Abstract
Studies of puberty have focused primarily on changes in hormones and on observable physical bodily characteristics. Little is known, however, about the nature of the relation between pubertal status and brain physiology. This is particularly important given findings that have linked the onset of puberty with both changes in cognitive functioning and increases in the incidence of depression and anxiety. The present study examined relations between pubertal stage, as assessed by Tanner Staging, and brain anatomy in a sample of 54 girls aged 9 - 15 years. Brain morphometric analysis was conducted using high-resolution magnetic resonance imaging (MRI). The hippocampus and amygdala were manually traced on MRI scans in all participants. Stepwise regression analyses were conducted with total intracranial volume (ICV), age, and pubertal status as the predictor variables and hippocampus and amygdala volumes as outcome variables. Pubertal status was significantly associated with left amygdala volume, after controlling for both age and intracranial volume (ICV). In addition, puberty was related to right hippocampus and amygdala volumes, after controlling for ICV. In contrast, no significant associations were found between age and hippocampal and amygdala volumes after controlling for pubertal status and ICV. These findings highlight the importance of the relation between pubertal status and morphometry of the hippocampus and amygdala, and of limbic and subcortical structures that have been implicated in emotional and social behavior.
Keywords: Puberty, Brain Development, Girls
1. Introduction
The onset of puberty is often delineated by changes in physical body characteristics; there are several neuroendocrine-related developments, however, that occur prior to observable changes in physical characteristics. For example, the release of gonadotropin releasing hormone (GnRH) is signaled by the hypothalamus in a pulsatile fashion and initiates the cascade of events that have been postulated to characterize the onset of puberty (Sizonenko, 1978). Following the release of GnRH, the pituitary responds by releasing luteinizing and follicular stimulating hormones that cause the release of estrogen and testosterone by the ovaries and lead to the development of secondary sexual characteristics (Apter, 1980). Importantly, these dramatic changes in hormonal physiology and in bodily characteristics during puberty coincide, particularly in girls, with an increased vulnerability to behavioral problems and dysregulation of emotional behaviors, such as depression, anxiety, and addictive behaviors (e.g., Angold & Costello, 2006; Chambers et al., 2003; Costello et al., 2007; Reardon et al., 2009). More specifically, an increase in the prevalence of changes in mood has been linked to reproduction in females (Parry & Haynes, 2000). This increase in vulnerability to change in mood in girls during and after puberty has been hypothesized to involve changes in neuroanatomy of limbic structures, such as the hippocampus and amygdala, and underscores the importance of gaining a comprehensive understanding of this stage of development and its relation to these neuroanatomic structures (Chen et al., 2010; Caetano et al., 2007; MacMillans et al., 2003; Rosso et al., 2005).
In this context, it is possible that this vulnerability is associated with the simultaneous (and possibly interrelated) changes in brain morphology that mirror puberty. For example, investigators have reported changes in cortical complexity, global and region-specific decreases in cortical gray matter, and increases in white matter with chronological age during childhood and adolescence (Blanton et al., 2001; Giedd et al., 1999; Toga et al., 2006). Moreover, girls show decreases in cortical gray matter earlier than do boys, paralleling the sex difference in the timing of puberty (Giedd et al., 2004; Papadimitriou, 2001). It is important to note, however, that there are few direct in vivo investigations of the relation between puberty and brain maturation. Using a dichotomous Tanner staging variable in girls, Bramen et al (2010) examined pubertal changes in mesial temporal lobe structures as well as cortical gray matter and reported significant decreases in amygdala volume with increasing pubertal stage. Peper et al (2009) also examined relations between brain morphometry and puberty in girls and found significant decreases in gray matter in the temporal lobe with increasing estradiol levels. Interestingly, however, no puberty-associated effects were found in the hippocampus for either study even though the hippocampus is known to have sex steroid hormone receptors and is influenced by the release of sex steroid hormones (Beyenburg et al., 2000; Gould et al., 2000).
There is considerable variation in chronological age regarding the onset, transition, and completion of puberty. For example, dramatic changes in skeletal and height physiology can occur at a young chronological age in one child and much later in another child, indicating that chronological age may not be a sensitive index. Indeed, pubertal stage is a better predictor of such physiological changes than is chronological age (Baccetti et al., 2006; Dorn et al., 2006).
This finding raises the important question of whether pubertal stage is also a stronger predictor than is chronological age in brain morphology.
The present study was designed to examine associations of pubertal stage, as assessed by Tanner Staging as a measurement of gonadarche (Dorn et al., 2006), and brain anatomy in a sample of girls, ages 9 to 15 years. Given the abundance of sex steroid hormone receptors in the hippocampus and amygdala and the onset of sex steroid hormone release with puberty, we hypothesized that pubertal stage would be a stronger predictor than would age of changes in volumes of the hippocampus and amygdala.
2. Experimental Procedures
2.1. Participants
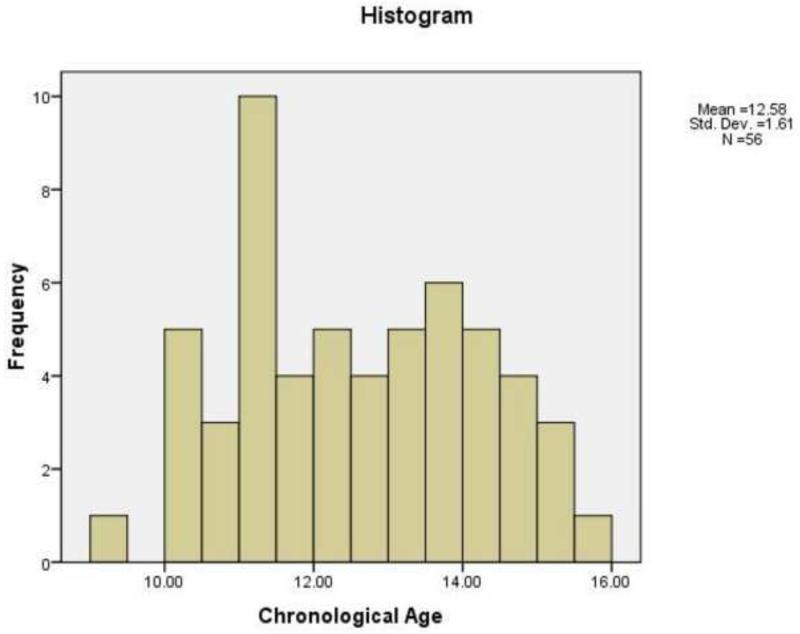
Participants in this study were 54 girls (mean age 12.602 years; SD: 1.632; minimum and maximum: 9.32 and 15.58 years; (Frequency histogram presented in Figure 1) who were recruited with their mothers through advertisements posted in numerous locations within the local community (e.g., internet bulletin boards, university kiosks, supermarkets). Sixty-seven percent were Caucasian-American, 15% Bi-racial-American, 7% Asian-American, 6% Hispanic-American, and 6% African-American. Institutional Review Board approval was obtained for this project. At least one parent of each participant gave written informed consent for the child's participation, and each child gave written assent. To eliminate possible effects of psychiatric disorders on brain morphology, all girls were administered the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS-PL; Kaufman et al., 2000) to assess current and past psychiatric diagnoses. The K-SADS-PL generates reliable and valid child psychiatric diagnoses (Kaufman et al., 1997). Girls with a current or past episode of a psychiatric Axis I disorder were excluded from the study.
Figure 1.
Frequency histogram of the age distribution of individuals.
2.2. Pubertal Assessment
Tanner Staging (Tanner & Whitehouse, 1976) was used to examine pubertal status, with Tanner stage 1 representing an absence of secondary sexual characteristics and Tanner stage 5 indicating physiological sexual maturity. Tanner Breast and Tanner Hair assessments were included separately in the analyses. Whereas stages of Tanner Hair are postulated to reflect increases in testosterone, Tanner Breast is associated more strongly with increases in estrogen during puberty (Penfold et al., 1981; Shirtcliff et al., 2009). Mean ages and standard deviations for Tanner Breast and Hair Stages are presented in Table 1.
Table 1.
Mean age, standard deviations (in years), and frequencies for Tanner breast and hair stages.
| TANNER STAGE | TANNER BREAST (MEAN AGE ± SD; FREQUENCY) | TANNER HAIR (MEAN AGE ± SD; FREQUENCY) |
|---|---|---|
| 1 | 10.278 ± .851 4 |
10.403 ± .883 6 |
| 2 | 11.182 ± .842 6 |
11.295 ± .633 8 |
| 3 | 12.424 ± 1.442 23 |
12.670 ± 1.441 11 |
| 4 | 13.505 ± 1.414 16 |
12.883 ± 1.286 19 |
| 5 | 14.090 ± .530 5 |
14.357 ± .991 10 |
2.3. Menarche
Twenty-one of the 49 participants in the sample who provided menarche status had started menarche. The mean age of girls who had not started menarche was 11.59 years (SD = 1.09) and of girls who had started menarche was 13.78 years (SD = 1.29; t (47) =6.43, p <.001).
2.4. Socioeconomic status variables
Socioeconomic status (SES) data included total household income and the school education of the mother and father. To examine the effects of SES on menarche status, an ordinal logistic regression was conducted using SES variables as independent measures and menarche status as the dependent variable.
2.5. Brain Assessment
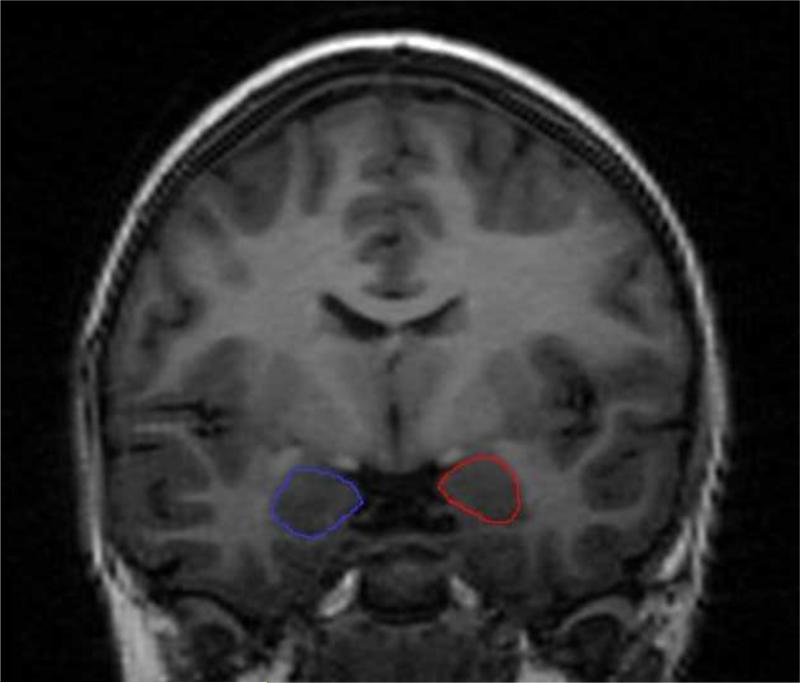
Sagittal T1-weighted 3D IR-prep SPGR magnetic resonance imaging (MRI) scans were collected from all subjects using an image acquisition matrix with 0.86×0.86×1.5mm3 voxels (TI/FA 400ms/15 deg.) and acquired utilizing a 1.5T MRI scanner. Each scan was interpolated to 1 mm3 voxels and normalized to the CCHMC female pediatric brain template (http://www.irc.cchmc.org) using a rigid body registration based on mutual information, as implemented in SPM2 to correct for possible head tilt differences. Total intracranial volume (ICV) and ICV gray, white, and CSF tissue types were automatically measured after the removal of extra-cerebral tissue voxels using BrainSuite2 (http://www.loni.ucla.edu/software). The hippocampus and amygdala were manually traced in successive 1 mm slices in the coronal viewing plane (an example tracing of the amygdala is presented in Figure 2). Both the axial and sagittal viewing planes were utilized to corroborate anatomy (for details on the anatomic guidelines see Levitt et al., 2001). Prior to manual tracings of all MRI scans, both intra- and inter-rater reliability were conducted. Intra-rater reliability had a coefficient of variation of 3%. The intra-class correlation coefficient for inter-rater reliability was .93 for the hippocampus and .89 for the amygdala. All morphometric analyses for the study were conducted by one rater blind to age and pubertal stage.
Figure 2.
Example tracings of the left and right amygdala using structural MRI.
2.6. Statistical Analyses
A stepwise linear regression analyses was conducted using total ICV, chronological age, and puberty as independent variables and volumes of the hippocampus and amygdala as dependent variables. First, total ICV was entered into the model, followed by pubertal stage (Tanner-H and Tanner-B, independently), followed by age to examine associations with hippocampus and amygdala volumes. To compare the relative strengths of the effects of age and puberty, a second analysis was conducted, first entering total ICV into the model, followed by age, followed by pubertal status (Tanner-H and Tanner-B, independently) to predict hippocampus and amygdala volumes. Finally, one-way (by menarche status) analyses of covariance (ANCOVAs) were conducted on hippocampus and amygdala volumes, with total ICV and age as covariates.
3. Results
3.1. Menarche
There were no significant differences between girls who had started their menses and girls who had not started menses in total ICV gray or white matter or in volumes of the right or left hippocampus or amygdala (see Table 2). There were also no significant effects of SES variables on menarche status.
Table 2.
Means and standard deviations (in cm3) of hippocampus and amygdala volumes by menarche status.
| MENARCHE | RIGHT HIPPOCAMPUS | LEFT HIPPOCAMPUS | RIGHT AMYGDALA | LEFT AMYGDALA |
|---|---|---|---|---|
| No | 2.541 ± .329 | 2.241 ± .278 | 1.933 ± .339 | 1.781 ± .299 |
| Yes | 2.453 ± .274 | 2.170 ± .222 | 1.792 ± .346 | 1.766 ± .277 |
3.2. Hippocampus and amygdala
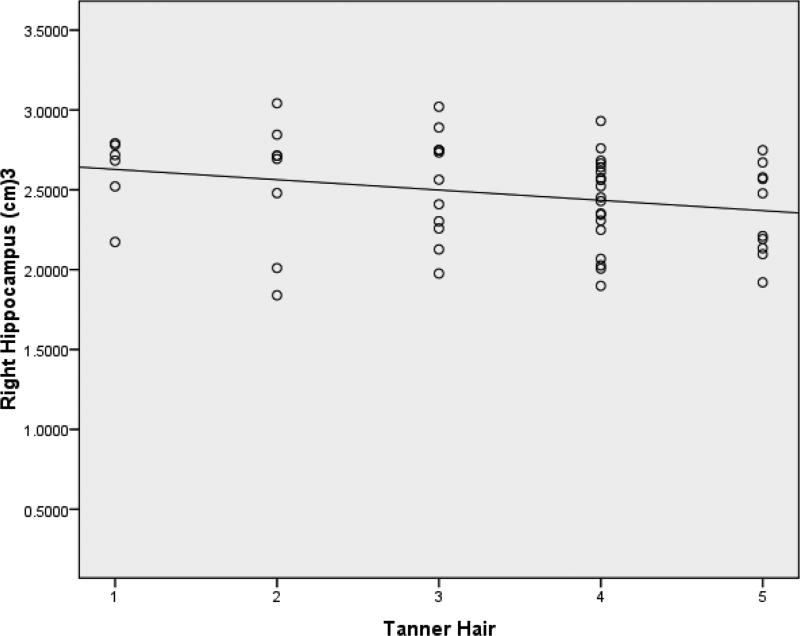
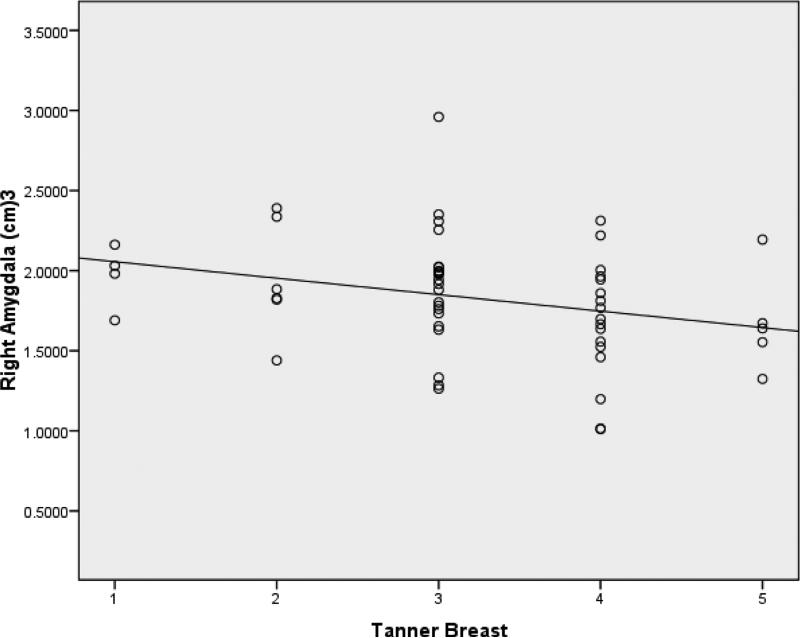
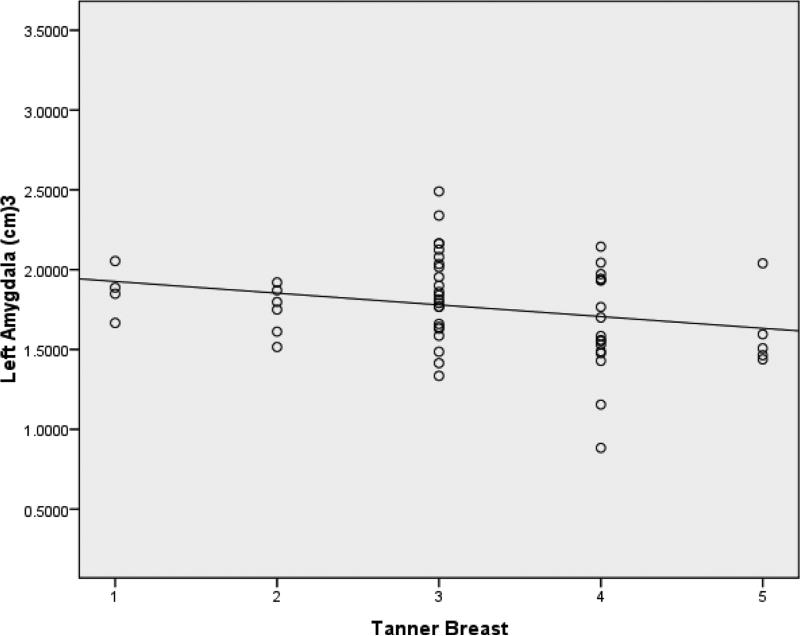
Means and standard deviations for volumes of the hippocampus and amygdala at each Tanner stage are presented in Table 3. In all regression models, ICV was found to be a significant predictor of volumes of both the right and left hippocampus after controlling for age and pubertal stage (β=.291, t=2.203, p=.032; β =.291, t=2.24, p=.030). Even after controlling for ICV, however, Tanner-H was a significant predictor of right hippocampal volume (β =-.295, t=-2.297, p = .026) (Figure 3). Age was not found to be a significant predictor of either left or right hippocampal volume before or after controlling for ICV. Controlling for ICV, Tanner-B was found to be significantly associated with right amygdala volume (β =-.292, t=-2.211, p = .031) (Figure 4). In addition, controlling for ICV and age, Tanner-B was found to predict left amygdala volume (β =-.408, t=-2.406, p =.020) (Figure 5). Age was not found to predict left or right amygdala volumes before or after controlling for ICV.
Table 3.
Means and standard deviations (in cm3) for hippocampus and amygdala volumes by Tanner breast and hair stages.
| TANNER STAGE | RIGHT HIPPOCAMPUS | LEFT HIPPOCAMPUS | RIGHT AMYGDALA | LEFT AMYGDALA |
|---|---|---|---|---|
| Tanner-breast 1 | 2.377 ± .450 | 2.123 ± .217 | 1.966 ± .199 | 1.864 ± .159 |
| Tanner-breast 2 | 2.703 ± .171 | 2.341 ± .145 | 1.950 ± .358 | 1.744 ± .155 |
| Tanner-breast 3 | 2.546 ± .313 | 2.278 ± .274 | 1.906 ± .369 | 1.864 ± .290 |
| Tanner-breast 4 | 2.372 ± .253 | 2.094 ± .210 | 1.674 ± .382 | 1.701 ± .272 |
| Tanner-breast 5 | 2.371 ± .350 | 2.157 ± .254 | 1.677 ± .320 | 1.609 ± .248 |
| Tanner-hair 1 | 2.611 ± .236 | 2.340 ± .228 | 1.918 ± .146 | 1.750 ± .164 |
| Tanner-hair 2 | 2.542 ± .415 | 2.200 ± .239 | 1.864 ± .389 | 1.783 ± .188 |
| Tanner-hair 3 | 2.582 ± .312 | 2.230 ± .269 | 1.915 ± .385 | 1.932 ± .326 |
| Tanner-hair 4 | 2.425 ± .281 | 2.200 ± .273 | 1.745 ± .443 | 1.754 ± .289 |
| Tanner-hair 5 | 2.360 ± .282 | 2.127 ± .207 | 1.794 ± .279 | 1.671 ± .237 |
Figure 3.
After controlling for ICV, Tanner-H was a predictor of right hippocampal volume.
Figure 4.
After controlling for ICV, Tanner-B was found to predict right amygdala volume.
Figure 5.
After controlling for ICV and age, Tanner-B was found to predict left amygdala volume.
4. Discussion
Given the importance of the role of the amygdala and hippocampus in changes in behaviors that develop with the onset of puberty, such as depression and anxiety, we examined the associations of pubertal stage and age with amygdala and hippocampus volumes in young girls. We found that, after controlling for ICV, increasing Tanner-Hair stage was associated with lower volume in the right hippocampus and increasing Tanner-Breast stage was associated with lower volume of the right and left amygdala. Age was not a significant predictor of hippocampus or amygdala volumes.
Our finding that puberty, rather than chronological age, predicted changes in the hippocampus and amygdala could be due to chronobiology. Adolescents show greater phase delay and increased sleep/wake cycles in their sleep patterns than do children (Carskadon et al., 1993), possibly as a result of the stronger relation of melatonin secretion with puberty than with chronological age (Salti et al., 2000). This well-known pubertal shift in sleep patterns in adolescents, leading them to want to stay up later, may decouple the synchronicity or entrainment of daily circadian rhythms and angle the timing of the biological clock towards other endogenous rhythms rather than the ~24-hour sleep/wake cycle, such as the release of hormones and, in effect, alter brain morphology in a non-chronological age-dependent manner. Gonadal hormones have “activational effects” on circadian rhythms and the circadian pacemaker (Hagenauer et al., 2009). This, in theory, could alter the sleep/wake patterns (or biological clock) to align with gonadal pubertal changes rather than with daily circadian rhythms and influence brain chronobiology. Brain chronobiology includes the hippocampus and amygdala, two brain networks that have been characterized to be influenced by the circadian system (Vandewalle et al., 2009).
Our finding of decreasing volume of the right hippocampus with increasing Tanner-Hair pubertal stage in healthy girls suggests that testosterone plays a role in the maturation of this region. Indeed androgen sensitivity is found to occur in the hippocampus and could be the cause of the association between pubertal status and change in volume of the hippocampus (Beyenburg et al., 2000). The association of hippocampal volume with pubertal stage may also reflect an important change in autobiographical memories during this developmental stage (Cabeza et al., 2004). For example older children and adolescents are able to provide more retrospective autobiographical as well as future narratives. In a meta-analysis, Van Patten (2004) describes that the relation of mesial temporal lobe volumes and cognition, and in particular, memory, is affected by age of the participants. Thus, whereas in older individuals memory is positively related to volume, in younger individuals memory and volume are negatively related. This suggests that smaller volumes in children and adolescents are an indicator of an increase in cognitive performance. Furthermore, in a recent study of parental nurturance and hippocampal volumes, Rao et al. (2010) found that increased parental nurturance is related to decreased hippocampal volumes in children and adolescents. A decrease in hippocampal volumes may be one positive indicator of outcomes in children and adolescents. Describing autographical memories is related to the ability to regulate emotions (Habermas et al., 2009; McLean, 2005). Overgeneralized autobiographical memories have been conceptualized as a risk factor for depression (Rice & Rawal, 2011). In this context, the adaptive use of autobiographical memories may be impaired in Major Depression (Conway & Pleydell-Pearce, 2000; Habermas et al., 2009). Given that depressive symptoms develop in girls during puberty, this formulation is consistent with both a difficulty in emotion regulation and reductions in the volume of the right hippocampus (Joormann & Gotlib, 2010; Maller et al., 2007).
We also found a significant relation between the amygdala and pubertal stage. Our finding that Tanner-Breast stage is associated with amygdala volume is consistent with studies in humans that describe the expression of α-estrogen receptors in the amygdala nuclei (Osterlund & Hurd, 2001). Correctly extracting socially and emotionally salient information from faces is thought to involve amygdala function (Adolphs et al., 1998; Adolphs, 2003; 2010; Sergerie et al., 2006); indeed, the amygdala may aid in the social construction of one's self through automatic feedback of social responses from others, especially during teen years when peers take on special significance in the development of the self (Spear, 2000). Plus, investigators implicate the right amygdala in self-relatedness to facial stimuli, suggesting a close link between the self and evaluation of emotions (Northoff et al., 2007). Our finding of an association of pubertal stage and the left amygdala is consistent with these findings. The left amygdala is proposed to involve the retrieval of emotional memories (Sergerie et al., 2006). Such retrieval of emotional memories is influenced by mood, which affects accessing both negative and positive memories (Haas & Canli, 2008). Interestingly, healthy adolescents are characterized by fluctuations in mood that are not related to negative symptoms (Larson et al., 1980), which could explain, in part, changes in the left amygdala that relate to the release of estrogen that occurs during puberty as α, yet not β, estrogen receptors are implicated in brain regions involved in emotions (Osterlund & Hurd, 2001).
As mentioned previously, the prevalence of a change in mood and anxiety behaviors in girls increases during the transition through puberty (Hankin et al., 1998). Changes in neural morphology at this time may predispose increased variation in neurodevelopment and could exacerbate the normal changes in autobiographical memories, mood, and social behaviors that accompany maturation. For example, puberty is associated with symptoms of anxiety, depression, and stress during adolescence (Huerta & Brizuela-Gamiño, 2002). More specifically, major depression is reported to involve dysfunction of the amygdala and hippocampus (Gotlib & Hamilton, 2008; Hamilton & Gotlib, 2008), anatomic structures found in the present study to differ volumetrically with pubertal stage. Hormone replacement therapy has neuroprotective effects on both mood and brain anatomy, suggesting that sex-steroid mechanisms play a role in mood (Ha et al., 2007; Stephens et al, 2006).
Conclusions
In closing, we should note three limitations of this study. First, we used Tanner staging to assess pubertal status rather than assessing sex steroid hormones such as estrogen, progesterone, and testosterone, which could provide more detailed information regarding the relation between brain morphology and pubertal development (see Dorn et al., 2006). However, strong correlations between Tanner staging and levels of these hormones have been documented in prior studies (Shirtcliff et al., 2009). Second, we did not assess the phase of menstrual cycle during which the girls were scanned; this should be examined in future studies. Finally, we had relatively small sample sizem cc s, particularly in Tanner Stages 1 and 5. These sample sizes precluded an examination of the effects of ethnicity on the relation between puberty and brain morphology. This is an important area for future research. Despite these limitations, however, the present findings highlight the significance of pubertal status as an important developmental milestone associated with volumes of the right hippocampus and amygdala. Future research should examine more explicitly the mechanisms underlying the associations between pubertal status and neuroanatomical development and begin to explore the nature of the relations among brain physiology, socio-cognitive functioning, onset of emotional disorders, and puberty.
Highlights.
Little is known regarding the relation of puberty and brain development
Puberty was found to be a significant predictor of changes in the hippocampus and amygdala
This highlights the importance of the developmental stage of puberty
Acknowledgements
We would like to thank Melissa L. Henry, Yamanda Wright, and Hannah Burley for their contributions to this scientific manuscript. This research was supported by National Institute of Mental Health Grant MH74849 awarded to Ian H. Gotlib.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393:470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Cognitive neuroscience of human social behavior. Nature Reviews Neuroscience. 2003;4:165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- Adolphs R. What does the amygdala contribute to social cognition? Annals of the New York Academy of the Sciences. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angold A, Costello EJ. Puberty and depression. Child and Adolescent Psychiatric Clinics of North America. 2006;15:919–937. doi: 10.1016/j.chc.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Apter D. Serum steroids and pituitary hormones in female puberty: a partly longitudinal study. Clinical Endocrinology. 1980;12:107–120. doi: 10.1111/j.1365-2265.1980.tb02125.x. [DOI] [PubMed] [Google Scholar]
- Baccetti T, Franchi L, De Toffol L, Ghiozzi B, Cozza P. The diagnostic performance of chronologic age in the assessment of skeletal maturity. Progress in Orthodontics. 2006;7:176–188. [PubMed] [Google Scholar]
- Beyenburg S, Watzka M, Clusmann H, Blumcke I, Bidlingmaier F, Elger CE, Stoffel-Wagner B. Androgen receptor mRNA expression in the human hippocampus. Neuroscience Letters. 2000;294:25–28. doi: 10.1016/s0304-3940(00)01542-1. [DOI] [PubMed] [Google Scholar]
- Blanton RE, Levitt JG, Thompson PM, Narr KL, Capetillo-Cunliffe L, Nobel A, Singerman JD, McCracken JT, Toga AW. Mapping cortical asymmetry and complexity patterns in normal children. Psychiatry Research Neuroimaging. 2001;107:29–43. doi: 10.1016/s0925-4927(01)00091-9. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends in Cognitive Science. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Prince SE, Daselaar SM, Greenberg DL, Budde M, Dolcos F, LaBar KS, Rubin DC. Brain activity during episodic retrieval of autobiographical and laboratory events: an fMRI study using a novel photo paradigm. Journal of Cognitive Neuroscience. 2004;16:1583–1594. doi: 10.1162/0898929042568578. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Vieiera C, Acebo C. Association between puberty and delayed phase preference. Sleep. 1993;16:258–262. doi: 10.1093/sleep/16.3.258. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. American Journal of Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M, Cole SR. The Development of Children. 2nd edition Scientific American Books; New York, NY.: 1993. [Google Scholar]
- Costello EJ, Sung M, Worthman C, Angold A. Pubertal maturation and the development of alcohol use and abuse. Drug and Alcohol Dependency. 2007;88:S50–59. doi: 10.1016/j.drugalcdep.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Annals of the New York Academy of Sciences. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Dorn LD, Dahl RE, Woodward HR, Biro F. Defining the boundaries of early adolescence: a user's guide to assessing pubertal status and pubertal timing in research with adolescents. Applied Developmental Science. 2006;10:30–56. [Google Scholar]
- Forbes EE, Williamson DE, Ryan ND, Dahl RE. Positive and negative affect in depression: influence of sex and puberty. Annals of the New York Academy of Sciences. 2004;1021:341–347. doi: 10.1196/annals.1308.042. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Hamilton JP. Neuroimaging and depression: Current status and unresolved issues. Current Directions in Psychological Science. 2008;17:159–163. [Google Scholar]
- Ha DM, Xu J, Janowsky JS. Preliminary evidence that long-term estrogen use reduces white matter loss in aging. Neurobioogy of Aging. 2007;28:1936–1940. doi: 10.1016/j.neurobiolaging.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BW, Canli T. Emotional memory function, personality structure and psychopathology: a neural system approach to the identification of vulnerability markers. Brain Research and Reviews. 2008;58:71–84. doi: 10.1016/j.brainresrev.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenauer MH, Perryman JI, Lee TM, Carskadon MA. Adolescent changes in the homeostatic and circadian regulation of sleep. Developmental Neuroscience. 2009;31:276–284. doi: 10.1159/000216538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JA, Parry BL, Blumenthal SJ. The menstrual cycle in context, I: Affective syndromes associated with reproductive hormonal changes. The Journal of Clinical Psychiatry. 1988;49:474–480. [PubMed] [Google Scholar]
- Hamilton JP, Gotlib IH. Neural substrates of increased memory sensitivity for negative stimuli in major depression. Biological Psychiatry. 2008;63:1155–1162. doi: 10.1016/j.biopsych.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KE. Development of depression from preadolescence to young adulthood: emerging gender differences in a 10-year longitudinal study. Journal of Abnormal Psychology. 1998;107:128–140. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- Hausmann M, Tegenthoff M, Sänger J, Janssen F, Güntürkün O, Schwenkreis P. Transcallosal inhibition across the menstrual cycle: a TMS study. Clinical Neurophysiology. 117:26–32. doi: 10.1016/j.clinph.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Hausmann M, Güntürkün O. Steroid fluctuations modify functional cerebral asymmetries: the hypothesis of progesterone-mediated interhemispheric decoupling. Neuropsychologia. 2000;38:1362–1374. doi: 10.1016/s0028-3932(00)00045-2. [DOI] [PubMed] [Google Scholar]
- Huerta R, Brizuela-Gamiño OL. Interaction of pubertal status, mood and self-esteem in adolescent girls. Journal of Reproductive Medicine. 2002;47:217–25. [PubMed] [Google Scholar]
- Joormann J, Gotlib IH. Emotion regulation in depression: relation to cognitive inhibition. Cognition and Emotion. 2010;24:281–298. doi: 10.1080/02699930903407948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karádi K, Kállai J, Köver F, Nemes J, Makäny T, Nagy F. Endogenous testosterone concentration, mental rotation, and size of the corpus callosum in a sample of young Hungarian women. Perceptual and Motor Skills. 2006;102:445–453. doi: 10.2466/pms.102.2.445-453. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent DA, Ryan ND, Rao U. K-SADS-PL. Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39:1208. doi: 10.1097/00004583-200010000-00002. [DOI] [PubMed] [Google Scholar]
- Larson R, Csikszentmjhalyi M, Graef R. Mood variability and the psychosocial adjustment. Journal of Youth and Adolescence. 1980;9:469–490. doi: 10.1007/BF02089885. [DOI] [PubMed] [Google Scholar]
- Levitt JG, Blanton RE, Caplan R, Asarnow R, Guthrie D, Toga AW, Capetillo-Cunliffe L, McCracken JT. Medial temporal lobe in childhood-onset schizophrenia. Psychiatry Research Neuroimaging. 2001;108:17–27. doi: 10.1016/s0925-4927(01)00108-1. [DOI] [PubMed] [Google Scholar]
- Maller JJ, Daskalakis ZJ, Fitzgerald PB. Hippocampal volumetrics in depression: the importance of the posterior tail. Hippocampus. 2007;17:1023–1027. doi: 10.1002/hipo.20339. [DOI] [PubMed] [Google Scholar]
- Mclean KC. Late adolescent development identity development: narrative meaning making and memory telling. Developmental Psychology. 2005;41:683–691. doi: 10.1037/0012-1649.41.4.683. [DOI] [PubMed] [Google Scholar]
- Northoff G, Schneider F, Rotte M, Matthiae C, Tempelmann C, Wiebking C, Bermpohl F, Heinzel A, Danos P, Heinze HJ, Bogerts B, Walter M, Panskeep J. Differential parametric modulation of self-relatedness and emotions in different brain regions. Human Brain Mapping. 2007 Dec 6; doi: 10.1002/hbm.20510. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund MK, Hurd YL. Estrogen receptors in the human forebrain and the relation to neuropsychiatric disorders. Progress in Neurobiology. 2001;64:251–267. doi: 10.1016/s0301-0082(00)00059-9. [DOI] [PubMed] [Google Scholar]
- Parry B, Haynes P. Mood disorders and the reproductive cycle. The Journal of Gender Specific Medicine. 2000;3:53–58. [PubMed] [Google Scholar]
- Papadimitriou A. Sex differences in the secular changes in pubertal maturation. Pediatrics. 2001;108:E65. doi: 10.1542/peds.108.4.e65. [DOI] [PubMed] [Google Scholar]
- Pasupathi M, Wainryb C. On telling the whole story: facts and interpretations in autobiographical memory narratives from childhood through midadolescence. Developmental Psychology. 2010;46:735–746. doi: 10.1037/a0018897. [DOI] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends in Cognitive Science. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Penfold JL, Smeaton TC, Gilliland JM, Boulton TJ, Thomsett MJ, Preece MA, Vimpani A. Indices of serum androgens in normal puberty: Correlations of two indices with chronological age, bone age, and pubertal development in boys and girls. Clinical Endocrinology. 1981;15:183–192. doi: 10.1111/j.1365-2265.1981.tb00653.x. [DOI] [PubMed] [Google Scholar]
- Rao H, Betancourt L, Giannetta JM, Brodsky NL, Korczykowski M, Avants BB, Gee JC, Wang J, Hurt H, Detre JA, Farah MJ. Early parental care is important for hippocampal maturation: evidence from brain morphology in humans. Neuroimage. 2010;49:1144–1150. doi: 10.1016/j.neuroimage.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice F, Rawal A. Can basic research help in the prevention of childhood and adolescent depression? Examining a cognitive and emotional regulation approach. Depression Research and Treatment. 2011 doi: 10.1155/2011/871245. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergerie K, Lepage M, Armony JL. A process-specific functional disassocation of the amygdala in emotional memory. Journal of Cognitive Neuroscience. 2006;18:1359–1367. doi: 10.1162/jocn.2006.18.8.1359. [DOI] [PubMed] [Google Scholar]
- Sizonenko PC. Endocrinology in preadolescents and adolescents. I. Hormonal changes during normal puberty. American Journal of Diseases of Children. 1978;132:704–712. doi: 10.1001/archpedi.1978.02120320064015. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. Neuroimage. 1999;9:587–597. doi: 10.1006/nimg.1999.0436. [DOI] [PubMed] [Google Scholar]
- Spear L. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stephens C, Pachana NA, Bristow V. The effect of hormone replacement therapy on mood and everyday memory in younger mid-life women. Psychology, Health, and Medicine. 2006;11:461–469. doi: 10.1080/13548500600678180. [DOI] [PubMed] [Google Scholar]
- Tanner JM, Whitehouse RH. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Archives of Disease in Childhood. 1976;51:170–179. doi: 10.1136/adc.51.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Giedd JN, Woods RP, Macdonald D, Evans AC, Toga AW. Growth patterns in the developing brain detected by using continuum mechanical tensor maps. Nature. 2000;404:190–193. doi: 10.1038/35004593. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends in Neuroscience. 2006;29:148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urdin T, Klein S. Early adolescence: a review of the literature. The U.S. Dept of Education, Office of Educational Research and Development; 1998. [Google Scholar]
- Vandewalle G, Maquet P, Dijk D-J. Light as a modulator of cognitive brain function. Trends in Cognitive Sciences. 2009;13:429–438. doi: 10.1016/j.tics.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Petten Van. Relationship between hippocampal volume and memory ability in healthy individuals across the life span: review and meta-analysis. Neuropsychologia. 2004;42:1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]