Abstract
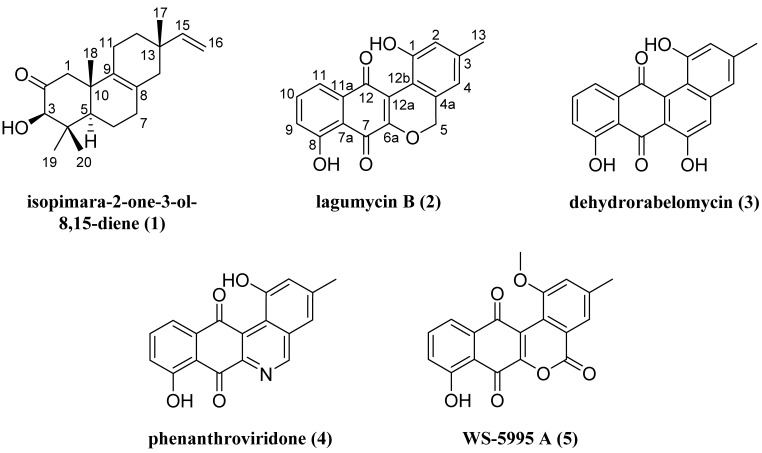
A screening of our actinomycete fraction library against the NCI-60 SKOV3 human tumor cell line led to the isolation of isopimara-2-one-3-ol-8,15-diene (1), lagumycin B (2), dehydrorabelomycin (3), phenanthroviridone (4), and WS-5995 A (5). These secondary metabolites were produced by a Micromonospora sp. isolated from sediment collected off the Cát Bà peninsula in the East Sea of Vietnam. Compound 1 is a novel Δ8,9-pimarane diterpene, representing one of approximately 20 actinomycete-produced diterpenes reported to date, while compound 2 is an angucycline antibiotic that has yet to receive formal characterization. The structures of 1 and 2 were elucidated by combined NMR and MS analysis and the absolute configuration of 1 was assigned by analysis of NOESY NMR and CD spectroscopic data. Compounds 2–5 exhibited varying degrees of cytotoxicity against a panel of cancerous and non-cancerous cell lines. Overall, this study highlights our collaborative efforts to discover novel biologically active molecules from the large, underexplored, and biodiversity-rich waters of Vietnam’s East Sea.
Keywords: actinomycete, marine, Micromonospora, ovarian cancer, Vietnam, diterpene
1. Introduction
Natural products and their synthetic derivatives are essential toward the discovery of drugs, accounting for greater than 74% of marketed small molecule therapies [1,2]. In particular, actinomycete bacteria have provided us with an abundance of bioactive compounds, including more than half of marketed antibiotics and many clinically useful anticancer drugs [2]. Of the different classes of molecules produced by actinomycetes, the terpenes are a structurally diverse group of natural products with approximately 60,000 members. A recent study unveiled that the capacity of actinomycetes to produce diterpenes has been significantly underestimated, where 25 of 100 randomly selected strains were identified as potential diterpene producers [3]. Despite this, only an estimated twenty diterpenes of actinomycete origin have been reported to date [4]. This is an exceedingly scarce fraction of the nearly 12,000 diterpenes described in the peer-reviewed literature, which are predominantly plant- and fungal-derived. In the current study, we report the rare isolation of a diterpene from an actinomycete strain.
Our program explores the potential of marine and fresh water-derived actinomycete secondary metabolites to serve as antibiotic and anticancer drug leads [5,6]. As part of this program, the University of Illinois at Chicago has partnered with the Vietnam Academy of Science and Technology to explore the potential of Vietnam’s East Sea to provide such leads. The East Sea in Vietnam covers an area of approximately three-million km2 and traces 3000 km of coastline. This stretch is comprised of a multitude of microenvironments covering depths from 200 m to 5000 m. The marine biodiversity within the East Sea is considered to be some of the most extensive in the world, yet it remains poorly understood and explored. In the current study, through an in vitro growth inhibition screening of our fraction library against the NCI-60 SKOV3 human tumor cell line, we identified an actinomycete isolated from sediment collected off the Cát Bà peninsula in the East Sea of Vietnam. From this strain we isolated and characterized the novel diterpene isopimara-2-one-3-ol-8,15-diene (1) and lagumycin B (2), an angucycline that has yet to receive full structural characterization in the peer-reviewed literature (Figure 1) [7]. In addition, we identified the previously reported angucyclines dehydrorabelomycin (3) [8,9], phenanthroviridone (4) [10,11], and WS-5995 A (5) [12,13]. Herein we present the structure elucidation and biological activity of these compounds.
Figure 1.
Structures of compounds 1–5.
2. Results and Discussion
2.1. Structure Elucidation of Isopimara-2-one-3-ol-8,15-diene (1) and Lagumycin B (2)
Following a series of chromatographic experiments, 1 was obtained as a colorless solid. Combined HRMS and NMR experiments allowed for the assignment of the molecular formula as C20H30O2, indicative of six degrees of unsaturation. 1H and 13C NMR data are shown in Table 1. In the 1H NMR spectrum, we observed evidence for four sp3 quaternary carbon-bound methyl groups, one vinyl group, an oxymethine geminal to a hydroxy group, a pair of geminal hydrogens adjacent to a carbonyl, and additional methylene and methine groups on 1 that constituted the remainder of a pimarane diterpene core skeleton.
Table 1.
1H and 13C NMR data (CDCl3) of 1.
| Position | 13C, Type a | 1H, Mult. (J, Hz) b,c |
|---|---|---|
| 1ax | 49.8, CH2 | 2.25, d (12.3) |
| 1eq | 2.58, d (12.3) | |
| 2 | 211.8,C | |
| 3 | 83.0, CH | 3.91, d (4.0) |
| 3-OH | 3.44, d (4.0) | |
| 4 | 45.3, C | |
| 5 | 50.3, CH | 1.78, m |
| 6 | 18.8, CH2 | 1.58, m |
| 1.80, m | ||
| 7 | 32.3, CH2 | 2.03, m |
| 8 | 126.4, C | |
| 9 | 134.3, C | |
| 10 | 43.8, C | |
| 11ax | 21.4, CH2 | 1.76, m |
| 11eq | 1.88, m | |
| 12eq | 34.8, CH2 | 1.33, m |
| 12ax | 1.53, m | |
| 13 | 35.3, C | |
| 14 | 41.6, CH2 | 1.76, m |
| 1.88, m | ||
| 15 | 145.9, CH | 5.72, dd (17.5, 10.7) |
| 16 | 111.2, CH2 | 4.85, dd (17.5, 1.4) |
| 4.92, dd (10.7, 1.4) | ||
| 17 | 28.3, CH3 | 0.98, s |
| 18 | 20.6, CH3 | 0.93, s |
| 19 | 29.3, CH3 | 1.21, s |
| 20 | 16.5, CH3 | 0.72, s |
a 226.2 MHz; b 600 MHz; c s = singlet; d = doublet; dd = doublet of doublets; m = multiplet.
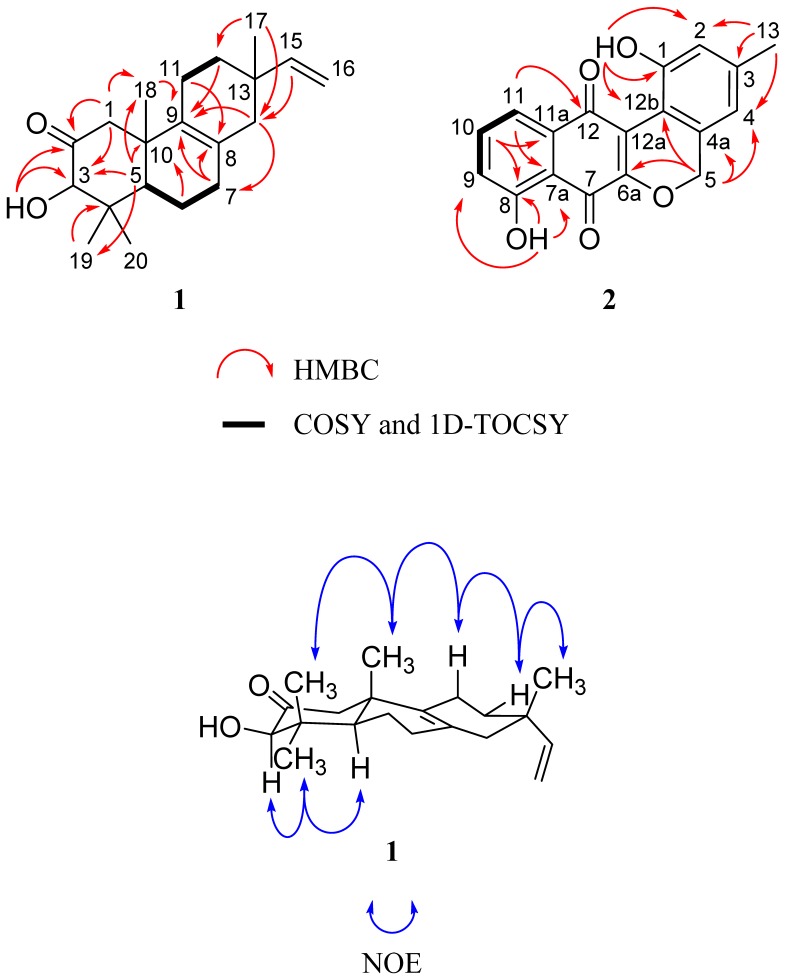
Analysis of 13C- DEPTQ and HSQC NMR data for 1 indicated the presence of one carbonyl carbon (δC 211.8, C-2), two fully-substituted endocyclic alkene carbons (δC 126.4, C-8; 134.3, C-9), one vinyl methylene carbon (δC 111.2, C-16), one vinyl methine carbon (δC 145.9, C-15), one oxymethine carbon (δC 83.0, C-3), and fourteen additional sp3 carbons. Given that the molecular formula of 1 afforded six degrees of unsaturation and the molecule contained one carbonyl, one vinyl, and one endocyclic alkene group, the remaining three degrees were satisfied by the tricyclic pimarane ring system. Key HMBC, COSY, and 1D-TOCSY correlations are given in Figure 2.
Figure 2.
Key 2D NMR correlations of 1 and 2.
The connectivity of the two distinct spin systems of C-11–C-12 and C-5–C-6–C-7 was determined by interpretation of the COSY NMR spectrum. Confirmation of these assignments was supported by a series of 1D-TOCSY experiments that exploited the solvent effect of C6D6 on lower frequency chemical shifts, which served to deconvolute the signal overlap observed when 1H NMR experiments were run in CDCl3 (Table S2 and Figures S5–S8) [14]. At this point in the elucidation process, we used HMBC correlations of methyl groups in 1 to piece together the tricyclic diterpene core. Geminal methyl groups were confirmed at C-4 based on HMBC correlations from H3-19 and H3-20 to C-3, C-4, and C-5. Signal H3-18 exhibited correlations to C-1, C-9, and C-10, suggesting a bond to the quaternary C-10. The connectivity of ring A was further established by observation of HMBC correlations from H2-1 to C-3, C-5, C-9, C-10, and C-18, suggesting that this methylene was between the C-2 carbonyl and C-10. Additionally, the oxymethine signal (H-3) showed HMBC couplings to C-2, C-4, C-19, and C-20, while the hydroxy signal (3-OH) displayed correlations to C-2 and C-3. This supported that H-3 and 3-OH were connected to the same carbon, which was positioned between the carbonyl at C-2 and the quaternary C-4. Couplings were also observed from H-5 to C-4 and C-10, solidifying the fusion of rings A and B. The connectivity of the C-5–C-6–C-7 spin system from ring A to the remainder of the core was established by observation of HMBC correlations from H2-7 to C-8 and C-9. Correlations from H2-14 to C-7, C-8, C-9, C-12, C-15, and C-17 placed it between the alkene C-8 and quaternary C-13. Resonance H3-17 showed HMBC correlations to C-12, C-13, C-14, and C-15, placing it at position 13. HMBC correlations between the vinyl methine hydrogen (H-15) and C-13, C-14, and C-17 supported this, while correlations from H2-16 to C-15 and C-13 provided further evidence for connectivity of the vinyl group to C-13. The remaining connectivity of ring C was satisfied by HMBC correlations from H2-11 to C-8, C-9, and C-13.
A 2D-NOESY NMR experiment was employed in order to determine the relative stereochemistry of 1, revealing correlations between H3-20 and H3-18, H3-18 and H-11ax, H-11ax and H-12eq, and H-12eq and H3-17; this suggested that these hydrogens projected out from the same face of the molecule. This orientation was supported by the observation of NOESY correlations between hydrogens H-3 and H3-19, and H3-19 and H-5, projecting from the opposite face of the molecule (Figure 2).
To identify the absolute configuration of centers in 1, a CD spectrum was acquired and analyzed (Figure S11). Compound 1 exhibited a positive Cotton effect at the n → π* carbonyl transition of 290 nm, indicating that the β-axial methyl group (C-18) was in the rear upper left octant when the octant rule was applied [15]. Thus, the absolute configuration of stereocenters in 1 was elucidated as 3R, 5R, 10S, and 13S, establishing the structure of 1 as shown.
Following a series of chromatographic steps, 2 was obtained as a pale orange solid. Combined HRMS and NMR experiments allowed for the assignment of the molecular formula as C18H12O5, indicative of thirteen degrees of unsaturation. The UV absorption profile (maxima of 204, 280, 310, and 410 nm) of 2 displayed characteristics of an extended aromatic ring system similar to the previously reported angucycline isolates 3, 4, and 5. 1H and 13C NMR data are shown in Table 2. A singlet resonance at δH 5.19 (2H, H-5) observed in the 1H NMR spectrum indicated the presence of oxygenated methylene protons. Two highly deshielded resonances at δH 10.45 (1H, 1-OH) and δH 11.70 (1H, 8-OH) gave evidence of two hydroxy peri-protons. The observation of three aromatic signals at δH 7.30 (1H, H-9), δH 7.81 (1H, H-11), and δH 7.66 (1H, H-10) indicated the presence of a tri-substituted aromatic ring. Additionally, two singlet resonances were observed in the aromatic region at δH 6.54 (1H, H-4) and δH 6.85 (1H, H-2), indicating the presence of isolated hydrogens on a separate, tetrasubstituted aromatic system. Finally, a singlet resonance at δH 2.33 (3H, H3-13) evidenced one aromate-bound methyl group.
Table 2.
1H and 13C NMR data (CDCl3) of 2.
| Position | 13C, Type a | 1H, Mult. (J, Hz) b,c |
|---|---|---|
| 1 | 154.5, C | |
| 1-OH | 10.45, s | |
| 2 | 121.1, CH | 6.85, s |
| 3 | 143.7, C | |
| 4 | 118.2, CH | 6.54, s |
| 4a | 130.3, C | |
| 5 | 70.7, CH2 | 5.19, s |
| 6a | 157.0, C | |
| 7 | 183.4, C | |
| 7a | 113.5, C | |
| 8 | 161.7, C | |
| 8-OH | 11.70, s | |
| 9 | 125.2, CH | 7.30, d (8.1) |
| 10 | 137.2, CH | 7.66, t (8.1) |
| 11 | 121.3, CH | 7.81, d (8.1) |
| 11a | 132.2, C | |
| 12 | 186.6, C | |
| 12a | 124.4, C | |
| 12b | 109.9, C | |
| 13 | 21.3, CH3 | 2.33, s |
a 226.2 MHz; b 600 MHz; c s = singlet; d = doublet; t = triplet.
Analysis of 13C-DEPTQ NMR data suggested the presence of two quinone carbonyls (δC 186.6, C-12; 183.4, C-7), two hydroxy-substituted aromatic carbons (161.7, C-8; 154.5, C-1), one oxygenated methylene carbon (δC 70.7, C-5), one methyl carbon (δC 21.3, H3-13), and an additional twelve aromatic carbons. Given that the molecular formula afforded thirteen degrees of unsaturation and the molecule contained two carbonyls and seven double bonds, the remaining degrees were satisfied by the fused angucycline ring system.
Interpretation of COSY NMR data defined one distinct aromatic spin system that connected C-9–C-10–C-11, supporting the aforementioned evidence for a 1,2,3-trisubstitued benzene moiety. A phenolic hydroxy peri-proton at δH 11.70 showed HMBC correlations to C-7a, C-8, and C-9, establishing hydroxy connectivity to C-8. An aromatic hydrogen at H-10 exhibited an HMBC correlation to C-11a, completing assignments for the 1,2,3-trisubstitued benzene. An HMBC correlation observed between aromatic hydrogen H-11 and the carbonyl C-12 was critical to establishment of the phenol position relative to the central quinone ring. The positions of the C-7 and C-12 relative to the oxygenated carbon at C-6a were based on comparison to previously reported 13C chemical shift values of similarly substituted paraquinones [16,17]. The 1-OH peri-proton showed HMBC correlations to C-1, C-12b, and C-2, providing evidence for connectivity of the hydroxy group to C-1. The singlet aromatic methyl signal at H3-13 exhibited correlations to C-3, C-2, and C-4 in the HMBC spectrum, placing it meta to the hydroxy group at C-1. HMBC correlations from the oxygenated methylene at H2-5 to C-4a, C-4, C-12b, and C-6a established connectivity of the naphthoquinone fragment to the phenol moiety. Key COSY and HMBC correlations are given in Figure 2. Thus, the structure of 2 is as shown with the name lagumycin B, following the structure and nomenclature established in the 1995 dissertation by Balk-Bindseil and Laatsch [7].
The characterization of known compounds 3–5 was based on comparison of 1H NMR and HRMS data with those appearing in literature (Figures S18–S23) [8,9,10,11,12,13].
2.2. Cytotoxicity Evaluation of 1–5
Following their identification, compounds 1–5 were assessed for cytotoxicity in two high-grade ovarian cancer cell lines, OVCAR4 and Kuramochi [18]. Potential for selective cytotoxicity was evaluated using two non-cancerous mouse cell lines representing the putative progenitor cells of ovarian cancer, murine ovarian surface epithelial (MOSE) and murine oviductal epithelial (MOE). Screening of 1–5 against this panel revealed 3 and 4 to be non-specifically cytotoxic, which was in accord with their previously reported bioactivities [8,10,11,19,20,21]. Compound 2 exhibited up to 14-fold enhanced cytotoxicity against non-cancerous murine cell lines MOSE and MOE, with LC50 values of 9.80 µM and 10.8 µM, respectively. Generally speaking, this para-quinone scaffold is not an attractive lead, as higher toxicity in non-tumorigenic cell lines confers serious overall toxicity. In contrast, compound 5 showed approximately seven-fold greater activity toward Kuramochi ovarian cancer cells with an LC50 of 18.6 μM (Table 3). Previous studies reported that 5 inhibits tumor cell proliferation and viability in L1210 lymphocytic leukemia cells in vitro with an IC50 range of 0.24–0.65 µM [22]. Compound 1 was not significantly active in all bioassays in the current study.
Table 3.
In vitro cytotoxicity of 1–5.
| Compound | Cytotoxicity LC50 (µM) a | |||
|---|---|---|---|---|
| Kuramochi | OVCAR4 | MOSE | MOE | |
| 1 | >33.1 | >33.1 | >33.1 | >33.1 |
| 2 | >32.5 | >32.5 | 9.80 | 10.8 |
| 3 | 6.72 | 11.0 | 3.50 | 28.5 |
| 4 | 1.11 | 4.82 | 2.85 | 6.20 |
| 5 | 18.6 | 127 | >149 | >149 |
a Doxorubicin was used as the positive control and was lethal at the lowest concentration tested (0.078 µM).
Compounds 2–5 are classified as angucyclines, which are among the largest class of type II PKS natural products and are known to exhibit a wide variety of biological activities [23]. Compound 2 was initially isolated from marine Streptomyces sp. B8245 and reported in a dissertation, but it has yet to receive formal characterization [7]. Both 3 and 4 have previously been identified as intermediates in kinamycin biosynthesis [24,25]; this product was not detected in our fermentation extracts. Similarly, compound 3 was proven to be a biosynthetic intermediate of jadomycin and gilvocarcin in a Streptomyces lividans strain [26], though these were also not observed in secondary metabolite fractions produced by our Micromonospora strain. In previous reports, 3 exhibited no detectable activity when screened against a panel of Gram-negative and Gram-positive bacteria [27], but inhibited a wide range of cancer cell lines at micromolar potency. Compound 4 was reported to have a variety of antibiotic and cytotoxic properties and our data support these non-specific biological activities [10,11,21]. Finally, compound 5 was first isolated from a Streptomyces auranticolor strain and was reported to exhibit anticoccidial activity against the apicomplexan poultry parasite Eimeria tenella, while exhibiting no significant biological activity when screened against a panel that included two fungal species, a human parasite, and several Gram-negative and Gram-positive bacteria [12,13]. This compound was also studied more recently for its aforementioned antileukemic activity, where it inhibited proliferation leading to apoptosis through DNA cleavage, blockage of nucleoside transport and inhibition of DNA, RNA, and protein synthesis [22]. To our knowledge, this study is the first to report cytotoxicity of compound 5 in carcinomas. In most cases, 4 is significantly more cytotoxic than 3 and 5. Presumably, this is due to the pyridine function in 4. Additionally, a contributing factor to reduced cytotoxicity may be the loss of aromaticity in the adjacent anthraquinone ring (5), though further biological testing and structure activity analyses are required to support this.
3. Experimental Section
3.1. General Experimental Procedures
Optical rotation measurement was performed in MeOH using a 10.0 cm cell on a PerkinElmer 241 polarimeter at 25 °C. The UV spectra were measured on a Shimadzu Pharma Spec UV-1700 spectrophotometer. NMR spectra were obtained on a Bruker 600 MHz DRX NMR spectrometer equipped with an inverse 5 mm TXI cryogenic probe with z-axis pfg and XWINNMR version 3.5 operating software, and a 900 (226.2) MHz Bruker AVANCE NMR spectrometer equipped with an inverse 5 mm TCI cryogenic probe with z-axis pfg and TopSpin version 1.3 operating software at the University of Illinois at Chicago Center for Structural Biology. Chemical shifts (δ) are given in ppm and coupling constants (J) are reported in Hz. 1H and 13C NMR resonances of 1 and 2 are reported in Table 1 and Table 2, respectively. 1H and 13C NMR chemical shifts were referenced to the CDCl3 (δH 7.26 ppm and δC 77.0 ppm, respectively) and C6D6 solvent signals (δH 7.16 ppm and δC 128.1 ppm, respectively). High resolution mass spectra were obtained on a Waters Synapt QToF mass spectrometer at the University of Illinois at Chicago Research Resources Center (UIC RRC). High-performance liquid chromatography (HPLC-UV) data were obtained using a Hewlett-Packard series 1100 system controller and pumps with a Model G1315A diode array detector (DAD) equipped with a reversed-phase C18 column (Phenomenex Luna, 100 mm × 4.6 mm, 5 μm; Torrance, CA, USA) at a flow rate of 0.5 mL·min−1. Semi-preparative HPLC scale separations were performed using a Hewlett Packard Series 1050 system with a Phenomenex Luna semi-preparative C18 column (250 mm × 10 mm, 5 μm) at a flow rate of 2.4 mL·min−1. Preparative HPLC scale separations were performed using a Waters LC4000 System equipped with a Phenomenex Luna preparative C18 column (250 mm × 21.2 mm, 5 μm) at a flow rate of 16 mL·min−1. Silica gel column chromatography was conducted using Bonna-Angela Technologies Cleanert® silica gel (Wilmington, DE, USA) with an average particle size of 40–60 μm and an average pore size of 60 Å.
3.2. Collection and Identification of Actinomycete Strain G039
Strain G039 was isolated from a sediment sample collected by PONAR at a depth of 22 m, from ca. 3.3 miles off the coast southeast of Cát Bà Peninsula in Vietnam (20°41°30°′ N, 107°05°58°′ E) in July of 2011. Strain G039 (GenBank accession number KR703606) shared 99% 16S rRNA gene sequence identity with the type strain Micromonospora haikouensis (GenBank accession number NR117442) [28].
3.3. Fermentation and Extraction
Strain G039 was grown in 38 × 1 L portions in Fernbach flasks containing high nutrient media components in artificial seawater (10 g starch, 4 g yeast extract, 2 g peptone, 1 g calcium carbonate, 100 mg potassium bromide, 40 mg iron sulfate, and 33.3 g Instant Ocean® (Blacksburg, VA, USA) per liter of dH2O) for 7 days at 21 °C while shaking at 220 rpm. Sterilized Amberlite XAD-16 resin (15 g·L−1) was added to each flask to absorb the extracellular secondary metabolites. The culture medium and resin were shaken for 10 h and filtered using cheesecloth to remove the resin. The resin, cell mass, and cheesecloth were extracted with acetone overnight, concentrated under vacuum, and partitioned between water and ethyl acetate. The organic layer was dried under vacuum to afford 3.52 g of extract.
3.4. Isolation and Characterization of Isopimara-2-one-3-ol-8,15-diene (1) and Lagumycin B (2)
The organic layer from the liquid-liquid partition was fractionated using silica gel flash column chromatography (23 cm × 3.5 cm, 85 g silica) eluting with a step gradient solvent system of 200 mL 100% hexanes (HEX), 200 mL 90% HEX:10% dichloromethane (DCM), 200 mL 70% HEX:30% DCM, 200 mL 100% DCM, 200 mL 70% DCM:30% ethyl acetate (EtOAc), 200 mL 70% EtOAc:30% DCM, 200 mL 100% ethyl acetate, 200 mL 95% EtOAc:5% 2-propanol, 200 mL 90% EtOAc:10% methanol (MeOH), 200 mL 50% EtOAc:50% MeOH, 400 mL 95% MeOH:5% ammonium hydroxide (NH4OH) to afford eleven fractions. Upon re-screening, it was determined that fraction 3 contained the bioactive constituents, thus, it was further separated using RP-C18 preparative HPLC (16 mL·min−1, gradient of 50% aqueous MeOH to 100% MeOH over 25 min, followed by an isocratic flow of 100% MeOH for 15 min), to afford 13 fractions. Fractions 6 and 11 exhibited biological activity so they were further separated.
Fraction 6 was further purified using RP-C18 semi-preparative HPLC (2.4 mL·min−1, gradient of 70% aqueous MeOH to 80% MeOH for 37.5 min, followed by an isocratic flow of 100% ACN for 10 min) to afford phenanthroviridone (4, tR 37.5 min, 0.66 mg, 0.019% yield), lagumycin B (2, tR 40.2 min, 0.9 mg, 0.026% yield) and WS-5995 A (5, tR 42.0 min, 1.2 mg, 0.034% yield).
Fraction 11 was further separated using RP-C18 semi-preparative HPLC (2.4 mL·min−1, gradient of 80% aqueous MeOH to 90% MeOH for 37.5 min, followed by an isocratic flow of 100% ACN for 10 min) to afford 8 fractions. Fraction 2 was further purified using two iterations of RP-C18 semi-preparative HPLC (2.4 mL·min−1, gradient of 80% aqueous MeOH to 90% MeOH for 37.5 min, followed by an isocratic flow of 100% ACN for 10 min) to afford dehydrorabelomycin (3, tR 26.7 min, 0.4 mg, 0.011% yield). Purification of compound 3 also afforded isopimara-2-one-3-ol-8,15-diene (1, tR 27.5 min, 0.9 mg, 0.026% yield), eluting as a neighboring peak observable at 220 nm.
Isopimara-2-one-3-ol-8,15-diene (1): Colorless solid (0.9 mg). = +20.7 (c = 0.00053, MeOH). UV (MeOH) λmax (log ε) = 204 (3.67) and shoulders at 228 (3.39) and 242 (3.30) nm. CD (c = 0.0031, MeOH): λmax (Δε) = 225 (+65.2), 250 (−4.4), 290 (+54.4) nm (Figure S11). 1H NMR (900 MHz, CDCl3) and 13C NMR (226.2 MHz, CDCl3), see Table 1. HRESI-QToF MS m/z 303.2338 [M + H]+ (calcd. for C20H31O2: 303.2319), and m/z 325.2141 [M + Na]+ (calcd. for C20H30O2Na: 325.2138).
Lagumycin B (2): Pale orange solid (0.9 mg). UV (MeOH) λmax (log ε) = broad absorptions with maxima at 204 (3.57), 280 (2.86), 310 (2.73) and 410 (2.27) nm. 1H NMR (600 MHz, CDCl3) and 13C NMR (226.2 MHz, CDCl3), see Table 2. HRESI-QToF MS m/z 309.0773 [M + H]+ (calcd. for C18H13O5: 309.0757).
3.5. OVCAR4, Kuramochi, MOSE, MOE, and Vero Cytotoxicity Assays
Human ovarian OVCAR4 and Kuramochi cancer cells were maintained in RPMI 1640 (11875-093, Life-technologies; Carlsbad, CA, USA) supplemented with 10% FBS (16000-044, Life-technologies; Carlsbad, CA, USA) and 1% penicillin/streptomycin. Non-cancerous murine ovarian surface epithelium (MOSE) and murine oviductal epithelium (MOE) cells were maintained as previously reported [29]. Concentration-response experiments were performed as previously described for 72 h [6].
4. Conclusions
Of the five compounds isolated in this study, two were previously unreported. Compound 1 represents a relatively rare example of the isolation of a diterpene from an actinomycete strain. Since a previous report that mined the genomes of a small population of actinomycete strains suggested that this phylum has a far greater capacity to produce diterpenes (25 of 100 strains) than is represented in the peer reviewed literature, it is possible that this discrepancy is a product of these pathways remaining silent under laboratory growth conditions, or of the methods used to isolate natural products from bacteria, namely bioassay-guided fractionation [3]. One possible explanation of the latter phenomenon is that specific classes of diterpenes are conserved within Actinobacteria, and that these classes are not biologically active in the typical barrage of assays used to discover small molecule leads, though in the absence of extensive genome mining and subsequent structure identification-bioactivity experiments, this will remain speculation.
The novel diterpene isopimara-2-one-3-ol-8,15-diene (1) was isolated along with the angucycline lagumycin B (2), and three other angucyclines from a Micromonospora sp. collected in the East Sea of Vietnam. The angucyclines exhibited varying degrees of cytotoxicity against a panel of cancerous and non-cancerous cell lines, with the notable exception of WS-5995 A (5), which showed enhanced cytotoxicity against the Kuramochi cell line when compared to non-tumorigenic cell lines. Though not significantly active in our bioassays, the discovery of isopimara-2-one-3-ol-8,15-diene contributes to the growing but still relatively small number of diterpenes identified from actinomycetes. This study highlights our collaborative efforts to discover novel biologically active molecules from the large, underexplored, and biodiversity-rich waters of Vietnam’s East Sea.
Acknowledgments
The authors would like to acknowledge the Institute of Marine Environment and Resources at VAST for assistance in sample collection; Ying Gao, Mark Sadek, Skylar Carlson, and Maryam Elfeki for fraction library development and preliminary bioactivity screening of strain G039; Baojie Wan, Sanghyun Cho, and Scott G. Franzblau of the Institute for Tuberculosis Research (ITR) at the University of Illinois at Chicago for initial screening of strain G039 against Mycobacterium tuberculosis; Dejan Nikolic of the University of Illinois at Chicago Research Resources Center (UIC RRC) for assistance in acquisition of mass spectra; David Lankin for assistance in acquiring 13C-DEPTQ NMR and 1D-TOCSY NMR data, and Benjamin E. Ramirez and Gerd Prena of the University of Illinois at Chicago Center for Structural Biology (UIC CSB) for assistance in acquiring remaining NMR data and CD spectroscopic data, respectively. This project was funded under Department of Defense grant #W81XWH-13-1-0171, UIC Campus Research Board grant, and American Cancer Society (Illinois Division) grant #254871. MWM is supported by the Office of the Director, National Institutes of Health (OD) and National Center for Complementary and Integrative Health (NCCIH) (5T32AT007533). The authors also acknowledge the Ministry of Science and Technology of Vietnam (MOST) and the Vietnam Academy of Science and Technology (VAST) for financial support (Grant No.: VAST.TĐ.ĐAB.04/13-15).
Supplementary Files
Author Contributions
Conceived and designed the experiments: MWM, EÓ, JB, VCP, BTM. Performed the experiments: MWM, EÓ, UT, VCP, BTM. Analyzed the data: MWM, EÓ, JB, BTM. Wrote the paper: MWM, EÓ, JB, BTM.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Fenical W., Jensen P.R. Developing a new resource for drug discovery: Marine actinomycete bacteria. Nat. Chem. Biol. 2006;2:666–673. doi: 10.1038/nchembio841. [DOI] [PubMed] [Google Scholar]
- 2.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie P., Ma M., Rateb M.E., Shaaban K.A., Yu Z., Huang S.-X., Zhao L.-X., Zhu X., Yan Y., Peterson R.M., et al. Biosynthetic potential-based strain prioritization for natural product discovery: A showcase for diterpenoid-producing actinomycetes. J. Nat. Prod. 2014;77:377–387. doi: 10.1021/np401063s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smanski M.J., Peterson R.M., Huang S.-X., Shen B. Bacterial diterpene synthases: New opportunities for mechanistic enzymology and engineered biosynthesis. Curr. Opin. Chem. Biol. 2012;16:132–141. doi: 10.1016/j.cbpa.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mullowney M.W., Hwang C.H., Newsome A.G., Wei X., Tanouye U., Wan B., Carlson S., Barranis N.J., Ó hAinmhire E., Chen W.-L., et al. Diaza-anthracene antibiotics from a freshwater-derived actinomycete with selective antibacterial activity toward Mycobacterium tuberculosis. ACS Infect. Dis. 2015;1:168–174. doi: 10.1021/acsinfecdis.5b00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mullowney M.W., Ó hAinmhire E., Shaikh A., Wei X., Tanouye U., Santarsiero B.D., Burdette J.E., Murphy B.T. Diazaquinomycins E–G, novel diaza-anthracene analogs from a marine-derived Streptomyces sp. Mar. Drugs. 2014;12:3574–3586. doi: 10.3390/md12063574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balk-Bindseil W., Laatsch H. Ph.D. Thesis. University of Göttingen; Göttingen, Germany: 1995. Neue Naturstoffe aus dem Screening Mariner Actinomyceten: Isolierung und Strukturaufklärung der Lagumycine und des Oceamycins. [Google Scholar]
- 8.Yamashita N., Takashi H., Kazuo S., Haruo S. 6-Hydroxytetrangulol, a new CPP32 protease inducer produced by Streptomyces sp. J. Antibiot. 1998;1:79–81. doi: 10.7164/antibiotics.51.79. [DOI] [PubMed] [Google Scholar]
- 9.Liu W.C., Parker L., Slusarchyk S., Greenwood G.L., Grahm S.F., Meyers E. Isolation, characterization, and structure of rabelomycin, a new antibiotic. J. Antibiot. 1970;23:437–441. doi: 10.7164/antibiotics.23.437. [DOI] [PubMed] [Google Scholar]
- 10.Fendrich G., Zimmermann W., Gruner J., Auden J.A.L. Phenanthridine Derivatives, Process for the Preparation Thereof, and Compositions Containing Them. 5,093. U.S. Patent. 1990 Mar 3;
- 11.Gore M.P., Gould S.J., Weller D.D. Synthesis of putative intermediates in the biosynthesis of the kinamycin antibiotics: Total synthesis of phenanthroviridin aglycon and related compounds. J. Org. Chem. 1992;57:2774–2783. doi: 10.1021/jo00036a005. [DOI] [Google Scholar]
- 12.Ikushima H., Iguchi E., Kohsaka M., Aoki H., Imanaka H. Streptomyces auranticolor sp. nov., a new anticoccidial antibiotics producer. J. Antibiot. 1980;33:1103–1106. doi: 10.7164/antibiotics.33.1103. [DOI] [PubMed] [Google Scholar]
- 13.Ikushima H., Okamoto M., Tanaka H., Ohe O., Kohsaka M., Aoki H., Imanaka H. New anticoccidial antibiotics, WS-5995 A and B: I. Isolation and characterization. J. Antibiot. 1980;33:1107–1113. doi: 10.7164/antibiotics.33.1107. [DOI] [PubMed] [Google Scholar]
- 14.Crews P., Rodríguez J., Jaspars M. Organic structure Analysis. 2nd ed. Oxford University Press; New York, NJ, USA: 2010. p. 48. [Google Scholar]
- 15.Moffitt W., Woodward R.B., Moscowitz A., Klyne W., Djerassi C. Structure and the optical rotatory dispersion of saturated ketones. J. Am. Chem. Soc. 1961;83:4013–4018. doi: 10.1021/ja01480a015. [DOI] [Google Scholar]
- 16.Dawson B.A., Girard M., Kindack D., Fillion J., Awang D.V.C. 13C NMR of lapachol and some related naphthoquinones. Magn. Reson. Chem. 1989;27:1176–1177. doi: 10.1002/mrc.1260271212. [DOI] [Google Scholar]
- 17.Yamashita M., Kaneko M., Tokuda H., Nishimura K., Kumeda Y., Iida A. Synthesis and evaluation of bioactive naphthoquinones from the Brazilian medicinal plant, Tabebuia avellanedae. Bioorg. Med. Chem. 2009;17:6286–6291. doi: 10.1016/j.bmc.2009.07.039. [DOI] [PubMed] [Google Scholar]
- 18.Domcke S., Sinha R., Levine D.A., Sander C., Schultz N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat. Commun. 2013;4:1–10. doi: 10.1038/ncomms3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lombó F., Abdelfattah M.S., Braña A.F., Salas J.A., Rohr J., Méndez C. Elucidation of oxygenation steps during oviedomycin biosynthesis and generation of derivatives with increased antitumor activity. ChemBioChem. 2009;10:296–303. doi: 10.1002/cbic.200800425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamashita N. Manufacture of Dehydrorabelomycin with Streptomyces Species and Use of the Antibiotic Agent as Antitumor Agent. JP 10201494 A. Aug 4, 1998.
- 21.Zhang W., Liu Z., Li S., Lu Y., Chen Y., Zhang H., Zhang G., Zhu Y., Zhang G., Zhang W., et al. Fluostatins I–K from the South China Sea-derived Micromonospora rosaria SCSIO N160. J. Nat. Prod. 2012;75:1937–1943. doi: 10.1021/np300505y. [DOI] [PubMed] [Google Scholar]
- 22.Perchellet E.M., Sperfslage B.J., Qabaja G., Jones G.B., Perchellet J.P. Quinone isomers of the WS-5995 antibiotics: Synthetic antitumor agents that inhibit macromolecule synthesis, block nucleoside transport, induce DNA fragmentation, and decrease the growth and viability of L1210 leukemic cells more effectively than ellagic acid and genistein in vitro. Anticancer Drugs. 2001;12:401–417. doi: 10.1097/00001813-200106000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Kharel M.K., Pahari P., Shepherd M.D., Tibrewal N., Nybo S.E., Shaaban K.A., Rohr J. Angucyclines: Biosynthesis, mode-of-action, new natural products, and synthesis. Nat. Prod. Rep. 2012;29:264–325. doi: 10.1039/C1NP00068C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seaton P.J., Gould S.J. Kinamycin biosynthesis. Derivation by excision of an acetate unit from a single-chain decaketide intermediate. J. Am. Chem. Soc. 1987;109:5282–5284. doi: 10.1021/ja00251a045. [DOI] [Google Scholar]
- 25.Cone M.C., Hassan A.M., Gore M.P., Gould S.J., Borders D.B., Alluri M.R. Detection of phenanthroviridin aglycon in a UV mutant of Streptomyces murayamaensis. J. Org. Chem. 1994;59:1923–1924. doi: 10.1021/jo00086a056. [DOI] [Google Scholar]
- 26.Tibrewal N., Pahari P., Wang G., Kharel M.K., Morris C., Downey T., Hou Y., Bugni T.S., Rohr J. Baeyer–Villiger C–C bond cleavage reaction in gilvocarcin and jadomycin biosynthesis. J. Am. Chem. Soc. 2012;134:18181–18184. doi: 10.1021/ja3081154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cone M.C., Seaton P.J., Halley K.A., Gould S.J. New products related to kinamycin from Streptomyces murayamaensis. I. Taxonomy, production, isolation, and biological properties. J. Antibiot. 1989;42:179–188. doi: 10.7164/antibiotics.42.179. [DOI] [PubMed] [Google Scholar]
- 28.Xie Q.Y., Qu Z., Lin H.P., Li L., Hong K. Micromonospora haikouensis sp. nov., isolated from mangrove soil. Antonie Leeuwenhoek. 2012;101:649–655. doi: 10.1007/s10482-011-9682-y. [DOI] [PubMed] [Google Scholar]
- 29.Ó hAinmhire E., Quartuccio S.M., Cheng W., Ahmed R.A., King S.M., Burdette J.E. Mutation or loss of p53 differentially modifies TGFβ action in ovarian cancer. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0089553. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.