20 per million with large intercountry variation [3]. Based on the Italian Mesothelioma Registry, median latency between asbestos exposure and disease onset is 44.6 years and increases over time in a linear fashion [4]. We therefore expect incidence rates in European nations still to be rising, with peak incidences around 2020 or later [5]. While about 80% of MPM cases are related to asbestos exposure [6], germline mutations of the BRCA1-associated protein-1 (BAP1) predispose individuals to MPM [7].
Figure 1.
Malignant pleural mesothelioma vs. normal pleura: (a) normal pleura; (b) malignant pleural mesothelioma growth resulting in compression of the lung.
MPM is a very heterogeneous and highly chemoresistant tumor; therefore, management remains a clinical challenge. The best response rates are being reported after chemotherapy with cisplatin and pemetrexed [8]. Several aspects of mesothelioma treatment are under controversial discussion, in particular, extent and best type of surgery, need for radiotherapy and neoadjuvant as compared to adjuvant treatment. For less advanced disease, multimodality treatment, including neoadjuvant chemotherapy with cisplatin and pemetrexed, followed by extrapleural pneumonectomy with or without chemotherapy, is offered [9]. For patients with more advanced disease, the combination of cisplatin and pemetrexed has become the standard treatment, as supported by a phase 3 study [8].
Best survival data are reported from groups combining macroscopic complete resection achieved by extrapleural pneumonectomy or pleurectomy/decortication, chemo- and radio-therapy in a multimodal treatment [10,11,12,13]. However, the nature of the pleura makes it virtually impossible to resect with adequate safety margins. To increase the local tumor control and decrease recurrence, local adjuvant strategies have been developed. They include intracavitary treatment with photodynamic therapy [14] or chemotherapy [15,16].
For patients who cannot benefit from multimodality treatment, other treatment options, including immunotherapy, as well as targeting oncogenic pathways activated in MPM are under evaluation in the clinic (reviewed in [17]). One pathway that is activated in MPM and that we were the first to describe in this disease is the Hedgehog pathway (Hh) [18].
2. The Hedgehog Signaling Pathway in MPM
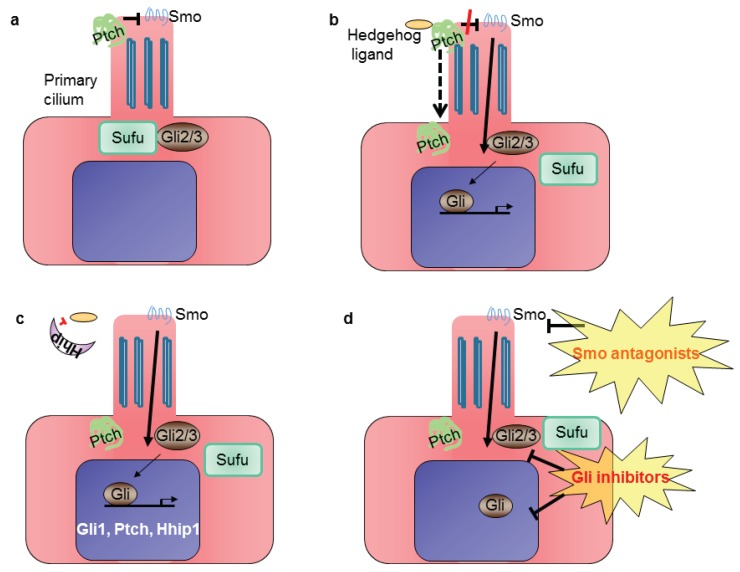
Classical Hh core signaling components (reviewed in [19]) are the Hedgehog ligands (Sonic Hedgehog, Shh; desert Hedgehog, Dhh; Indian Hedgehog, Ihh), which upon binding to the transmembrane receptor patched (Ptch) causes Ptch to remove its inhibitory influence on the G protein-coupled receptor smoothened (Smo) (Figure 2). Activation of Smo then leads to nuclear translocation of the glioma-associated protein (Gli) family of transcription factors and induction of Hh target genes, such as Gli1 and Hedgehog interacting protein (Hhip). The latter competes with Ptch by binding to Hh ligands [20]. Gli protein levels and activities are primarily regulated by suppressor of fused (Sufu). Sufu is a major negative regulator of mammalian Hh signaling. Loss of Sufu in mammals leads to global Hh pathway activation and early embryonic lethality [21,22]. In the absence of the Hedgehog family of ligands, Gli-mediated transcription is inhibited. In vertebrates, Hh signaling occurs in the primary cilium, a non-motile flagellar-like organelle present on growth-arrested cells [23].
Figure 2.
Simplified scheme of Hh signaling in mammalian cells: (a) in the absence of Hedgehog ligand, patched 1 (Ptch) localizes to the primary cilium, where it prevents activation of smoothened (Smo); (b) Hh signaling is activated upon binding of the ligand to the Ptch receptor, which leaves the cilium, releases the inhibition of Smo and leads to glioma-associated (Gli) transcription factors’ translocation into the nucleus; (c) Gli activates the expression of target genes Gli1, Ptch and Hedgehog interacting protein (Hhip1); (d) the pathway can be inhibited at the level of Smo or Gli.
The Hh signaling pathway is essential for embryonic mesothelial development [24]. In the adult, Hh continues to signal to discrete populations of stem and progenitor cells within various organs where it is considered to have a role in maintaining homeostasis after injury (reviewed in [19]). The Hh signaling pathway is inactive in most adult tissues, including mesothelium [18], but consistent with re-activation of developmental pathways in cancer, the Hh pathway has been shown to become upregulated in MPM tumors. We were the first to show SHH gene expression in human MPM tumor tissues along with increased expression levels of HHIP and GLI1 [18]. High GLI1 gene expression was associated with shorter overall survival in MPM patients [18]. Another study reported that high SMO and SHH expression levels were associated with worse survival of MPM patients [25].
The importance of the Hh pathway in MPM has been recently reinforced by data obtained in samples from 85 patients profiled for molecular differences between tumor cells and healthy cells by large consortia, such as The Cancer Genome Atlas (TCGA). Since 2006, TCGA has explored these differences in many cancer types using a variety of platforms using single-nucleotide polymorphism, small RNA transcriptome, exome and methylation data from sequencing and microarrays. Systematic analysis of TCGA datasets was generated through the Broad’s Genome Data Analysis Center (GDAC; http://gdac.broadinstitute.org/). The pipelines run in a computational framework called Firehose, which also generates analysis reports [26]. The Hh pathway appears in the top 10 pathways deregulated in MPM (Table 1) [27]. Moreover, based on the mRNA expression profile, the 85 tumors clustered into four groups [28], one of which is characterized by overexpression of Hh target genes GLI1, HHIP and PTCH2. For 39 of these patients, clinical outcome is also available, and the Hh target gene-expressing group is one of the two groups with the worst overall survival (median 6.6 months, [29]).
Table 1.
Malignant pleural mesothelioma (MPM) specific pathway perturbations reported in The Cancer Genome Atlas (TCGA).
| Pathway Name | Average No. of Perturbations |
|---|---|
| WT1 (Nephrin/Neph1/Yap/TEAD) | 18 |
| Ephrin A reverse signaling | 13 |
| Syndecan-1-mediated signaling | 11 |
| Hedgehog signaling | 10 |
| IL-4-mediated signaling | 10 |
| Signaling regulated by Ret | 10 |
| Ephrin B reverse signaling | 10 |
| Endothelins | 10 |
| CDKN2A (Rb pathway) | 10 |
| Glucocorticoid receptor regulatory network | 9 |
Altogether, published work and data mining indicate that deregulated Hh signaling defines a subgroup of patients with bad clinical outcome.
With the exception of the fact that Hedgehog signaling is essential for embryonic mesothelium development and that re-activation of embryonic signaling is frequently observed in tumors, the factors driving aberrant Hh pathway activity in MPM are not known yet. A study using a panel of seven MPM cell lines demonstrated a missense mutation in the SUFU gene, leading to T411M amino acid change, coupled with three base pair (CTG) insertions in the SMO gene, resulting in an additional amino acid 23L_24GinsL in the signal peptide region in one cell line [30]. The 3-bp insertion in SMO was also detected in one MPM patient out of 14 patients analyzed. Transfection of a SUFU cDNA harboring the T411M missense mutation suppressed Gli-reporter gene downregulation as observed with wild-type SUFU [30], although it is unclear whether such results were observed with similar levels of SUFU protein expressed. Deletion of PTCH1 exons 8–23 was observed in another cell line [30]. Deletion of chromosome 9q22.32, containing the PTCH1 gene, is observed in medulloblastoma and basal cell carcinoma (reviewed in [31]) and various other cancers [32,33,34], consistent with the loss of tumor suppressor function. The functional impact of the insertion in the SMO gene still remains to be investigated. This data indicate that mutations of the Hh pathway rarely exist in MPM.
As mentioned above, the Hh pathway is important for development and tissue repair, and it is also active in cancer cells with the stem cell phenotype (reviewed in [35,36]). We had hypothesized that cancer cells with the stem cell phenotype are present in MPM, a cancer type with a high chemoresistant and relapse rate. Therefore, we used a functional assay, which identifies a small and distinct subset of cells, called a “side population” (SP), with phenotypic markers of multipotent hematopoietic stem cells after staining bone marrow with the DNA staining dye Hoechst 33342 [37]. The SP is due to the expression of functional ATP-binding cassette (ABC) transporters [38]. When living cells are stained with Hoechst 33342, SP cells efflux the DNA staining dye via their ABC transporters. When cells are co-incubated with ABC transporter inhibitors verapamil or fumitremorgin C (FTC), Hoechst 33342 is no longer effluxed, leading to a shift in the dual emission wavelength fluorescence-activated cell sorting (FACS) analysis upon which the SP can be identified. The ABCG2 drug transporter is responsible for the SP in the bone marrow [38,39]. We detected side population cells of MPM having precursor characteristics and showing the enrichment of tumor initiating and PTCH1 expression compared to the non-side population [40].
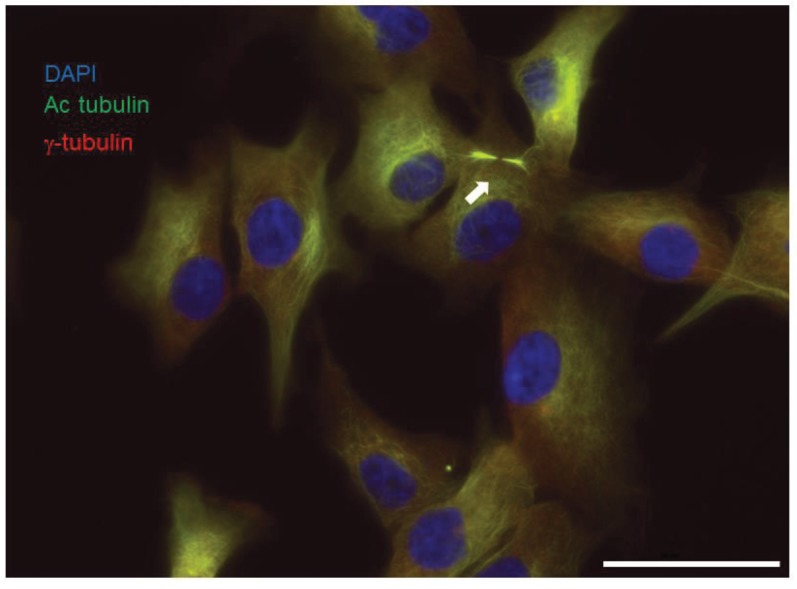
Stem cell signaling is, as also observed by others [41], not maintained when primary tumors are grown in the presence of serum; therefore, we set up primary human MPM in the absence of serum and in the presence of their own conditioned medium plus specific growth factors [42,43] and in 3% oxygen. The culture conditions in 3% instead of 20% oxygen were implemented because they are more physiologic [44]. In these conditions, we could observe the presence of primary cilia (Figure 3), probably linked to the fact that about 35% of the cells are quiescent [45]. Autocrine regulation of the Hh pathway in a ligand-dependent fashion was also observed [18]. Indeed, secretion of desert Hedgehog ligand (DHH) was observed to stimulate MPM growth by regulating Yes-associated protein 1, a transcription co-activator controlled by the Hippo pathway [18]. Consistent with the Hh pathway being overexpressed in a subgroup of MPM as detailed above, only four out of six of the primary MPM cell lines cultured in these conditions responded to the inhibition of the Hh pathway by the Smo antagonist, cyclopamine. The treatment with another Smo antagonist, HhAntag, increased the number of growth-arrested cells in an Hh-responsive cell line in vitro [45] and reduced the tumor growth implanted subcutaneously in immunodeficient mice by approximately 40% [18]. Thus, the Hh pathway activation in MPM may originate from increased pathway activity in a ligand-dependent manner during the regeneration and repair of mesothelium after tissue damage. Nevertheless, it should be noted that a small group of MPM patients may harbor mutations of the pathway.
Figure 3.
Primary culture of mesothelioma grown in the absence of serum and in 3% oxygen develop primary cilia. Primary mesothelioma cells were fixed in 4% paraformaldehyde, then were permeabilized in 0.1% saponin. Primary cilia (arrow) were detected using anti-acetylated tubulin (Sigma T 6793, 1:50), anti-gamma tubulin (Sigma T 5192, 1:1000) and Alexa 488 and Cy5-coupled secondary antibodies, respectively. Nuclei were stained with DAPI. Scale bar: 50 μm.
In addition, we recently detected paracrine activation of Hh signaling in MPM. Analysis of GLI1 immunoreactivity in a small cohort of MPM patients revealed heterogeneous expression in both tumor and stroma fractions [46]. In order to investigate in the most complete way the role of Hh signaling in both tumor and stroma, we used a rat immunocompetent mesothelioma model showing activation of Hh signaling [46]. We observed downregulation of the Hh pathway mostly in the stromal part after the treatment with an Hh antagonist [46]. Downregulation of the Hh pathway was associated with reduced expression of factors essential for promoting tumor growth and with reduced tumor volume [46]. The same finding has been detected in pancreatic and colorectal cancers [47,48,49]. The role of the stromal Hh pathway in tumor maintenance was clearly demonstrated in pancreatic cancer, where tumor growth was reduced when growing in a microenvironment lacking Gli1 compared to the wild-type [49]. The role of the microenvironment should not be neglected, especially in MPM, given the number of MPM-specific pathway perturbations associated with the tumor to stroma interaction reported in The Cancer Genome Atlas (TCGA) (Table 1).
A number of clinical trials for Hh inhibitors, mainly Smo antagonists, have been conducted to date (reviewed in [36,50]). Three mesothelioma patients were enrolled in the phase 1 clinical trial for Smo inhibitor vismodegib and showed no response [51]; however, in that study, activation of the Hh pathway in the tumors was not investigated. As for other targeted therapy, there is a need for proper predictive biomarkers. In medulloblastoma, another cancer where a subgroup of patients show Hh activation, a five-gene expression signature was used to select patients who received Hh inhibitor, and 66% showed objective responses [52]. A phase 3 trial in patients with recurrent medulloblastoma is ongoing to validate this assay for predicting the response to Hh pathway inhibition. It is not known whether this gene signature would be the same in mesothelioma.
Several lines of evidence suggest existing crosstalk between Gli1 and other signal transduction pathways (reviewed in [53]) in an Hh ligand-independent manner. For example, an activated mTOR pathway, which we and others have shown to be associated with the worst clinical outcome in mesothelioma [54,55], promotes Gli1 transcriptional activity [56]. It is not clear yet what the role of non-canonical Hh activation in MPM is. Nevertheless, novel therapies targeting the Hedgehog pathway downstream of Smo have been developed. Several studies reported that Gli1 inhibition, either by agents, such as arsenic trioxide, which prevents Gli2 localization to primary cilia [57], inhibits GLI1 transcriptional activity [58] and suppresses Gli transcription via induction of DNA damage [59] or GANT61, which prevents Gli1-DNA binding in living cells [60], but it also has many other mechanisms of action (reviewed in [61]), resulting in growth arrest and induction of cell death in MPM cells in vitro [62,63,64].
3. Conclusions
In summary, several lines of evidence indicate that Hh signaling is activated in a subgroup of MPM patients and is associated with the worst clinical outcome. In a limited number of them, activation may result from either mutation in components of the pathway, while for the others, it seems to be resulting from ligand-driven activation. The identification of biomarkers and parallel or compensatory non-canonical signaling may be useful for exploring mechanism-based therapeutic combinations.
Acknowledgments
We are grateful to Rolf Stahel for critical reading of the manuscript. Emanuela Felley-Bosco is supported by the Stiftung für Angewandte Krebsforschung, the Krebsliga Zürich and the Swiss National Science Foundation (CRSII3_147697); Isabelle Opitz is supported by the Swiss National Science Foundation (PP00P3_133657).
Author Contributions
All authors contributed to this manuscript and approved the final version.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Park E.K., Takahashi K., Hoshuyama T., Cheng T.J., Delgermaa V., le G.V., Sorahan T. Global magnitude of reported and unreported mesothelioma. Environ. Health Perspect. 2011;119:514–518. doi: 10.1289/ehp.1002845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagner J.C., Sleggs C.A., Marchand P. Diffuse pleural mesothelioma and asbestos exposure in the north western cape province. Br. J. Ind. Med. 1960;17:260–271. doi: 10.1136/oem.17.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lianes P., Remon J., Bover I., Isla D. Seom guidelines for the treatment of malignant pleural mesothelioma. Clin. Transl. Oncol. 2011;13:569–573. doi: 10.1007/s12094-011-0699-5. [DOI] [PubMed] [Google Scholar]
- 4.Marinaccio A., Binazzi A., Cauzillo G., Cavone D., Zotti R.D., Ferrante P., Gennaro V., Gorini G., Menegozzo M., Mensi C., et al. Analysis of latency time and its determinants in asbestos related malignant mesothelioma cases of the italian register. Eur. J. Cancer. 2007;43:2722–2728. doi: 10.1016/j.ejca.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Peto J., Decarli A., La Vecchia C., Levi F., Negri E. The european mesothelioma epidemic. Br. J. Cancer. 1999;79:666–672. doi: 10.1038/sj.bjc.6690105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carbone M., Kratzke R.A., Testa J.R. The pathogenesis of mesothelioma. Semin. Oncol. 2002;29:2–17. doi: 10.1053/sonc.2002.30227. [DOI] [PubMed] [Google Scholar]
- 7.Testa J.R., Cheung M., Pei J., Below J.E., Tan Y., Sementino E., Cox N.J., Dogan A.U., Pass H.I., Trusa S., et al. Germline bap1 mutations predispose to malignant mesothelioma. Nat. Genet. 2011;43:1022–1025. doi: 10.1038/ng.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogelzang N.J., Rusthoven J.J., Symanowski J., Denham C., Kaukel E., Ruffie P., Gatzemeier U., Boyer M., Emri S., Manegold C., et al. Phase iii study of pemetrexed in combination with cisplatin vs. cisplatin alone in patients with malignant pleural mesothelioma. J. Clin. Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 9.Weder W., Kestenholz P., Taverna C., Bodis S., Lardinois D., Jerman M., Stahel R.A. Neoadjuvant chemotherapy followed by extrapleural pneumonectomy in malignant pleural mesothelioma. J. Clin. Oncol. 2004;22:3451–3457. doi: 10.1200/JCO.2004.10.071. [DOI] [PubMed] [Google Scholar]
- 10.Rusch V., Baldini E.H., Bueno R., de Perrot M., Flores R., Hasegawa S., Klepetko W., Krug L., Lang-Lazdunski L., Pass H., et al. The role of surgical cytoreduction in the treatment of malignant pleural mesothelioma: Meeting summary of the international mesothelioma interest group congress, 11–14 September 2012, Boston, Mass. J. Thorac. Cardiovasc. Surg. 2013;145:909–910. doi: 10.1016/j.jtcvs.2013.01.039. [DOI] [PubMed] [Google Scholar]
- 11.Cao C., Tian D.H., Pataky K.A., Yan T.D. Systematic review of pleurectomy in the treatment of malignant pleural mesothelioma. Lung Cancer. 2013;81:319–327. doi: 10.1016/j.lungcan.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 12.Cao C.Q., Yan T.D., Bannon P.G., McCaughan B.C. A systematic review of extrapleural pneumonectomy for malignant pleural mesothelioma. J. Thorac. Oncol. 2010;5:1692–1703. doi: 10.1097/JTO.0b013e3181ed0489. [DOI] [PubMed] [Google Scholar]
- 13.Cao C., Tian D., Manganas C., Matthews P., Yan T.D. Systematic review of trimodality therapy for patients with malignant pleural mesothelioma. Ann. Cardiothorac. Surg. 2012;1:428–437. doi: 10.3978/j.issn.2225-319X.2012.11.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedberg J.S. Photodynamic therapy for malignant pleural mesothelioma: The future of treatment? Expert Rev. Respir. Med. 2011;5:49–63. doi: 10.1586/ers.11.1. [DOI] [PubMed] [Google Scholar]
- 15.Tilleman T.R., Richards W.G., Zellos L., Johnson B.E., Jaklitsch M.T., Mueller J., Yeap B.Y., Mujoomdar A.A., Ducko C.T., Bueno R., et al. Extrapleural pneumonectomy followed by intracavitary intraoperative hyperthermic cisplatin with pharmacologic cytoprotection for treatment of malignant pleural mesothelioma: A phase II prospective study. J. Thorac. Cardiovasc. Surg. 2009;138:405–411. doi: 10.1016/j.jtcvs.2009.02.046. [DOI] [PubMed] [Google Scholar]
- 16.Opitz I., Lauk O., Meerang M., Bommeli C., Jetter A., Günther D., Stahel R., Weder W. Mo 14.05 intracavitary cisplatin-fibrin chemotherapy after resection for malignant pleural mesothelioma patients (influence-meso)—Preliminary results. J. Thorac. Oncol. 2013;8:343. [Google Scholar]
- 17.Stahel R.A., Weder W., Felley-Bosco E., Petrausch U., Curioni-Fontecedro A., Schmitt-Opitz I., Peters S. Searching for targets for the systemic therapy of mesothelioma. Ann. Oncol. 2015 doi: 10.1093/annonc/mdv101. [DOI] [PubMed] [Google Scholar]
- 18.Shi Y., Moura U., Opitz I., Soltermann A., Rehrauer H., Thies S., Weder W., Stahel R.A., Felley-Bosco E. Role of hedgehog signaling in malignant pleural mesothelioma. Clin. Cancer Res. 2012;18:4646–4656. doi: 10.1158/1078-0432.CCR-12-0599. [DOI] [PubMed] [Google Scholar]
- 19.Petrova R., Joyner A.L. Roles for hedgehog signaling in adult organ homeostasis and repair. Development. 2014;141:3445–3457. doi: 10.1242/dev.083691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chuang P.T., McMahon A.P. Vertebrate hedgehog signalling modulated by induction of a hedgehog-binding protein. Nature. 1999;397:617–621. doi: 10.1038/17611. [DOI] [PubMed] [Google Scholar]
- 21.Svard J., Heby-Henricson K., Persson-Lek M., Rozell B., Lauth M., Bergstrom A., Ericson J., Toftgard R., Teglund S. Genetic elimination of suppressor of fused reveals an essential repressor function in the mammalian hedgehog signaling pathway. Dev. Cell. 2006;10:187–197. doi: 10.1016/j.devcel.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 22.Cooper A.F., Yu K.P., Brueckner M., Brailey L.L., Johnson L., McGrath J.M., Bale A.E. Cardiac and cns defects in a mouse with targeted disruption of suppressor of fused. Development. 2005;132:4407–4417. doi: 10.1242/dev.02021. [DOI] [PubMed] [Google Scholar]
- 23.Corbit K.C., Aanstad P., Singla V., Norman A.R., Stainier D.Y., Reiter J.F. Vertebrate smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 24.Dixit R., Ai X., Fine A. Derivation of lung mesenchymal lineages from the fetal mesothelium requires hedgehog signaling for mesothelial cell entry. Development. 2013;140:4398–4406. doi: 10.1242/dev.098079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y., He J., Zhang F., Li H., Yue D., Wang C., Jablons D.M., He B., Lui N. SMO expression level correlates with overall survival in patients with malignant pleural mesothelioma. J. Exp. Clin. Cancer Res. 2013 doi: 10.1186/1756-9966-32-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gehlenborg N., Noble M.S., Getz G., Chin L., Park P.J. Nozzle: A report generation toolkit for data analysis pipelines. Bioinformatics. 2013;29:1089–1091. doi: 10.1093/bioinformatics/btt085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Broad institute of mit and harvard; [(accessed on 12 May 2015)]. Broad institute tcga genome data analysis center (2014): Paradigm pathway analysis of mrnaseq expression and copy number data. Available online: http://gdac.broadinstitute.org/runs/analyses__2014_10_17/reports/cancer/MESO-TP/Pathway_Paradigm_RNASeq_And_Copy_Number/nozzle.html. [Google Scholar]
- 28.Broad institute of mit and harvard; [(accessed on 12 May 2015)]. Broad institute tcga genome data analysis center (2014): Clustering of mrnaseq gene expression: Consensus nmf. Available online: http://gdac.broadinstitute.org/runs/analyses__2014_10_17/reports/cancer/MESO-TP/mRNAseq_Clustering_Consensus_Plus/nozzle.html. [Google Scholar]
- 29.Broad institute of mit and harvard; [(accessed on 12 May 2015)]. Broad institute tcga genome data analysis center (2014): Correlation between aggregated molecular cancer subtypes and selected clinical features. Available online: http://gdac.broadinstitute.org/runs/analyses__2014_10_17/reports/cancer/MESO-TP/Correlate_Clinical_vs_Molecular_Subtypes/nozzle.html. [Google Scholar]
- 30.Lim C.B., Prele C.M., Cheah H.M., Cheng Y.Y., Klebe S., Reid G., Watkins D.N., Baltic S., Thompson P.J., Mutsaers S.E. Mutational analysis of hedgehog signaling pathway genes in human malignant mesothelioma. PLoS ONE. 2013;8:e66685. doi: 10.1371/journal.pone.0066685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teglund S., Toftgard R. Hedgehog beyond medulloblastoma and basal cell carcinoma. Biochim. Biophys. Acta. 2010;1805:181–208. doi: 10.1016/j.bbcan.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Knuutila S., Aalto Y., Autio K., Bjorkqvist A.M., El-Rifai W., Hemmer S., Huhta T., Kettunen E., Kiuru-Kuhlefelt S., Larramendy M.L., et al. DNA copy number losses in human neoplasms. Am. J. Pathol. 1999;155:683–694. doi: 10.1016/S0002-9440(10)65166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minobe K., Onda M., Iida A., Kasumi F., Sakamoto G., Nakamura Y., Emi M. Allelic loss on chromosome 9q is associated with lymph node metastasis of primary breast cancer. Jpn. J. Cancer Res. 1998;89:916–922. doi: 10.1111/j.1349-7006.1998.tb00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schultz D.C., Vanderveer L., Buetow K.H., Boente M.P., Ozols R.F., Hamilton T.C., Godwin A.K. Characterization of chromosome 9 in human ovarian neoplasia identifies frequent genetic imbalance on 9q and rare alterations involving 9p, including cdkn2. Cancer Res. 1995;55:2150–2157. [PubMed] [Google Scholar]
- 35.Islam F., Gopalan V., Smith R.A., Lam A.K. Translational potential of cancer stem cells: A review of the detection of cancer stem cells and their roles in cancer recurrence and cancer treatment. Exp. Cell Res. 2015;335:135–147. doi: 10.1016/j.yexcr.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 36.Takebe N., Miele L., Harris P.J., Jeong W., Bando H., Kahn M., Yang S.X., Ivy S.P. Targeting notch, hedgehog, and wnt pathways in cancer stem cells: Clinical update. Nat. Rev. Clin. Oncol. 2015 doi: 10.1038/nrclinonc.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goodell M.A., Brose K., Paradis G., Conner A.S., Mulligan R.C. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J. Exp. Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou S., Schuetz J.D., Bunting K.D., Colapietro A.M., Sampath J., Morris J.J., Lagutina I., Grosveld G.C., Osawa M., Nakauchi H., et al. The abc transporter bcrp1/abcg2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat. Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 39.Scharenberg C.W., Harkey M.A., Torok-Storb B. The abcg2 transporter is an efficient hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood. 2002;99:507–512. doi: 10.1182/blood.V99.2.507. [DOI] [PubMed] [Google Scholar]
- 40.Frei C., Opitz I., Soltermann A., Fischer B., Moura U., Rehrauer H., Weder W., Stahel R., Felley-Bosco E. Pleural mesothelioma side populations have a precursor phenotype. Carcinogenesis. 2011;32:1324–1332. doi: 10.1093/carcin/bgr127. [DOI] [PubMed] [Google Scholar]
- 41.Sasai K., Romer J.T., Lee Y., Finkelstein D., Fuller C., McKinnon P.J., Curran T. Shh pathway activity is down-regulated in cultured medulloblastoma cells: Implications for preclinical studies. Cancer Res. 2006;66:4215–4222. doi: 10.1158/0008-5472.CAN-05-4505. [DOI] [PubMed] [Google Scholar]
- 42.Ricci-Vitiani L., Lombardi D.G., Pilozzi E., Biffoni M., Todaro M., Peschle C., de Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 43.Clement V., Sanchez P., de Tribolet N., Radovanovic I., Ruiz I.A.A. Hedgehog-gli1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr. Biol. 2007;17:165–172. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parrinello S., Samper E., Krtolica A., Goldstein J., Melov S., Campisi J. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat. Cell Biol. 2003;5:741–747. doi: 10.1038/ncb1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Renganathan A., Kresoja-Rakic J., Echeverry N., Ziltener G., Vrugt B., Opitz I., Stahel R.A., Felley-Bosco E. GAS5 long non-coding RNA in malignant pleural mesothelioma. Mol. Cancer. 2014 doi: 10.1186/1476-4598-13-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meerang M., Bérard K., Felley-Bosco E., Vrugt B., Boss A., Kenkel D., Broggini-Tenzel A., Stahel R., Lauk O., Arni S., et al. Effects of vismodegib in an orthotopic immunocompetent rat model of malignant pleural mesothelioma. 2015. Unpublished data.
- 47.Yauch R.L., Gould S.E., Scales S.J., Tang T., Tian H., Ahn C.P., Marshall D., Fu L., Januario T., Kallop D., et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–410. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 48.Bailey J.M., Mohr A.M., Hollingsworth M.A. Sonic hedgehog paracrine signaling regulates metastasis and lymphangiogenesis in pancreatic cancer. Oncogene. 2009;28:3513–3525. doi: 10.1038/onc.2009.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mills L.D., Zhang Y., Marler R.J., Herreros-Villanueva M., Zhang L., Almada L.L., Couch F., Wetmore C., Pasca di Magliano M., Fernandez-Zapico M.E. Loss of the transcription factor gli1 identifies a signaling network in the tumor microenvironment mediating kras oncogene-induced transformation. J. Biol. Chem. 2013;288:11786–11794. doi: 10.1074/jbc.M112.438846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amakye D., Jagani Z., Dorsch M. Unraveling the therapeutic potential of the hedgehog pathway in cancer. Nat. Med. 2013;19:1410–1422. doi: 10.1038/nm.3389. [DOI] [PubMed] [Google Scholar]
- 51.LoRusso P.M., Rudin C.M., Reddy J.C., Tibes R., Weiss G.J., Borad M.J., Hann C.L., Brahmer J.R., Chang I., Darbonne W.C., et al. Phase i trial of hedgehog pathway inhibitor vismodegib (gdc-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin. Cancer Res. 2011;17:2502–2511. doi: 10.1158/1078-0432.CCR-10-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shou Y., Robinson D.M., Amakye D.D., Rose K.L., Cho Y.J., Ligon K.L., Sharp T., Haider A.S., Bandaru R., Ando Y., et al. A five-gene hedgehog signature developed as a patient preselection tool for hedgehog inhibitor therapy in medulloblastoma. Clin. Cancer Res. 2015;21:585–593. doi: 10.1158/1078-0432.CCR-13-1711. [DOI] [PubMed] [Google Scholar]
- 53.Aberger F., Ruiz I.A.A. Context-dependent signal integration by the gli code: The oncogenic load, pathways, modifiers and implications for cancer therapy. Semin. Cell Dev. Biol. 2014;33:93–104. doi: 10.1016/j.semcdb.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cedres S., Montero M.A., Martinez P., Martinez A., Rodriguez-Freixinos V., Torrejon D., Gabaldon A., Salcedo M., Ramon Y.C.S., Felip E. Exploratory analysis of activation of pten-pi3k pathway and downstream proteins in malignant pleural mesothelioma (mpm) Lung Cancer. 2012;77:192–198. doi: 10.1016/j.lungcan.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 55.Bitanihirwe B.K., Meerang M., Friess M., Soltermann A., Frischknecht L., Thies S., Felley-Bosco E., Tsao M.S., Allo G., de Perrot M., et al. Pi3k/mtor signaling in mesothelioma patients treated with induction chemotherapy followed by extrapleural pneumonectomy. J. Thorac. Oncol. 2014;9:239–247. doi: 10.1097/JTO.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y., Ding Q., Yen C.J., Xia W., Izzo J.G., Lang J.Y., Li C.W., Hsu J.L., Miller S.A., Wang X., et al. The crosstalk of mtor/s6k1 and hedgehog pathways. Cancer Cell. 2012;21:374–387. doi: 10.1016/j.ccr.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim J., Lee J.J., Kim J., Gardner D., Beachy P.A. Arsenic antagonizes the hedgehog pathway by preventing ciliary accumulation and reducing stability of the Gli2 transcriptional effector. Proc. Natl. Acad. Sci. USA. 2010;107:13432–13437. doi: 10.1073/pnas.1006822107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beauchamp E.M., Ringer L., Bulut G., Sajwan K.P., Hall M.D., Lee Y.C., Peaceman D., Ozdemirli M., Rodriguez O., Macdonald T.J., et al. Arsenic trioxide inhibits human cancer cell growth and tumor development in mice by blocking hedgehog/gli pathway. J. Clin. Investig. 2011;121:148–160. doi: 10.1172/JCI42874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakamura S., Nagano S., Nagao H., Ishidou Y., Yokouchi M., Abematsu M., Yamamoto T., Komiya S., Setoguchi T. Arsenic trioxide prevents osteosarcoma growth by inhibition of gli transcription via DNA damage accumulation. PLoS ONE. 2013;8:e69466. doi: 10.1371/journal.pone.0069466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lauth M., Bergstrom A., Shimokawa T., Toftgard R. Inhibition of gli-mediated transcription and tumor cell growth by small-molecule antagonists. Proc. Natl. Acad. Sci. USA. 2007;104:8455–8460. doi: 10.1073/pnas.0609699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gonnissen A., Isebaert S., Haustermans K. Targeting the hedgehog signaling pathway in cancer: Beyond smoothened. Oncotarget. 2015;6:13899–13913. doi: 10.18632/oncotarget.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li H., Lui N., Cheng T., Tseng H.H., Yue D., Giroux-Leprieur E., Do H.T., Sheng Q., Jin J.Q., Luh T.W., et al. Gli as a novel therapeutic target in malignant pleural mesothelioma. PLoS ONE. 2013;8:e57346. doi: 10.1371/journal.pone.0057346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lim C.B., Prele C.M., Baltic S., Arthur P.G., Creaney J., Watkins D.N., Thompson P.J., Mutsaers S.E. Mitochondria-derived reactive oxygen species drive GANT61-induced mesothelioma cell apoptosis. Oncotarget. 2015;6:1519–1530. doi: 10.18632/oncotarget.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.You M., Varona-Santos J., Singh S., Robbins D.J., Savaraj N., Nguyen D.M. Targeting of the hedgehog signal transduction pathway suppresses survival of malignant pleural mesothelioma cells in vitro. J. Thorac. Cardiovasc. Surg. 2014;147:508–516. doi: 10.1016/j.jtcvs.2013.08.035. [DOI] [PubMed] [Google Scholar]