Synopsis
Acute kidney injury in a clinical diagnosis guided by standard criteria based on changes in serum creatinine, urine output or both. Severity of acute kidney injury is determined by the magnitude of increase in serum creatinine or decrease in urine output. Patients manifesting both oliguria and azotemia and those in which these impairments are persistent are more likely to have worse disease and worse outcomes. Both short- and long-term outcomes are worse when patients have some stage of AKI by both criteria. Duration of AKI was also a significant predictor of long-term outcomes irrespective of severity. New biomarkers for AKI may substantially aid in the risk assessment and evaluation of patients at risk for AKI.
Keywords: acute kidney injury, renal replacement therapy, dialysis, clinical trials, biomarkers, renal recovery, mortality
Introduction
Acute kidney injury (AKI) is a clinical diagnosis. Already in ancient times it was noted that the failure to pass urine was lethal if untreated and might be due to either “an empty bladder” or an obstruction. Indeed, urinary catheters were used as early as 3000 BC. It was Galen who first established the kidneys as the source of urine and as organs that “filtered the blood”.1 Prior to this, it was generally believed that urine was made in the bladder from food and drink. Progress in the clinical assessment of renal function was quite limited from the time of Galen until the 18th century when urea was discovered—however, it would be more than a century later before increases is blood urea and serum creatinine would be used to quantify azotemia (“azote” is a very old name for nitrogen). Azotemia results from reductions in glomerular filtration rate (GFR) and together with oliguria (“small” urine) or anuria (no urine) form the cardinal features of kidney failure.
However, azotemia and oliguria represent not only disease but a normal response of the kidney to extracellular volume depletion or a decreased renal blood flow. Conversely, a “normal” urine output and GFR in the face of volume depletion could only be viewed as renal dysfunction. Thus, changes in urine output and GFR are neither necessary nor sufficient for the diagnosis of renal pathology.2 Still, they serve as the backbone for our existing diagnostic criteria.3
Criteria for AKI
Little progress was made in the understanding of AKI throughout the first two millennia AD. Although the term nephritis dates back to the 16th century it wasn’t really until the late 19th century that Bright described renal failure (Bright’s disease) and included both acute and chronic forms.4 A century later Bywaters and Beall described “acute renal failure” following crush injury.5 Throughout the remainder of the 20th century however acute renal failure had no widely accepted biochemical definition. As many a 60 different definitions littered the field. In 2004 the RIFLE criteria (Risk Injury Failure Loss End-stage renal disease) were put forth by the Acute Dialysis Quality Initiative.6 RIFLE included either change in serum creatinine or urine output as criteria recognizing that AKI could be non-oliguric but at the same time creatinine may not increase as rapidly as urine output falls and it is therefore better to have both criteria available. It was not understood at the time, the degree to which urine output and creatinine criteria interact—see “creatinine and urine output” below. One shortcoming of the RIFLE criteria was it’s application in patients with preexisting CKD. In patients with elevated baseline creatinines, the proportional changes required by RIFLE seemed excessive. For example, while a patient with a baseline creatinine of 1.0 mg/dl would fulfill criteria for AKI with an increase to 1.5, a patient with a baseline of 2.0 mg/dl would need to reach 3.o. Furthermore, the higher the baseline creatinine the longer the time required to reach a 50% increase. In essence it does not feel credible that a patient with a baseline of 2.6 mg/dl would need to increase to 3.9 and take 3 days to do it just to get to RIFLE-R! For this reason the AKI network (AKIN) proposed a modification to RIFLE that would also classify AKI when only a small increase in creatinine (0.3 mg/dl or greater) is observed in a short period of time (48 hours or less).7 Finally, in effort to harmonize RIFLE, AKIN and pRIFLE (a modification for pediatrics), Kidney Disease Improving Global Outcomes (KDIGO) proposed a unified version of these rules (table 1).3
Table 1.
Criteria and Staging for Acute Kidney Injury
| Stage | Serum creatinine | Urine output |
|---|---|---|
| 1 | 1.5–1.9 times baseline OR ≥0.3 mg/dl (>26.5 μmol/l) increase |
<0.5 ml/kg/h for 6–12 hours |
| 2 | 2.0–2.9 times baseline | <0.5 ml/kg/h for ≥12 hours |
| 3 | 3.0 times baseline OR Increase in serum creatinine to ≥4.0 mg/dl (353.6 μmol/l) OR Initiation of renal replacement therapy OR, In patients <18 years, decrease in eGFR to <35 ml/min per 1.73 m2 |
<0.3 ml/kg/h for ≥24 hours OR Anuria for ≥12 hours |
Minimum criteria for Acute Kidney Injury include an Increase in SCr by ≥0.3 mg/dl (>26.5 μmol/l) observed within 48 hours; or an Increase in SCr to ≥1.5 times baseline, which is known or presumed to have occurred within the prior 7 days; or Urine volume <0.5 ml/kg/h for 6 hours.
The purpose of standardized criteria for AKI
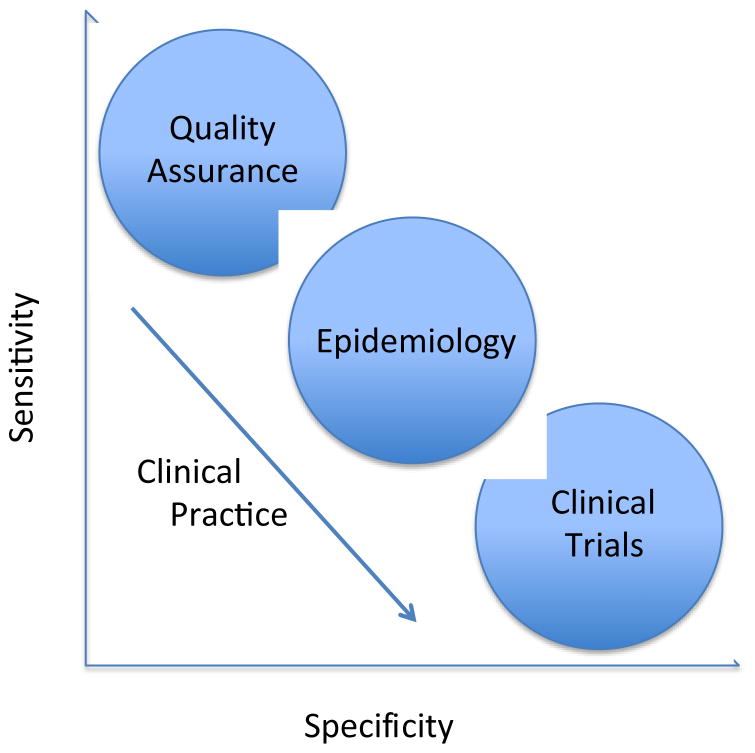
If AKI is clinical diagnosis, why are standard criteria desirable? The answer to this question comes in two parts. First, even though clinical judgment is required, a framework for the clinical diagnosis is needed. In general we don’t base our diagnoses on pure speculation, we consider a set of diagnostic features and use these to guide our judgment. These criteria are not “cook book” but they do serve as a frame of reference so that the average patient with the disease in question will fulfill the criteria put forth. Second, standardized criteria for diagnosis of AKI serve multiple purposes (Figure 1) and it is neither feasible nor desirable to have a clinical adjudication for all of these. For example in large epidemiologic studies it would not be practical to examine each patient. In these studies we accept diagnostic constructs as long they achieve reasonable sensitivity and specificity for the disease in question. However, diagnostic criteria, just like a diagnostic test, have test characteristics and specific “cut points” are chosen to maximize sensitivity, specificity or some degree of both. For quality improvement one might be interested in casting the widest possible net—maximizing sensitivity. If certain things can be done for all patients with “possible AKI” like avoiding unnecessary nephrotoxic medications we’d want to identify these patients. Conversely, for ascertaining outcomes in clinical trials we tend to favor specificity over sensitivity.
Figure 1.
Sensitivity/specificity tradeoffs for various applications of clinical definitions. For research and quality improvement, fixed thresholds are usually needed, while for clinical application diagnoses can be more flexible depending on the actions they elicit.
For clinical use, our preference for maximizing sensitivity or specificity depends on the clinical actions we intend to take. The decision to admit a patient with chest pain to the hospital is best supported by tests that are highly sensitive because our chief concern is about missing a myocardial infarction. Giving that same patient thrombolytic therapy calls for higher specificity. Importantly however, there is another feature that exists in clinical practice that clinical studies or quality improvement projects usual don’t enjoy—time. For clinical studies and for most quality improvement projects, a diagnosis is fixed. In other words a patient either has AKI or they don’t. For clinical purposes we have the luxury of provisional diagnoses. As more information becomes available we can and do change our diagnoses. Thus, it may be very appropriate to use a set of diagnostic criteria that are very sensitive for our initial evaluation and to require greater specificity for our final diagnosis. Over time we can include the patient’s clinical course and response to therapy in our assessment (Figure 2).
Figure 2.
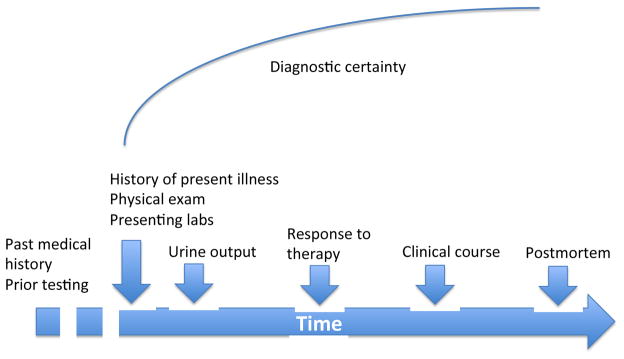
Diagnostic certainty. Diagnostic certainty is usually low at the outset of a clinical evaluation but improves with time as more information and diagnostic testing results become available.
Baseline renal function
A reference serum creatinine is used to apply the diagnostic criteria shown in table 1 and to stage patients. When determining the most suitable reference creatinine, the first consideration is the timing of the acute illness believed to be the cause of the AKI. For example, in a patient admitted on Friday with unstable angina who then has three daily serum creatinine measures, all essentially the same, before undergoing cardiac surgery on Monday, there is need to have a historical baseline in order to evaluate the serum creatinine on post-op day 1. In this example, the pre-operative serum creatinine is a suitable reference. By contrast, consider the patient who presents with a 2-day history of fever and cough and an elevated creatinine. Lets say the creatinine continues to increase after admission. If there is an increase of at least 0.3mg/dl over a period of 48hours or less (any 48 hour period not only the first 48 hours) the patient will meet criteria for AKI. However, let’s assume that our patient’s creatinine reaches 2.2 mg/dl. What stage is the AKI? Staging is important because the stage correlates with clinical outcomes like receipt of renal replacement therapy and mortality.8–10 A serum creatinine might mean stage 3 AKI, for example in a patient with a reference creatinine of 0.7 mg/dl, or it could be stage 1 in a patient with a reference creatinine of 1.4 mg/dl. Thus the reference is extremely important. The best reference creatinine for a patient presenting with AKI will not be the admission value since it is likely already abnormal (unless the patient presented only with oliguria). Therefore a baseline creatinine obtained prior to the current illness but still recent would be ideal. Unfortunately, patients rarely have the intuition to get their creatinine checked just before developing AKI. As such we are left with deciding between various less ideal baseline values or no value at all. Various studies have shown that even an old baseline (up to one year prior) is better than nothing.11,12 When multiple baseline values are available, particularly when no clear pattern is discernable, a median is probably the most representative.11 However, even here, judgment can be important. In a patient whose last 6 serum creatinines (one each month for the last 6 months), have been slowly rising, the most recent creatinine is probably the best reference. Similarly, some prior baselines might have been in the setting of prior episodes of AKI and it might be possible to select a more representative value out of the series of prior values if the history is known. In other words, the best reference creatinine is the one that the clinician believes is most representative of the patient’s premorbid renal function.
One of the most difficult clinical problems can be the assessment of a patient with abnormal renal function and an uncertain past medical history. The problem is not dissimilar to the cardiac patient with an abnormal but non-diagnostic ECG (e.g., non-specific T-wave abnormalities) and no prior ECG on record for comparison. Importantly, a patient presenting with previously unknown kidney disease might have chronic kidney disease (CKD), AKI, or both. In any case, the patient does have “something” and it is incumbent on the heath care system to determine what and to manage it appropriately. Ancillary tests like renal ultrasounds can be helpful to determine kidney size and examination of the urine can provide other clues. For example, a 40 y.o. white female presenting with an acute illness and a serum creatinine of 2.0 mg/dl who has normal kidney size on ultrasound and unremarkable urine sediment has AKI until proven otherwise. Conversely, a similar patient with small kidneys and albuminuria has some element of CKD—she may well have AKI on CKD however. Obviously clinical judgment is required in these cases and what might serve as a provisional diagnosis might well change over time.3
If a patient presents with a clinical history compatible with AKI and an abnormal creatinine with no evidence of CKD by history or exam, the best reference creatinine may be a derived one. Since a normal creatinine may vary by more than two-fold based on demographics (especially age, race and sex) it is not appropriate to use a single normal value for all patients. Instead, the patient’s demographics can be fitted into the an estimated GFR equations such as the Modification of Diet in Renal Disease (MDRD) equation using a GFR of 75mL/min/1.73m2 (Table 2).6 This approach has been validated in multiple studies one showing that it tends to overestimate the severity of AKI11 while another shows just the opposite.12 Differences are likely the result of the frequency of undetected CKD in the population.
Table 2.
Estimated baseline creatinine
| Age (years) | Black males (mg/dl [μmol/l]) | Other males (mg/dl [μmol/l]) | Black females (mg/dl [μmol/l]) | Other females (mg/dl [μmol/l]) |
|---|---|---|---|---|
| 20–24 | 1.5 (133) | 1.0 (88) | 1.3 (115) | 1.2 (106) |
| 25–29 | 1.5 (133) | 1.2 (106) | 1.1 (97) | 1.0 (88) |
| 30–39 | 1.4 (124) | 1.2 (106) | 1.1 (97) | 0.9 (80) |
| 40–54 | 1.3 (115) | 1.1 (97) | 1.0 (88) | 0.9 (80) |
| 55–65 | 1.3 (115) | 1.1 (97) | 1.0 (88) | 0.8 (71) |
| >65 | 1.2 (106) | 1.0 (88) | 0.9 (80) | 0.8 (71) |
Estimated glomerular filtration rate = 75 (ml/min per 1.73 m2) = 186 × (serum creatinine [SCr]) − 1.154 × (age) − 0.203 × (0.742 if female) × (1.210 if black) = exp (5.228 − 1.154 × In [SCr]) − 0.203 × In (age) − (0.299 if female) + (0.192 if black). From Bellomo et al. Crit Care 2004; 8:R204–R212, used with permission.
Serum creatinine and urine output
Older systems to classify AKI, and non-renal-specific organ failure scores like the Sepsis-related Organ Failure Assessment (SOFA)13 use fixed thresholds for serum creatinine (e.g. 2.0 mg/dl) to classify renal “organ failure”. This approach is not appropriate for AKI for two reasons. First, normal creatinine may vary by as much as 2-fold depending on age, race and sex (see Table 2). Second, a fixed creatinine does not distinguish between acute and chronic abnormalities. Thus, modern methods to quantify severity of AKI are based on relative azotemia, defined by an increase in serum creatinine, or oliguria defined by a decrease in urine output (Table 1). However, patients manifesting both oliguria and azotemia and those in which these impairments are persistent are more likely to have worse disease and therefore worse outcomes.14
Recently, using a large heterogeneous series of patients cared for over an eight-year period, we have examined the associations between AKI and both short-term and long-term outcomes as functions of serum creatinine and urine output criteria both alone and in combination.14 Our results demonstrated that despite relatively minor differences in baseline characteristics, patients meeting both serum creatinine and urine outout criteria for AKI have dramatically worse outcomes compared to patients who manifest AKI solely or predominantly by one criterion. Indeed as seen in Table 3, hospital mortality was <18% and RRT was <3.5% for the 11,897 (37.1%) patients manifesting AKI by only one parameter. Meanwhile, mortality reached 51.1% and RRT 55.3% for the 2,200 (6.9%) patients meeting stage 3 criteria by both serum creatinine and urine output. Even stage 3 criteria in one domain with stage 1 criteria in another was associated with >30% hospital mortality and >10% use of RRT.14 These results establish the absolute necessity for urine output assessment for staging of AKI. They also appear to contrast with prior work by Ralib and colleagues who found that the oliguria threshold of 0.5ml/kg/hr was not predictive of survival whereas 0.3ml/kg/hr was.15 These authors did not examine the effects of serum creatinine and urine output together and their sample size was only 725 patients, limiting their statistical power. Other investigators have found urine output to be a sensitive and early marker for AKI and to be associated with adverse outcomes in critically ill patients.16 Urine output is also affected by renal tubular function as evidenced by response to a “furosemide stress test”.17 Importantly, 1-year outcomes parallel hospital outcomes for the various combinations of serum creatinine and urine output criteria. Indeed the survival curves continue to separate for much of the year following an AKI event.14
Table 3.
Relationship between Urine Output and Serum Creatinine Criteria and Clinical Outcomes.
| KDIGO Stage | Urine Output Only
|
|||||
|---|---|---|---|---|---|---|
| No AKI | Stage 1 | Stage 2 | Stage 3 | Total | ||
|
| ||||||
| Serum Creatinine Only | No AKI | 8,179 | 3,158 | 5,421 | 440 | 17,198 |
| Dead | 4.3% | 5.3% | 7.9% | 17.7% | 5.9% | |
| RRT | 0.0% | 0.0% | 0.1% | 1.1% | 0.1% | |
|
| ||||||
| Stage 1 | 1,889 | 1,262 | 3,485 | 842 | 7,478 | |
| Dead | 8.0% | 11.3% | 13.0% | 32.1% | 13.6% | |
| RRT | 0.3% | 0.7% | 0.6% | 10.9% | 1.7% | |
|
| ||||||
| Stage 2 | 618 | 476 | 1,533 | 831 | 3,458 | |
| Dead | 11.3% | 23.9% | 21.5% | 44.2% | 25.5% | |
| RRT | 1.0% | 1.3% | 1.7% | 21.7% | 6.3% | |
|
| ||||||
| Stage 3 | 371 | 321 | 1,019 | 2,200 | 3,911 | |
| Dead | 11.6% | 38.6% | 28.0% | 51.1% | 40.3% | |
| RRT | 3.2% | 17.8% | 14.2% | 55.3% | 36.6% | |
|
| ||||||
| Total | 11,057 | 5,217 | 11,458 | 4,313 | 32,045 | |
| Dead | 5.6% | 10.5% | 13.0% | 42.6% | 14.0% | |
| RRT | 0.3% | 1.4% | 1.7% | 34.6% | 5.6% | |
Shown are the number of patients, % hospital mortality, and % renal replacement therapy (RRT) for patients by maximum acute kidney injury (AKI) criteria (UO, SC, or both). Colors denote similar outcome patterns.
Kellum JA, Sileanu FE, Murugan R, et al. Classifying AKI by Urine Output versus Serum Creatinine Level J Am Soc Nephrol 2015 Jan 7 Epub; with permission.
In addition, isolated oliguria (no creatinine criteria present) is surprisingly frequent and appears to be associated with a long-term hazard. Stage 2 and 3 AKI by urine output criteria alone are associated with decreased 1-year survival. Several studies have emphasized the importance of fluid overload both in terms of its effect on clinical outcomes18–20 and on serum creatinine measurements.21 It is likely that most oliguric patients are volume overloaded and it is reasonable to deduce that this represents an adverse effect on survival. It is also conceivable that volume overload masks some degree of azotemia and thus profound oliguria is not just an early indicator of AKI but may be the only indicator.
It is also clear that AKI persistence has a substantial influence on outcome. For example, we found that 4 days at stage 3 AKI results in an approximately 30% rate of death or dialysis at 1 year while it requires more than a week at stage 1 to incur the same hazard.14 Similarly, Coca et al. demonstrated that duration of AKI based on creatinine following surgery was independently associated with subsequent outcome.22 Thus, risk for death or dialysis following AKI is greatest for patients that meet both serum creatinine and urine output criteria and for those in whom the abnormalities persist longer. However even a brief episode of isolated oliguria without subsequent azotemia appears to be associated with decreased 1-year survival.
Apart from clinical use, trials of diagnostics and therapeutics for AKI can be challenging for several reasons.23–25 The selection of short-term AKI end-points requires an understanding of the relationship between AKI severity and duration and long-term outcomes. In the critically ill, AKI is very common—upwards of 75% of patients manifesting the syndrome when defined by the full KDIGO criteria.26 However, spontaneous resolution (or rapid response to treatment) occurs in some patients. Such patients may be less appropriate for enrollment in clinical trials of novel therapeutics. Similarly, for various clinical trial applications, it may be important to select endpoints that are more closely tied to clinical outcomes.
Novel biomarkers
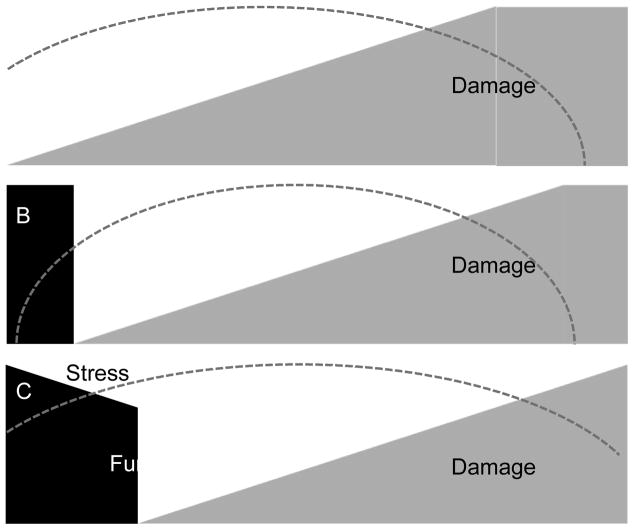
Over the last decade a number of novel biomarkers have been evaluated for their capacity to detect kidney damage and predict the development of AKI (for a recent review see the chapter from Chen and Koyner in this volume of Clinics27). Most novel markers were developed for their capacity to detect damage and as such they can provide additional insight into AKI, complementary to functional tests such as serum creatinine and urine output.28 Note, that the relationship between decreasing function and increasing damage is not as straightforward as might be assumed (Figure 3). The characteristic pattern whereby damage proceeds loss of function (panel A) may be seen in some cases of AKI and affords an opportunity to detect “subclinical” AKI before function start to fall. The problem is that other patterns also occur. For example functional decline may start to occur right along side damage (panel B) or in some cases function may start to decline even before damage (panel C). This makes damage markers hard to use to forecast AKI. However, other markers might actually measure “stress” occurring at the cellular level before damage or loss of function.
Figure 3.
Various clinical scenarios of Acute Kidney Injury based in function, damage and stress. The change in kidney function (e.g., glomerular filtration rate) is shown in black while damage is shown in grey. Panel A depicts the “classic” case where damage increases and is followed by a decline in function only after some time (time shown on the x-axis). Panels B and C show alternate scenarios where function may change coincidental to or even before damage. The dashed arc represents renal cell stress.
In 2013 we reported the results of a prospective, observational, international investigation (Sapphire study) of tissue inhibitor of metalloproteinases–2 (TIMP-2) and insulin-like growth factor binding protein 7 (IGFBP7) in a heterogeneous group of critically ill patients.29 In the validation phase we enrolled 744 adults without evidence of AKI. The primary endpoint was moderate-severe AKI (KDIGO stage 2–3)3 within 12 hours of sample. The area under the receiver operating characteristic curve (AUC) was 0.80 for [TIMP-2]•[IGFBP7] and these markers were significantly superior to all previously described markers of AKI (p<0.002) including neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule (KIM)-1, none of which achieved an AUC > 0.72.29 Two subsequent studies, Opal30 and Topaz,31 using the same endpoint in new cohorts confirmed the test characteristics for predicting AKI.
One of the reasons that [TIMP-2]•[IGFBP7] works for predicting AKI is that the markers relate to a cellular defense mechanism known as cell-cycle arrest. Each phase of the cell cycle has a specific function that is required for appropriate cell proliferation. Quiescent cells are normally in G0. In order for cells to divide and begin the process of repair, they must enter and exit each phase of the cell cycle on schedule.32–34 If the cell exits a phase too soon, or stays in a phase too long, the normal repair and recovery process can become maladaptive.33 For instance, if epithelial cells remain arrested in G1 or G2, it favors a hypertrophic and fibrotic phenotype.32,34 Conversely, exit from cell cycle in late G1 leads to apoptosis.35 Cyclins and cyclin-dependent kinases, cyclin-dependent kinase inhibitors control each phase of the cell cycle.33 The cell uses cell-cycle arrest as a protective mechanism to avoid cell-division when potentially damaged.33,36 By initiating cell-cycle arrest, cells can thus avoid cell division during stress and injury, which is protective. However, if the cells do not re-initiate the cell-cycle and remain arrested at G1 or G2 (or possibly other phases of cell cycle), a fibrotic phenotype can ensue. By detecting cell-cycle arrest markers in the urine we may actually be detecting cell stress (depicted as the dashed lines in figure 3). This stress may or may not lead to damage and functional decline but it is the earliest possible point the process can be detected.
Diagnostic uncertainty and future classification systems
No diagnostic criteria based on serum creatinine and urine output will ever be perfect. Some patients will meet these criteria and not have AKI. For example, a vegetarian with a baseline serum creatinine of 0.4 mg/dl who develops a creatinine of 0.6 after a large protein load may not have any kidney abnormality at all. A patient with short-term dehydration will experience oliguria and yet kidney injury is unlikely in absence of underlying disease or acute nephrotoxic exposures (e.g., myoglobin, radiocontrast). A fairly common scenario in hospitalized patients is to see the serum creatinine fall sharply on the first hospital day. Then over the next 48 hours the creatinine rebounds to baseline value. The increase in serum creatinine over this 48 hours may reach 0.3mg/dl and thus meet AKI criteria. AKI should not be diagnosed in a vacuum and clinical context should always be considered. Conversely, some patients with AKI may not fulfill the diagnostic criteria. A patient receiving large volume resuscitation or massive transfusion may not achieve the changes in serum creatinine especially early on. Similarly patients receiving large amounts of diuretics may maintain urine output at least for a time. Clinical judgment works both ways and should always be exercised in evaluating a patient with suspected AKI. Importantly, some investigators have shown that small absolute changes in serum creatinine in patients with low baseline creatinine are less significant than larger changes in the same relative magnitude in patients with high baseline levels.37 However, our study in critically ill patients found that in those with very low baseline creatinine, AKI is nevertheless associated with adverse long-term outcomes.14
Novel biomarkers of kidney damage or stress will add information to help clinicians arrive a prompt and accurate diagnoses. In the future we may well talk not just about the stage of AKI but the associated biomarker pattern. Patients with the same stage of AKI but with very different urinary [TIMP-2]•[IGFBP7] levels have different long-term outcomes (death or dialysis).38 In the future we may well speak of “stress positive/damage negative” AKI the way we currently speak of non-ST elevation myocardial infarction or “BRCA1 positive breast cancer”.26,39
Key Points.
The criteria for acute kidney are based on changes in serum creatinine and urine output. Standardized criteria such as KDIGO criteria allow for uniform implementation of guidelines and reliable estimates of incidence and outcomes.
However, acute kidney injury (AKI) remains a clinical diagnosis and clinical judgment is necessary to apply diagnostic criteria and to evaluate the changing clinical status of the patient.
Baseline renal function is also based on clinical judgment and is best determined by prior serum creatinine measurements; when none are available estimating equations can be used with caution.
Both serum creatinine and urine output provide independent and complementary information on renal function. Novel biomarkers can provide information on kidney damage and the latest markers can assess kidney stress.
In the near future, function, damage and stress may all be used to define AKI.
Acknowledgments
This work was supported in part by R01DK070910 and R01DK083961 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
Footnotes
Disclosure: None
The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of NIDDK or NIH.
Competing Financial Interest
JAK has received grant support and consulting fees from Alere, Astute Medical, Bard and numerous companies developing treatments for Acute Kidney Injury.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Diamandopoulos A. Twelve centuries of nephrological writings in the Graeco-Roman world of the Eastern Mediterranean (from Hippocrates to Aetius Amidanus) Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 1999;14 (Suppl 2):2–9. doi: 10.1093/ndt/14.suppl_2.2. [DOI] [PubMed] [Google Scholar]
- 2.Kellum JA. Acute kidney injury. Crit Care Med. 2008;36:S141–5. doi: 10.1097/CCM.0b013e318168c4a4. [DOI] [PubMed] [Google Scholar]
- 3.KDIGO AKIWG: Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline for Acute Kidney Injury. Kidney inter., Suppl 2012;2 : 1–138. 1–141, 2012
- 4.Eknoyan G. Emergence of the concept of acute kidney injury. Advances in Chronic Kidney Disease. 2008;15:308–313. doi: 10.1053/j.ackd.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Bywaters EG, Beall D. Crush Injuries with Impairment of Renal Function. Br Med J. 1941;1:427–432. doi: 10.1136/bmj.1.4185.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute Dialysis Quality Initiative workgroup: Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group; 2004. pp. R204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. 2007. p. R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoste EAJ, Clermont G, Kersten A, Venkataraman R, Angus DC, De Bacquer D, Kellum JA. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73. doi: 10.1186/cc4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients*. Crit Care Med. 2006;34:1913–1917. doi: 10.1097/01.CCM.0000224227.70642.4F. [DOI] [PubMed] [Google Scholar]
- 10.Sileanu FE, Murugan R, Lucko N, Clermont G, Kane-Gill SL, Handler SM, Kellum JA. AKI in Low-Risk versus High-Risk Patients in Intensive Care. Clin J Am Soc Nephrol [Internet] 2014 doi: 10.2215/CJN.03200314. Available from: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=25424992&retmode=ref&cmd=prlinks. [DOI] [PMC free article] [PubMed]
- 11.Siew ED, Matheny ME, Ikizler TA, Lewis JB, Miller RA, Waitman LR, Go AS, Parikh CR, Peterson JF. Commonly used surrogates for baseline renal function affect the classification and prognosis of acute kidney injury. Kidney Int. 2010;77:536–542. doi: 10.1038/ki.2009.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Závada J, Hoste E, Cartin-Ceba R, Calzavacca P, Gajic O, Clermont G, Bellomo R, Kellum JA AKI6 investigators. A comparison of three methods to estimate baseline creatinine for RIFLE classification. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2010;25:3911–3918. doi: 10.1093/ndt/gfp766. [DOI] [PubMed] [Google Scholar]
- 13.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. 1996. pp. 707–710. [DOI] [PubMed] [Google Scholar]
- 14.Kellum JA, Sileanu FE, Murugan R, Lucko N, Shaw AD, Clermont G. Classifying AKI by Urine Output versus Serum Creatinine Level. Journal of the American Society of Nephrology. 2015 doi: 10.1681/ASN.2014070724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ralib AM, Pickering JW, Shaw GM, Endre ZH. The urine output definition of acute kidney injury is too liberal. Crit Care. 2013;17:R112. doi: 10.1186/cc12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macedo E, Malhotra R, Bouchard J, Wynn SK, Mehta RL. Oliguria is an early predictor of higher mortality in critically ill patients. Kidney Int. 2011;80:760–767. doi: 10.1038/ki.2011.150. [DOI] [PubMed] [Google Scholar]
- 17.Chawla LS, Davison DL, Brasha-Mitchell E, Koyner JL, Arthur JM, Shaw AD, Tumlin JA, Trevino SA, Kimmel PL, Seneff MG. Development and standardization of a furosemide stress test to predict the severity of acute kidney injury. Crit Care. 2013;17:R207. doi: 10.1186/cc13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutherland SM, Zappitelli M, Alexander SR, Chua AN, Brophy PD, Bunchman TE, Hackbarth R, Somers MJG, Baum M, Symons JM, Flores FX, Benfield M, Askenazi D, Chand D, Fortenberry JD, Mahan JD, McBryde K, Blowey D, Goldstein SL. Fluid Overload and Mortality in Children Receiving Continuous Renal Replacement Therapy: The Prospective Pediatric Continuous Renal Replacement Therapy Registry. Am J Kidney Dis. 2010;55:316–325. doi: 10.1053/j.ajkd.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 19.Bouchard J, Soroko SB, Chertow GM, Himmelfarb J, Ikizler TA, Paganini EP, Mehta RL. Program to Improve Care in Acute Renal Disease (PICARD) Study Group: Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76:422–427. doi: 10.1038/ki.2009.159. [DOI] [PubMed] [Google Scholar]
- 20.Boyd JH, Forbes J, Nakada T-A, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39:259–265. doi: 10.1097/CCM.0b013e3181feeb15. [DOI] [PubMed] [Google Scholar]
- 21.Liu KD, Thompson BT, Ancukiewicz M, Steingrub JS, Douglas IS, Matthay MA, Wright P, Peterson MW, Rock P, Hyzy RC, Anzueto A, Truwit JD National Institutes of Health National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network. Acute kidney injury in patients with acute lung injury: impact of fluid accumulation on classification of acute kidney injury and associated outcomes. Crit Care Med. 2011;39:2665–2671. doi: 10.1097/CCM.0b013e318228234b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coca SG, King JT, Rosenthal RA, Perkal MF, Parikh CR. The duration of postoperative acute kidney injury is an additional parameter predicting long-term survival in diabetic veterans. Kidney Int. 2010;78:926–933. doi: 10.1038/ki.2010.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palevsky PM, Molitoris BA, Okusa MD, Levin A, Waikar SS, Wald R, Chertow GM, Murray PT, Parikh CR, Shaw AD, Go AS, Faubel SG, Kellum JA, Chinchilli VM, Liu KD, Cheung AK, Weisbord SD, Chawla LS, Kaufman JS, Devarajan P, Toto RM, Hsu C-Y, Greene T, Mehta RL, Stokes JB, Thompson AM, Thompson BT, Westenfelder CS, Tumlin JA, Warnock DG, Shah SV, Xie Y, Duggan EG, Kimmel PL, Star RA. Design of clinical trials in acute kidney injury: report from an NIDDK workshop on trial methodology. 2012:844–850. doi: 10.2215/CJN.12791211. [DOI] [PubMed] [Google Scholar]
- 24.Okusa MD, Molitoris BA, Palevsky PM, Chinchilli VM, Liu KD, Cheung AK, Weisbord SD, Faubel S, Kellum JA, Wald R, Chertow GM, Levin A, Waikar SS, Murray PT, Parikh CR, Shaw AD, Go AS, Chawla LS, Kaufman JS, Devarajan P, Toto RM, Hsu C-Y, Greene TH, Mehta RL, Stokes JB, Thompson AM, Thompson BT, Westenfelder CS, Tumlin JA, Warnock DG, Shah SV, Xie Y, Duggan EG, Kimmel PL, Star RA. Design of clinical trials in acute kidney injury: a report from an NIDDK workshop--prevention trials. 2012:851–855. doi: 10.2215/CJN.12811211. [DOI] [PubMed] [Google Scholar]
- 25.Molitoris BA, Okusa MD, Palevsky PM, Chawla LS, Kaufman JS, Devarajan P, Toto RM, Hsu C-Y, Greene TH, Faubel SG, Kellum JA, Wald R, Chertow GM, Levin A, Waikar SS, Murray PT, Parikh CR, Shaw AD, Go AS, Chinchilli VM, Liu KD, Cheung AK, Weisbord SD, Mehta RL, Stokes JB, Thompson AM, Thompson BT, Westenfelder CS, Tumlin JA, Warnock DG, Shah SV, Xie Y, Duggan EG, Kimmel PL, Star RA. Design of clinical trials in AKI: a report from an NIDDK workshop. Trials of patients with sepsis and in selected hospital settings. 2012:856–860. doi: 10.2215/CJN.12821211. [DOI] [PubMed] [Google Scholar]
- 26.Murugan R, Kellum JA. Acute kidney injury: what’s the prognosis? Nat Rev Nephrol. 2011;7:209–217. doi: 10.1038/nrneph.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Editor Please Provide.
- 28.Murray PT, Mehta RL, Shaw A, Ronco C, Endre Z, Kellum JA, Chawla LS, Cruz D, Ince C, Okusa MD. Potential use of biomarkers in acute kidney injury: report and summary of recommendations from the 10th Acute Dialysis Quality Initiative consensus conference. Kidney Int. 2014;85:513–521. doi: 10.1038/ki.2013.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, Bihorac A, Birkhahn R, Cely CM, Chawla LS, Davison DL, Feldkamp T, Forni LG, Gong MN, Gunnerson KJ, Haase M, Hackett J, Honore PM, Hoste EA, Joannes-Boyau O, Joannidis M, Kim P, Koyner JL, Laskowitz DT, Lissauer ME, Marx G, McCullough PA, Mullaney S, Ostermann M, Rimmele T, Shapiro NI, Shaw AD, Shi J, Sprague AM, Vincent J-L, Vinsonneau C, Wagner L, Walker MG, Wilkerson RG, Zacharowski K, Kellum JA. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17:R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoste EAJ, McCullough PA, Kashani K, Chawla LS, Joannidis M, Shaw AD, Feldkamp T, Uettwiller-Geiger DL, McCarthy P, Shi J, Walker MG, Kellum JA for the Sapphire Investigators. Derivation and validation of cutoffs for clinical use of cell cycle arrest biomarkers. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2014;29:2054–2061. doi: 10.1093/ndt/gfu292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bihorac A, Chawla LS, Shaw AD, Al-Khafaji A, Davison DL, Demuth GE, Fitzgerald R, Gong MN, Graham DD, Gunnerson K, Heung M, Jortani S, Kleerup E, Koyner JL, Krell K, Letourneau J, Lissauer M, Miner J, Nguyen HB, Ortega LM, Self WH, Sellman R, Shi J, Straseski J, Szalados JE, Wilber ST, Walker MG, Wilson J, Wunderink R, Zimmerman J, Kellum JA. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med. 2014;189:932–939. doi: 10.1164/rccm.201401-0077OC. [DOI] [PubMed] [Google Scholar]
- 32.Preisig PA, Franch HA. Renal epithelial cell hyperplasia and hypertrophy. Semin Nephrol. 1995;15:327–340. [PubMed] [Google Scholar]
- 33.Shankland SJ. Cell cycle regulatory proteins in glomerular disease. Kidney Int. 1999;56:1208–1215. doi: 10.1046/j.1523-1755.1999.00709.x. [DOI] [PubMed] [Google Scholar]
- 34.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nature Medicine. 2010;16:535–543. doi: 10.1038/nm.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meikrantz W, Schlegel R. Apoptosis and the cell cycle. J Cell Biochem. 1995;58:160–174. doi: 10.1002/jcb.240580205. [DOI] [PubMed] [Google Scholar]
- 36.Megyesi J, Safirstein RL, Price PM. Induction of p21WAF1/CIP1/SDI1 in kidney tubule cells affects the course of cisplatin-induced acute renal failure. J Clin Invest. 1998;101:777–782. doi: 10.1172/JCI1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol. 2014;9:12–20. doi: 10.2215/CJN.02730313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koyner JL, Shaw AD, Chawla LS, Hoste EAJ, Bihorac A, Kashani K, Haase M, Shi J, Kellum JA. Tissue Inhibitor Metalloproteinase-2 (TIMP-2) and IGF-Binding Protein-7 (IGFBP7) Levels Are Associated with Adverse Long-Term Outcomes in Patients with AKI. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2014060556. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kellum JA, Devarajan P. What can we expect from biomarkers for acute kidney injury? Biomark Med. 2014;8:1239–1245. doi: 10.2217/bmm.14.82. [DOI] [PMC free article] [PubMed] [Google Scholar]