Abstract
Two new furan derivatives, hypofurans A and B (1 and 2), and three new cyclopentenone derivatives, hypocrenones A–C (3–5), along with seven known compounds (6–12), were isolated from a marine fungus Hypocrea koningii PF04 associated with the sponge Phakellia fusca. Among them, compounds 10 and 11 were obtained for the first time as natural products. The planar structures of compounds 1–5 were elucidated by analysis of their spectroscopic data. Meanwhile, the absolute configuration of 1 was determined as 2R,3R by the comparison of the experimental and calculated electronic circular dichroism (ECD) spectra. All the isolates were evaluated for their antibacterial and antioxidant activity. Compounds 1, 10, and 12 all showed modest antibacterial activity against Staphylococcus aureus ATCC25923 (MIC, 32 μg/mL). In addition, compounds 1, 10 and 11 exhibited moderate DPPH radical scavenging capacity with IC50 values of 27.4, 16.8, and 61.7 µg/mL, respectively.
Keywords: Hypocrea koningii, sponge-associated fungus, furan derivatives, cyclopentenone derivatives, antibacterial, antioxidant
1. Introduction
Marine fungi harbor the potential to generate a multitude of structurally novel chemicals with diverse biological activities in part owing to harsh habitats [1,2]. More than one thousand new natural products have hitherto been harvested from marine-derived fungi. Excitingly, some of them with clinically relevant pharmacological activities will be probably developed into viable drug candidates exemplified by halimide, which entered into phase II clinical trials of cancer chemotherapy [3,4,5,6]. As an epitome of particular marine habitats, sponges often possess remarkable microbial biomass and diversity, and their microbial symbionts represent a precious wellspring of new scaffolds for drug discovery [7]. Hence, we focused on the sponge-derived fungus Hypocrea koningii PF04, which was associated with the marine sponge Phakellia fusca collected from Yongxing Island in the South China Sea [8].
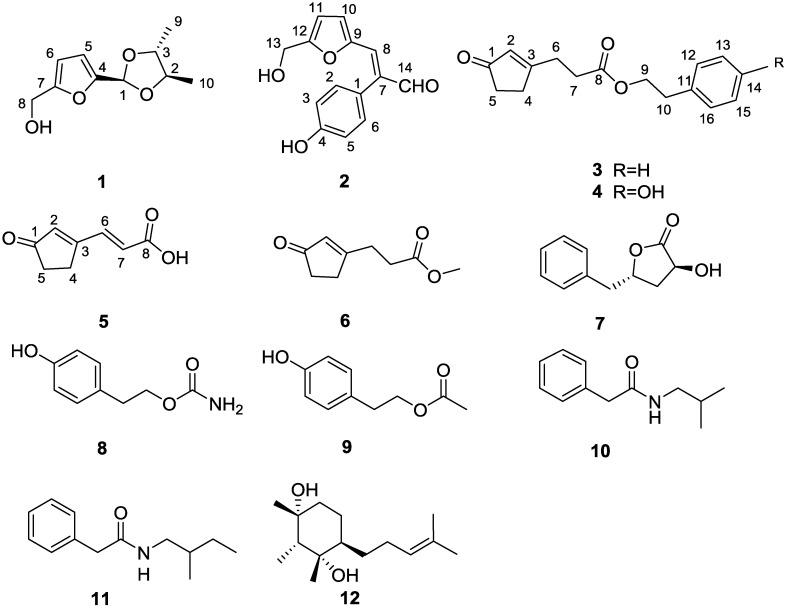
Members of the genus Hypocrea (also called Trichoderma), typically soil-borne or wood-decaying fungi, are renowned for opportunistic pathogens of immunocompromised humans as well as producers of industrial enzymes and biocontrol agents against plant pathogens [9,10]. According to previous reports on the chemical constituents of this genus, various types of novel secondary metabolites, including terpenoids [11], polyketides [12,13], alkaloids [14], peptides [15], have been documented. Notably, a large proportion of these metabolites exhibited therapeutic properties such as antimalarial [12,16], tyrosine kinase inhibitory [13], antimicrobial [11,14,15], and cytotoxic activities [12]. In our present search for bioactive constituents from H. koningii PF04, two new furan derivatives, hypofurans A and B (1 and 2) and three new cyclopentenone derivatives, hypocrenones A–C (3–5), together with seven known compounds, methyl-3-(3-oxocyclopent-1-enyl) propionate (6), harzialactone A (7), tyrosol carbamate (8), tyrosyl acetate (9), N-isobutyl-2-phenylacetamide (10), N-(2-methylbutyl)-2-phenylacetamide (11), and citrantifidiol (12) (Figure 1) were isolated from the ethyl acetate (EtOAc) extract of the solid-state culture. Among them, new compounds 1 and 2 are furan derivatives, and 3–6 encompass cyclopentenone subunits. Both furan and cyclopentenone moieties are prevalent in natural products and pharmaceuticals [17,18]. Herein, we described the details of the isolation, structure elucidation, possible biosynthetic pathways, and biological activities of these compounds.
Figure 1.
Chemical structures of compounds 1–12.
2. Results and Discussion
2.1. Structure Elucidation
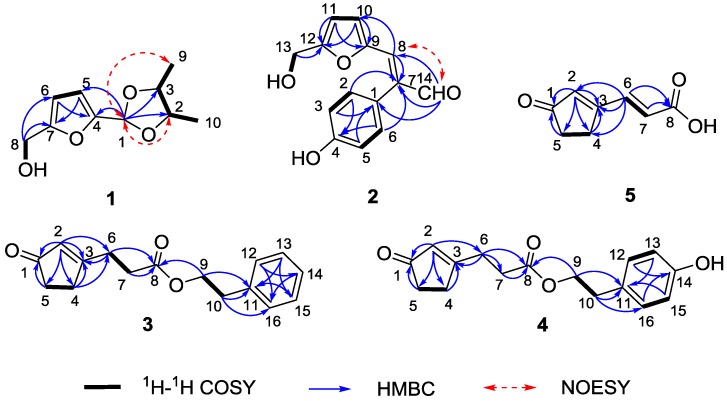
Hypofuran A (1) was afforded as a colorless oil. The molecular formula C10H14O4 was deduced from HRESIMS data (m/z 199.0965 for [M + H]+), indicative of four degrees of unsaturation. The IR spectrum of 1 showed characteristic absorptions for a hydroxy group (3386 cm−1) and a furan ring (3123, 1584, 1522, 888 cm−1) [19]. The 1H NMR spectrum (Table 1) showed a 2,5-disubstituted furan ring at δH 6.42 (d, H-5) and 6.24 (d, H-6), one acetal methine at δH 5.89 (s, H-1), an exchangeable proton at δH 5.23 (t, OH-8), one hydroxymethyl group at δH 4.37 (d, H-8), two oxygenated methines at δH 3.70 (m, H-3) and 3.67 (m, H-2), and two methyls at δH 1.27 (d, H-10) and 1.22 (d, H-9). The 13C NMR and DEPT data (Table 2) displayed 10 carbons, including two olefinic quaternary carbons, five methines (two olefinic ones), one oxymethylene, and two methyls. The hydroxymethyl group was located at C-7 (δC, 155.9) in the furan ring on the basis of HMBC correlations from H2-8 to C-6 and C-7. The hydroxymethylfuran ring accounted for three of four degrees of unsaturation, thus requiring an additional ring for 1. The remaining ring was assigned as a dioxolane ring from the HMBC correlations of H-1/C-2 and C-3 and COSY correlation of H-2/H-3. A closer examination of the COSY spectrum implied that two methyls were tethered to C-2 and C-3 of the dioxolane ring, respectively, which was confirmed by correlations of H-2/H3-10 and H-3/H3-9. Further HMBC correlations of H-1/C-4 and C-5 established the connection of the dioxolane ring to C-4 of the hydroxymethylfuran ring. Accordingly, the gross structure of hypofuran A (1) was elucidated as shown in Figure 1.
Table 1.
1H NMR Data (600 MHz, DMSO-d6) for Compounds 1–5 (J in Hz).
| Position | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 5.89, s | ||||
| 2 | 3.67, m | 6.99, d (8.5) | 5.91, t (1.6) | 5.87, s | 6.46, s |
| 3 | 3.70, m | 6.82, d (8.5) | |||
| 4 | 2.56, m | 2.53, m | 2.76, m | ||
| 5 | 6.42, d (3.2) | 6.82, d (8.5) | 2.39, m | 2.27, m | 2.41, m |
| 6 | 6.24, d (3.1) | 6.99, d (8.5) | 2.69, t (7.3) | 2.61, m | 7.61, d (15.9) |
| 7 | 2.60, t (7.3) | 2.61, m | 6.40, d (15.9) | ||
| 8 | 4.37, d (5.4) | 7.40, s | |||
| 9 | 1.22, d (5.8) | 4.33, t (7.0) | 4.17, t (6.9) | ||
| 10 | 1.27, d (5.8) | 6.19, d (3.5) | 2.95, t (7.0) | 2.76, t (6.9) | |
| 11 | 6.37, d (3.5) | ||||
| 12 | 7.21, d (7.0) | 7.02, d (8.4) | |||
| 13 | 4.36, d (4.9) | 7.31, t (7.5) | 6.68, d (8.4) | ||
| 14 | 9.63, s | 7.24, t (7.5) | |||
| 15 | 7.31, t (7.5) | 6.68, d (8.4) | |||
| 16 | 7.21, d (7.0) | 7.02, d (8.4) | |||
| 4-OH | 9.61, s | ||||
| 8-OH | 5.23, t (5.8) | 12.8, s | |||
| 13-OH | 5.34, t (5.7) | ||||
| 14-OH | 9.24, s |
Table 2.
13C NMR (150 MHz, DMSO-d6) Data for Compounds 1–5.
| Carbon | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 95.8, CH | 123.5, qC | 209.6, qC | 208.7, qC | 208.7, qC |
| 2 | 79.2, CH | 130.3, CH | 129.4, CH | 128.4, CH | 135.1, CH |
| 3 | 77.5, CH | 115.3, CH | 180.3, qC | 181.6, qC | 169.3, qC |
| 4 | 150.7, qC | 157.4, qC | 31.6, CH2 | 31.0, CH2 | 26.6, CH2 |
| 5 | 109.4, CH | 115.3, CH | 35.2, CH2 | 34.9, CH2 | 34.7, CH2 |
| 6 | 107.3, CH | 130.3, CH | 28.4, CH2 | 28.0, CH2 | 137.9, CH |
| 7 | 155.9, qC | 137.7, qC | 31.5, CH2 | 30.9, CH2 | 126.4, CH |
| 8 | 55.6, CH2 | 136.0, CH | 172.0, qC | 171.9, qC | 166.7, qC |
| 9 | 16.4, CH3 | 149.6, qC | 65.2, CH2 | 64.9, CH2 | |
| 10 | 16.5, CH3 | 117.0, CH | 35.0, CH2 | 33.5, CH2 | |
| 11 | 110.1, CH | 137.5, qC | 127.8, qC | ||
| 12 | 159.0, qC | 128.8, CH | 129.7, CH | ||
| 13 | 55.8, CH2 | 128.5, CH | 115.1, CH | ||
| 14 | 193.4, CH | 126.7, CH | 155.9, qC | ||
| 15 | 128.5, CH | 115.1, CH | |||
| 16 | 128.8, CH | 129.7, CH |
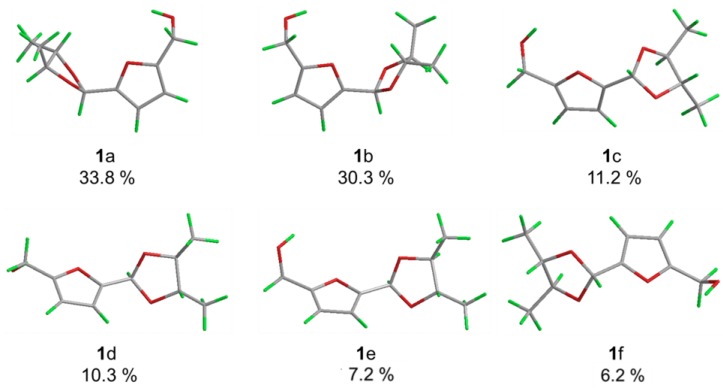
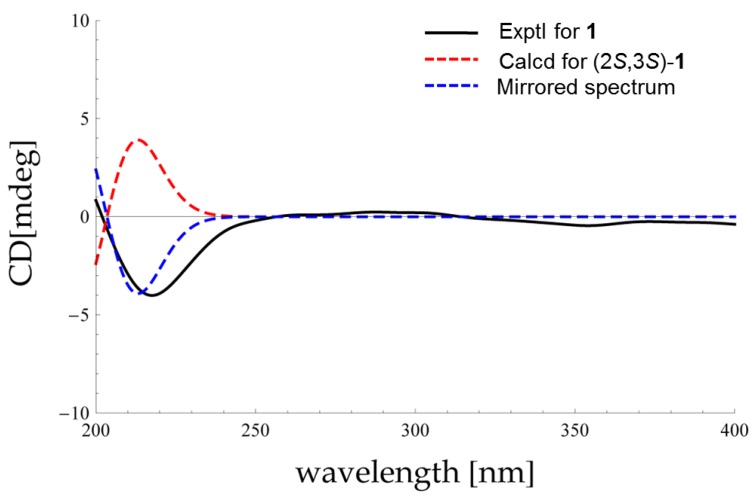
The relative stereochemistry of 1 was disclosed by a NOESY experiment (Figure 2). Two intense NOE interactions between H-1/H3-9 and H-1/H-2 suggested that H-1, H-2, and H3-9 were in the same orientation of the dioxolane ring while H-3 and H3-10 were cofacial. Thus, the relative configurations at C-2 and C-3 of 1 were assigned as 2S,3S or 2R,3R, respectively. In order to assign the absolute configuration of 1, we chose to compare its experimental electronic circular dichroism (ECD) spectrum with the correspondingly time-dependent density functional theory (TDDFT) calculated one, which has proved to be a powerful and reliable approach for determining the absolute configuration of natural products [20,21]. Six low energy conformers above 1% population were generated using conventional initial Merck Molecular Force Field (MMFF) and DFT geometry optimization method (Figure 3 and Supplementary Information), for which ECD spectra were calculated with the TZVP basis set and four different functionals (B3LYP, BH&HLYP, PBE0, CAM-B3LYP). The experimental ECD spectrum (MeOH) of 1 showed a strong negative Cotton effect around 218 nm. With all four methods, the mirror image curve of the Boltzmann-averaged ECD spectrum of (2S,3S)-1 was in good accordance with the experimental one, thus suggesting a (2R,3R) absolute configuration (Figure 4).
Figure 2.
Key COSY, HMBC, and NOESY correlations of compounds 1–5.
Figure 3.
Low energy conformations of 1.
Figure 4.
Comparison of experimental electronic circular dichroism (ECD) and calculated (MeOH) ECD spectra of 1.
Hypofuran B (2) was furnished as a yellow powder. The HRESIMS data (m/z 267.0634 for [M + Na]+) supported a molecular formula of C14H12O4. The IR absorption bands at 3404 cm−1 (a hydroxy group), together with 3020, 1614, 1516, 905 cm−1 (a furan ring), implying 2 possessed a hydroxymethylfuran ring [19]. The assumption was further supported by comparison of its corresponding NMR spectra of 1. The HMBC correlations of H-8 (δH, 7.40)/C-7 (δC, 137.7) and H-14 (δH, 9.63)/C-7 and C-8 (δC, 136.0) revealed that a formyl group (C-14, δC, 193.4) was linked to a vinyl group (C-7–C-8), constructing an acrylaldehyde moiety. Furthermore, the remaining characteristic signals at δH 9.61 (s, 4-OH), 6.99 (d, 8.5 Hz, 2H), and 6.82 (d, 8.5 Hz, 2H) were assignable to a para-hydroxyphenyl ring, established by the HMBC correlations of H-2 and H-6/C-4. The connectivity of the hydroxymethylfuran ring, acrylaldehyde moiety and para-hydroxyphenyl ring were accomplished by analysis of the HMBC spectrum. The crucial cross-peak of H-8/C-10 (δC, 117.0) clearly suggested that the hydroxymethylfuran ring was connected to the acrylaldehyde moiety, which was attached to the para-hydroxyphenyl ring based on the correlations of H-14/C-1 (δC, 123.5) as well as H-2 and H-6/C-7 in the HMBC spectrum. The geometry of the double bond (C-7–C-8) was determined as E by the NOESY correlation between H-14 and H-8 (Figure 2). According to the aforementioned information, the structure of 2 was unambiguously assigned as depicted in Figure 1.
Hypocrenone A (3) appeared as a colorless oil. It was assigned a molecular formula of C16H18O3, based on HRESIMS data for m/z 259.1329 [M + H]+. The IR spectrum indicated the presence of two carbonyl groups (1735, 1707 cm−1) and a benzene ring (1676 cm−1). Interpretation of the 13C NMR spectrum disclosed the existence of a conjugated ketone carbonyl carbon (δC 209.6, C-1), an ester carbonyl carbon (δC 172.0, C-8), two conjugated olefinic carbons (δC 180.3, C-3 and 129.4, C-2), a monosubstituted phenyl ring [δC 137.5 (C-11), 128.8 (C-12, C-16), 128.5 (C-13, C-15), and 126.7 (C-14)], an oxygenated methylene carbon (δC 65.2, C-9), and four methylene groups [δC 35.2 (C-5), 35.0 (C-10), 31.6 (C-4), and 28.4 (C-6)] (Table 2). The COSY cross-peak of H-4/H-5 in conjunction with HMBC correlations of H-2/C-1, C-3, C-4, and C-5, H-4/C-3 and C-8, and H2-5/C-1, C-3, and C-4 delineated a cyclopentenone moiety. Furthermore, the COSY cross-peaks of H2-6/H2-7 and H2-9/H2-10 suggested the presence of two spin systems, which were connected through an ester bond based on the HMBC correlations from H2-6, H2-7, and H2-9 to C-8 (Figure 2). Finally, C-6 was adjacent to the cyclopentenone moiety, as evident by the HMBC cross peaks of H-2 and H-4/C-6 and H-6/C-3, while C-10 was linked to the benzene ring, as indicated by the HMBC correlations of H-9 and H-10/C-11 and H-10/C-16, thereby establishing the structure of 3.
Hypocrenone B (4) was obtained as a colorless oil. Its molecular formula C16H18O4, was evidenced by HRESIMS data (m/z 297.1104 for [M + Na]+), which was one oxygen atom more than 3. The 1H NMR spectrum of 4 resembled that of compound 3 except for the presence of an exchangeable proton (δH, 9.24) and the conspicuous absence of an aromatic proton of 3 (Table 1). Further scrutiny of 1D NMR data, two sets of ortho-coupled aromatic proton signals [δH 7.02 and 6.68 (2H each, d, J = 8.4 Hz)] and six aromatic carbon signals, including an oxygenated one [δC 155.9 (C-14), 129.7 (C-12, C-16), 127.8 (C-11), and 115.1 (C-13, C-15)] were observed, indicating the presence of a para-hydroxyphenyl moiety in 4 instead of the phenyl ring in 3. The structure of 4 was further established by 2D NMR data (Figure 2) and named hypocrenone B.
Hypocrenone C (5) was yielded as a white powder. The HRESIMS spectrum of 5 exhibited a pseudomolecular ion peak at m/z 151.0394 [M − H]−, corresponding to its molecular formula C8H8O3. Diagnostic NMR data for 5 suggested the presence of a cyclopentenone moiety, identical with that of 3 and 4. Moreover, the COSY correlation of H-6 (δH 7.61)/H-7 (δH 6.40) and HMBC correlations from H-6 and H-7 to C-8 (δC 166.7), together with IR absorption band at 1714 cm−1 (carboxylic acid) allowed the unambiguous assignment of the acrylic acid moiety, which was adjacent to the C-3 of the cyclopentenone moiety. The connectivity was corroborated by a significant upfield shift for C-3 (δC 169.3, Δδ = -12.3) and the HMBC correlations of H-6/C-2 (δC 135.1), C-3 (δC 169.3), and C-4 (δC 26.6) (Figure 2). The E-configuration of the C-6/C-7 double bond was inferred from the large coupling constant value (15.9 Hz).
In addition, seven known compounds were identified as methyl-3-(3-oxocyclopent-1-enyl) propionate (6) [22], harzialactone A (7) [23], tyrosol carbamate (8) [24], tyrosyl acetate (9) [25], N-isobutyl-2-phenylacetamide (10) [26], N-(2-methylbutyl)-2-phenylacetamide (11) [27] and citrantifidiol (12) [28] by analysis and comparison of their spectroscopic data with the literature. Among them, compounds 10 and 11 have been previously synthesized but this article is the first report of their isolation as natural products.
2.2. Plausible Biosynthetic Pathways
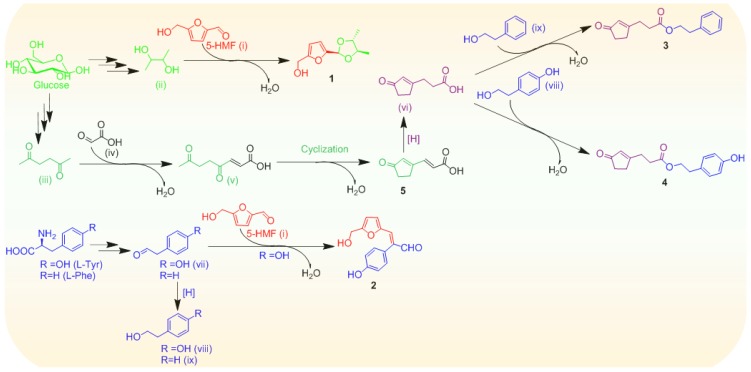
Possible biogenetic routes to compounds 1–5 were proposed as shown in Scheme 1. The biosynthetic precursors, probably including 5-hydroxymethyl furfural (5-HMF, (i)), 2,3-butanediol (ii) and hexane-2,5-dione (iii) could originate from hexose sugars (e.g., glucose) [29,30,31]. The 5-HMF (i) could be ligated to 2,3-butanediol (ii), resultantly providing 1. Hexane-2,5-dione (iii) might be condensed with glyoxylic acid (iv), followed by an aldol-type cyclization to form the cyclopentenone-containing metabolite 5, the hydrogenation of which perhaps produced the intermediate (vi). Furthermore, a C6-C2 unit obtained from either l-tyrosine or l-phenylalanine, presumably acted as a building block for the new compound assembly lines. l-tyrosine underwent decarboxylation and deamination, thereby providing 4-hydroxyphenylacetaldehyde (vii), and the further hydrogenation of the C-6/C-7 double bond formed 4-hydroxyethylphenol (viii) [32]. Analogously, l-phenylalanine might be converted to 2-phenylethanol (ix) [32]. Subsequently, 4-hydroxyphenylacetaldehyde (vii) could proceed aldol condensation with 5-HMF (i) to afford 2. The intermediates (ix) and (viii) were probably esterified with the substrate (vi) to yield 3 and 4, respectively. Overall, both cyclopentenone and furan moieties were most likely derived from sugar precursors, together with a C6-C2 unit elaborated from either l-tyrosine or l-phenylalanine, presumably constructing the framework of the new compounds.
Scheme 1.
Plausible biosynthetic pathways of 1–5.
2.3. Biological Activity
All the isolates (1–12) were evaluated for antibacterial activities against Gram-positive Staphylococcus aureus ATCC25923 and methicillin-resistant Staphylococcus aureus (MRSA) ATCC43300 as well as Gram-negative Escherichia coli ATCC25922. The MIC values for the compounds 1–12 were no less than 64 μg/mL against the tested pathogens, except for 1, 10 and 11. These three compounds selectively inhibited the growth of S. aureus with the same MIC value of 32 μg/mL. Meanwhile, Compounds 1–12 were also tested for antioxidant activities using DPPH radical scavenging assay. The results (Table 3) showed that compounds 1, 10 and 11 had moderate DPPH radical scavenging activities with IC50 values of 27.4 ± 7.4, 16.8 ± 4.3 and 61.7 ± 3.3 µg/mL, respectively, and the rest of the compounds did not show DPPH radical scavenging capacities (IC50 > 128 µg/mL).
Table 3.
DPPH radical scavenging activity of compounds 1–12 (IC50, µg/mL).
| Compound | IC50 | Compound | IC50 |
|---|---|---|---|
| 1 | 27.4 ± 7.4 | 8 | >128 |
| 2 | >128 | 9 | >128 |
| 3 | >128 | 10 | 16.8 ± 4.3 |
| 4 | >128 | 11 | 61.7 ± 3.3 |
| 5 | >128 | 12 | >128 |
| 6 | >128 | Ascorbic acid | 4.4 ± 0.4 |
| 7 | >128 |
3. Experimental Section
3.1. General Experimental Procedures
Optical rotations were measured on a Perkin-Elmer model 341 polarimeter (Perkin-Elmer Inc., Waltham, MA, USA). UV data were performed on a Hitachi U-3010 spectrophotometer (Hitachi Inc., Tokyo, Japan). IR (KBr) spectra were carried out on a Jasco FTIR-400 spectrometer (Jasco Inc., Tokyo, Japan). 1H, 13C, and 2D NMR spectra were obtained on a Varian 600 MHz spectrometer (Palo Alto, CA, USA). CD spectra were collected using a Jasco J-715 spectropolarimeter (Jasco Inc., Tokyo, Japan). HRESIMS and ESIMS data were acquired on an Agilent Technologies 6224 TOF LC-MS apparatus (Agilent Technologies Co., Ltd, Beijing, China) and a Waters Q-Tof micro YA019 mass spectrometer (Waters Corp., Milford, MA, USA). Preparative medium pressure liquid chromatography (MPLC) was carried out on Puriflash 450 Instruments (Interchim Company, Montlucon, France). Semi-preparative reversed-phase HPLC (RP-HPLC) was performed on a YMC-Pack Pro C18 RS column (5 μm, 250 × 10 mm id; YMC, Kyoto, Japan) with a Waters 1525 separation module equipped with a Waters 2998 Photodiode Array (PDA) detector. Column chromatography (CC) was performed on silica gel (200–300 mesh, Qingdao Haiyang Chemical Factory, Qingdao, China). Flash chromatographic column (ODS, 15 μm, Santai Technologies, Inc., Changzhou, China) was used for MPLC. Thin-Layer chromatography (TLC) analysis was performed on silica gel HSGF 254 plates and visualized by spraying with 10% anisaldehyde-H2SO4 reagent.
3.2. Fungal Material
The fungus H. koningii PF04 was isolated from the tissue of the sponge P. fusca collected from Yongxing Island in the South China Sea. The fungus was identified by its rDNA amplification and sequence analysis of the ITS region (GenBank accession no. FJ941853). A voucher strain was deposited at the School of Life Sciences and Biotechnology, Shanghai Jiao Tong University, Shanghai, China.
3.3. Fermentation
The strain was initially grown on PDA medium in a Petri dish for 7 days. A single colony was inoculated into seed medium (potato 200 g, dextrose 20 g, sea water 1000 mL) in 250 mL Erlenmeyer flasks on a rotatory shaker (180 rpm) at 25 °C for 48 h. Subsequently, the large scale fermentation was carried out in 50 × 250 mL Erlenmeyer flasks (80 g of rice and 120 mL of sea water), each of which was inoculated with seed medium (10 mL). The fungus PF04 was cultured under static conditions at 25 °C for 40 days.
3.4. Extraction and Isolation
The fermented material was extracted with acetone (3 × 5 L). The organic solvent was concentrated under reduced pressure and partitioned with EtOAc (1.5 L) and H2O (1.5 L) to yield the EtOAc extract (24.5 g). The extract was subjected to vacuum liquid chromatography (VLC) on silica gel column (6 × 15 cm, 200–300 mesh) using petroleum ether/EtOAc (20:1, 10:1, 8:1, 5:1, 4:1, 3:1, 2:1, 1:1, 0:1, v/v, gradient) to generate seven fractions (A–E). Fraction C (1.1 g) was further separated by MPLC with a gradient of MeOH/H2O (from 10% to 100% MeOH, 180 min) to afford ten subfractions (C1–C10) and the resulting subfraction C3 was further purified by RP-HPLC eluting 35% MeCN/H2O at a flow rate of 2 mL/min, to afford 8 (2.0 mg, tR = 26.6 min), 7 (3.4 mg, tR = 36.6 min), 9 (7.2 mg, tR = 46.4 min), and 6 (2.5 mg, tR = 66.3 min). Compounds 12 (3.0 mg, tR = 16.6 min), 3 (4.0 mg, tR = 43.4 min), and 11 (1.2 mg, tR = 45.6 min) were obtained by RP-HPLC (2.0 mL/min, 40% MeCN/H2O) from fraction C5. Fraction C7 was purified by RP-HPLC with an elution of 65% MeCN (2.0 mL/min) to yield 10 (8.6 mg, tR = 22.6 min). Fraction D (3.75 g) was separated by MPLC on to give eleven fractions (D1–D11). Fraction D4 were isolated by RP-HPLC eluting with 10% MeCN to afford 5 (2.0 mL/min, 2.5 mg, tR = 28.5 min). Fraction D10 was further purified by RP-HPLC eluting with 35% MeCN to obtain 1 (5.0 mg, tR = 36.6 min) and 4 (3.0 mg, tR = 38.6 min). Fraction D5 was further purified by RP-HPLC (35% MeCN in H2O, 2.0 mL/min) to afford 2 (3.0 mg, tR = 26.9 min).
Hypofuran A (1): colorless oil; [α] +2.00 (c 0.50, MeOH); UV (MeOH) (log ε) λmax 280 (4.68) nm; CD λmax (Δε) 218 (−22.80) nm; IR (KBr) νmax 3386, 3123, 2975, 2930, 2874, 2860, 1674, 1584, 1522, 1449, 1398, 1379, 1340, 1280, 1193, 1094, 1022, 989, 967, 888, 809, 777 cm−1; 1H and 13C NMR data, see Table 1 and Table 2; HRESIMS m/z 199.0965 [M + H]+ (calcd for C10H15O4, 199.0965).
Hypofuran B (2): yellow powder; UV (MeOH) (log ε) λmax 337 (4.61); IR (KBr) νmax 3404, 3020, 2959, 2934, 1717, 1614, 1516, 1447, 1435, 1396, 1358, 1310, 1262, 1224, 1173, 1087, 1044, 1009, 905, 831 cm−1; 1H and 13C NMR data, see Table 1 and Table 2; HRESIMS m/z 267.0634 [M + Na]+ (calcd for C14H12O4Na, 267.0633).
Hypocrenone A (3): colorless oil; UV (MeOH) (log ε) λmax 235 (4.68), 288 (3.88), 341 (3.72); IR (KBr) νmax 3441, 3064, 3029, 2959, 2926, 2860, 1735, 1707, 1676, 1616, 1496, 1435, 1339, 1282, 1240, 1174, 1090, 1053, 926, 846, 750, 701 cm−1; 1H and 13C NMR data, see Table 1 and Table 2; HRESIMS m/z 259.1329 [M + H]+ (calcd for C16H19O3, 259.1329).
Hypocrenone B (4): colorless oil; UV (MeOH) (log ε) λmax 232 (4.69), 278 (4.39), 341 (4.06); IR (KBr) νmax 3346, 3018, 2957, 2930, 2866, 1731, 1708, 1673, 1613, 1516, 1442, 1383, 1344, 1263, 1235, 1169, 1109, 1062, 1009, 833 cm−1; 1H and 13C NMR data, see Table 1 and Table 2; HRESIMS m/z 297.1104 [M + Na]+ (calcd for C16H18O4Na, 297.1103).
Hypocrenone C (5): white powder; UV (MeOH) (log ε) λmax 272 (4.57); IR (KBr) νmax 2925, 2858, 1714, 1654, 1625, 1569, 1513, 1434, 1398, 1356, 1294, 1266, 1232, 1102, 1045, 1018, 987, 928, 855, 825 cm−1; 1H and 13C NMR data, see Table 1 and Table 2; HRESIMS m/z 151.0394 [M − H]− (calcd for C8H7O3, 151.0395).
3.5. Antibacterial Activity Assay
The antibacterial activities were evaluated against three different bacteria (S. aureus ATCC25923, methicillin-resistant S. aureus (MRSA) ATCC43300 and E. coli ATCC25922) in 96-well microplates as described by Correa with some modifications [33]. The final concentrations of each compound in the wells were 256, 128, 64, 32, 16, 8, 4 and 2 μg/mL. Chloramphenicol was used as positive control. Each assay was carried out in triplicates.
3.6. DPPH Radical Scavenging Activity Assay
The DPPH free radical scavenging assay was performed by a modified method [34]. Briefly, the tested compounds were dissolved in methanol to serial concentrations. Each well in 96-well microplates containing 100 μL of sample solution and 100 μL of DPPH solution (methanol, 0.2 mM) was incubated at 37 °C for 30 min. Ascorbic acid was used as positive control. The blank control experiment was added methanol without any dissolved compound. The absorbance was measured at 517 nm on a microplate reader. DPPH scavenging rate (%) = (1 − absorbance of compound/absorbance of control) × 100. All experiments were taken in triplicates. The IC50 value (the concentration of a compound to scavenge 50% of DPPH radicals) was calculated from nonlinear regression analysis using the GraphPad Prism software 5.0 (GraphPad Software, San Diego, USA). Results are presented as the means ± SD.
3.7. Theoretical ECD Calculations
All calculations were performed with the Gaussian 09 program using various functionals (B3LYP, BH&HLYP, PBE0, CAM-B3LYP) and TZVP basis set. See Supplementary Information for more details of the DFT calculation.
4. Conclusions
Chemical investigation of the sponge-associated fungus Hypocrea koningii PF04 has resulted in the isolation and characterization of five new compounds, hypofurans A and B (1 and 2) and hypocrenones A–C (3–5), along with seven known secondary metabolites (6–12) encompassing two new natural products (10 and 11), representing a paradigm of chemical diversity. Structurally, hypofurans A was a furan derivative featuring a dioxolane ring where the absolute configuration was ascertained by comparing its experimental and calculated ECD spectra. With respect to the biogenetic relationships of all the new compounds, the plausible avenues furnishing them were postulated. In a small panel of antibacterial assays, compounds 1, 10 and 12 displayed modest inhibitory activities against S. aureus ATCC25923. In addition, compounds 1, 10 and 11 exhibited moderate DPPH radical scavenging activity. Collectively, this article showcased marine fungi served as new bioactive secondary metabolite producers.
Acknowledgments
This research was supported by the National Natural Science Fund for Distinguished Young Scholars of China (81225023), the National Natural Science Fund of China (No. 21172094, 41476121, 81302691, 41376155, 21372100, 81373321, 81302691, and U1301131). We are also grateful for the financial support of the National High Technology Research and Development Program of China (863 Projects, No. 2013AA092901 and 2013AA092902) and Public Science and Technology Research Funds Projects of Ocean (2015418024).
Supplementary Files
Author Contributions
All of the authors contributed to this work. L.-J.D. performed the experiments for the isolation, structure elucidation as well as biological evaluation. B.-B.G. carried out ECD Calculations. Z.-Y.L., Y.-X.L. isolated and provided the strain in this experiment. H.-B.Y., X.-J.L., and B.-N.H. advised and assisted Ding’s experiments. L.-J.D., B.-B.G., W.-H.J., W.Y., and W.-Z.T. shared the tasks of the manuscript preparation and revision. L.-J.D, Z.-Y.L., S.-H.X., and H.-W.L. conceived the research and supervised the work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Saleem M., Ali M.S., Hussain S., Jabbar A., Ashraf M., Lee Y.S. Marine natural products of fungal origin. Nat. Prod. Rep. 2007;24:1142–1152. doi: 10.1039/b607254m. [DOI] [PubMed] [Google Scholar]
- 2.Blunt J.W., Copp B.R., Keyzers R.A., Munro M.H., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2014;31:160–258. doi: 10.1039/c3np70117d. [DOI] [PubMed] [Google Scholar]
- 3.Rateb M.E., Ebel R. Secondary metabolites of fungi from marine habitats. Nat. Prod. Rep. 2011;28:290–344. doi: 10.1039/c0np00061b. [DOI] [PubMed] [Google Scholar]
- 4.Debbab A., Aly A.H., Proksch P. Bioactive secondary metabolites from endophytes and associated marine derived fungi. Fungal Divers. 2011;49:1–12. doi: 10.1007/s13225-011-0114-0. [DOI] [Google Scholar]
- 5.Bugni T.S., Ireland C.M. Marine-derived fungi: A chemically and biologically diverse group of microorganisms. Nat. Prod. Rep. 2004;21:143–163. doi: 10.1039/b301926h. [DOI] [PubMed] [Google Scholar]
- 6.Martins A., Vieira H., Gaspar H., Santos S. Marketed marine natural products in the pharmaceutical and cosmeceutical industries: Tips for success. Mar. Drugs. 2014;12:1066–1101. doi: 10.3390/md12021066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hentschel U., Piel J., Degnan S.M., Taylor M.W. Genomic insights into the marine sponge microbiome. Nat. Rev. Microbiol. 2012;10:641–654. doi: 10.1038/nrmicro2839. [DOI] [PubMed] [Google Scholar]
- 8.Ding B., Yin Y., Zhang F., Li Z. Recovery and phylogenetic diversity of culturable fungi associated with marine sponges Clathrina luteoculcitella and Holoxea sp. in the South China Sea. Mar. Biotechnol. 2011;13:713–721. doi: 10.1007/s10126-010-9333-8. [DOI] [PubMed] [Google Scholar]
- 9.Kopchinskiy A., Komon M., Kubicek C.P., Druzhinina I.S. TrichoBLAST: A multilocus database for Trichoderma and Hypocrea identifications. Mycol. Res. 2005;109:658–660. doi: 10.1017/S0953756205233397. [DOI] [PubMed] [Google Scholar]
- 10.Harman G.E., Howell C.R., Viterbo A., Chet I., Lorito M. Trichoderma species-opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004;2:43–56. doi: 10.1038/nrmicro797. [DOI] [PubMed] [Google Scholar]
- 11.Miao F.-P., Liang X.-R., Yin X.-L., Wang G., Ji N.-Y. Absolute configurations of unique harziane diterpenes from Trichoderma species. Org. Lett. 2012;14:3815–3817. doi: 10.1021/ol3014717. [DOI] [PubMed] [Google Scholar]
- 12.Berkaew P., Soonthornchareonnon N., Salasawadee K., Chanthaket R., Isaka M. Aurocitrin and related polyketide metabolites from the wood-decay fungus Hypocrea sp. BCC 14122. J. Nat. Prod. 2008;71:902–904. doi: 10.1021/np700740a. [DOI] [PubMed] [Google Scholar]
- 13.Ohkawa Y., Miki K., Suzuki T., Nishio K., Sugita T., Kinoshita K., Takahashi K., Koyama K. Antiangiogenic metabolites from a marine-derived fungus, Hypocrea vinosa. J. Nat. Prod. 2010;73:579–582. doi: 10.1021/np900698p. [DOI] [PubMed] [Google Scholar]
- 14.Wu B., Oesker V., Wiese J., Schmaljohann R., Imhoff J.F. Two new antibiotic pyridones produced by a marine fungus, Trichoderma sp. Strain MF106. Mar. Drugs. 2014;12:1208–1219. doi: 10.3390/md12031208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panizel I., Yarden O., Ilan M., Carmeli S. Eight new peptaibols from sponge-associated Trichoderma atroviride. Mar. Drugs. 2013;11:4937–4960. doi: 10.3390/md11124937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isaka M., Chinthanom P., Sappan M., Chanthaket R., Luangsa-ard J.J., Prabpai S., Kongsaeree P. Lanostane and hopane triterpenes from the entomopathogenic fungus Hypocrella sp. BCC 14524. J. Nat. Prod. 2011;74:2143–2150. doi: 10.1021/np200429b. [DOI] [PubMed] [Google Scholar]
- 17.Shen Y.-C., Prakash C.V.S., Kuo Y.-H. Three new furan derivatives and a new fatty acid from a Taiwanese marine sponge Plakortis simplex. J. Nat. Prod. 2001;64:324–327. doi: 10.1021/np000413d. [DOI] [PubMed] [Google Scholar]
- 18.Kikuchi H., Tsukitani Y., Iguchi K., Yamada Y. Clavulones, new type of prostanoids from the stolonifer Clavulariaviridis Quoy and Gaimard. Tetrahedron Lett. 1982;23:5171–5174. doi: 10.1016/S0040-4039(00)85788-3. [DOI] [Google Scholar]
- 19.Murray R.D.H., Jorge Z.D., Khan N.H., Shahjahan M., Quaisuddin M. Diosbulbin D and 8-epidiosbulbin E acetate, norclerodane diterpenoids from Dioscorea bulbifera tubers. Phytochemistry. 1984;23:623–625. doi: 10.1016/S0031-9422(00)80394-5. [DOI] [Google Scholar]
- 20.Sun K., Li Y., Guo L., Wang Y., Liu P., Zhu W. Indole diterpenoids and isocoumarin from the fungus, Aspergillus flavus, Isolated from the prawn, Penaeus vannamei. Mar. Drugs. 2014;12:3970–3981. doi: 10.3390/md12073970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bringmann G., Bruhn T., Maksimenka K., Hemberger Y. The assignment of absolute stereostructures through quantum chemical circular dichroism calculations. Eur. J. Org. Chem. 2009;2009:2717–2727. doi: 10.1002/ejoc.200801121. [DOI] [Google Scholar]
- 22.Sun S., Tian L., Wang Y., Wu H., Lu X., Pei Y. A novel natural product from the fermentation liquid of marine fungus Trichoderma atroviride G20–12. Asian J. Trad. Med. 2009;4:123–127. doi: 10.1080/10286020903193102. [DOI] [PubMed] [Google Scholar]
- 23.Amagata T., Usami Y., Minoura K., Ito T., Numata A. Cytotoxic substances produced by a fungal strain from a sponge: Physico-chemical properties and structures. J. Antibiot. 1998;51:33–40. doi: 10.7164/antibiotics.51.33. [DOI] [PubMed] [Google Scholar]
- 24.Gautschi J.T., Tenney K., Compton J., Crews P. Chemical investigations of a deep water marine-derived fungus: Simple amino acid derivatives from an Arthrinium sp. Nat. Prod. Commun. 2007;2:541–546. [Google Scholar]
- 25.Chen Q., Yang L., Zhang G., Wang F. Bioactivity-guided isolation of antiosteoporotic compounds from Ligustrum lucidum. Phytother. Res. 2013;27:973–979. doi: 10.1002/ptr.4820. [DOI] [PubMed] [Google Scholar]
- 26.Gernigon N., Al-Zoubi R.M., Hall D.G. Direct amidation of carboxylic acids catalyzed by ortho-iodo arylboronic acids: Catalyst optimization, scope, and preliminary mechanistic study supporting a peculiar halogen acceleration effect. J. Org. Chem. 2012;77:8386–8400. doi: 10.1021/jo3013258. [DOI] [PubMed] [Google Scholar]
- 27.Kang B., Fu Z., Hong S.H. Ruthenium-catalyzed redox-neutral and single-step amide synthesis from alcohol and nitrile with complete atom economy. J. Am. Chem. Soc. 2013;135:11704–11707. doi: 10.1021/ja404695t. [DOI] [PubMed] [Google Scholar]
- 28.Tarawneh A.H., León F., Radwan M.M., Rosa L.H., Cutler S.J. Secondary metabolites from the fungus Emericella nidulans. Nat. Prod. Commun. 2013;8:1285–1288. [PMC free article] [PubMed] [Google Scholar]
- 29.Ulbricht R.J., Northup S.J., Thomas J.A. A review of 5-hydroxymethylfurfural (HMF) in parenteral solutions. Toxicol. Sci. 1984;4:843–853. doi: 10.1093/toxsci/4.5.843. [DOI] [PubMed] [Google Scholar]
- 30.Celińska E., Grajek W. Biotechnological production of 2,3-butanediol-current state and prospects. Biotechnol. Adv. 2009;27:715–725. doi: 10.1016/j.biotechadv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Ferretti A., Flanagan V.P. Volatile constituents of whey powder subjected to accelerated browning. J. Dairy Sci. 1971;54:1764–1768. doi: 10.3168/jds.S0022-0302(71)86109-X. [DOI] [Google Scholar]
- 32.Kaminaga Y., Schnepp J., Peel G., Kish C.M., Ben-Nissan G., Weiss D., Orlova I., Lavie O., Rhodes D., Wood K. Plant phenylacetaldehyde synthase is a bifunctional homotetrameric enzyme that catalyzes phenylalanine decarboxylation and oxidation. J. Biol. Chem. 2006;281:23357–23366. doi: 10.1074/jbc.M602708200. [DOI] [PubMed] [Google Scholar]
- 33.Correa H., Aristizabal F., Duque C., Kerr R. Cytotoxic and antimicrobial activity of pseudopterosins and seco-pseudopterosins isolated from the octocoral Pseudopterogorgia elisabethae of San Andres and Providencia Islands (Southwest Caribbean Sea) Mar. Drugs. 2011;9:334–344. doi: 10.3390/md9030334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma K., Bao L., Han J., Jin T., Yang X., Zhao F., Li S., Song F., Liu M., Liu H. New benzoate derivatives and hirsutane type sesquiterpenoids with antimicrobial activity and cytotoxicity from the solid-state fermented rice by the medicinal mushroom Stereum hirsutum. Food Chem. 2014;143:239–245. doi: 10.1016/j.foodchem.2013.07.124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.