Abstract
Dracocephalum kotschyi is an essential oil containing plant found in Iran. In Iranian traditional medicine, D. kotschyi has been used as antispasmodic and analgesic but so far there is no pharmacological report about its antispasmodic activity. Therefore, the objective of this research was to study antispasmodic activity of the essential oil of D. kotschyi and two of its constituents namely limonene and α-terpineol. The essential oil was obtained from aerial parts of D. kotschyi using hydrodistillation method. The main components found in the essential oil were α-pinene (10%), neral (11%), geraniol (10%), α-citral (12%), limonene (9%) and α-terpineol (1.1%). For antispasmodic studies, a portion of rat ileum was suspended under 1 g tension in Tyrode's solution at 37 °C and gassed with O2. Effect of the D. kotschyi essential oil, limonene and α-terpineol were studied on ileum contractions induced by KCl (80 mM), acetylcholine (ACh, 500 nM) and electrical field stimulation (EFS). The essential oil, in a concentration dependent manner inhibited the response to KCl (IC50=51 ± 8.7 nl/ml), ACh (IC50=19 ± 2.7 nl/ml) and EFS (IC50=15 ± 0.5 nl/ml). Limonene and α-terpineol showed same pattern of inhibitory effect on ileum contraction. Their inhibitory effects were also concentration dependent. However, limonene was more potent than the essential oil while the α-terpineol was less potent than either limonene or the essential oil. From this experiment it was concluded that D. kotschyi essential oil has inhibitory effect on ileum contractions. Limonene contribute a major role in inhibitory effect of the essential oil while α-terpineol has weak antispasmodic activity.
Keywords: Dracocephalum kotschy, Essential oil, Limonene, α-terpineol, Ileum
INTRODUCTION
Dracocephalum kotschyi (D. kotschyi) is a wild flowering plant belonging to Labiatae family (1). This plant which is known as ZarinGiah in Iran is found in many parts of Khorasan province (1,2,3). D. kotschyi is an essential oil containing plant and its constituents includes limonene, verbenone, α-terpineol, caryophyllene, geranial and neral (4,5). In Iranian traditional medicine, D. kotschyi has been used as antispasmodic and analgesic (3,6). It has been reported that the analgesic activity might be due to presence of limonene and α-terpineol (3,6). Other constituents including calycopterin which is believed to have immunomodulatory effect and it has been suggested that it may have anti-inflammatory activity in arthritis (6,7,8). Furthermore, in animal model, D. kotschyi has shown to have antihyperlipidemic activity (9). Others suggested that this plant may also have antitumor activities (10).
So far, antispasmodic activity of a number of traditional medicine including Rosa damascene, Pycnocycla spinosa, Prangos ferulacea, Zataria multiflora, Satureja hortensis, Passiflora incarnata, Berberis integerrima, Crocus sativus, Ferula gummosa, Teucurium poliume, Melissa officinalis, and asa-foetida have been studied (11,12,13,14,15,16,17,18,19,20) but they have different potency of antispasmodic activity. Among these Pycnocycla spinosa was found to have most potent antispasmodic activity (13,14). D. kotschyi is another medicinal plant which has been used as antispasmodic agent in traditional medicine (3,6). However, the antispasmodic effect of D. kotschyi essential oil on gastrointestinal (GI) tract has not been studied by standard pharmacological methods. Therefore, the aim of this research was to investigate the effect of D. kotschyi essential oil and two of its components on ileum contraction, in order to evaluate their inhibitory effects on intestinal contraction.
MATERIALS AND METHODS
Plant materials and analysis of the essential oil
Dracocephalum kotschyi aerial parts were collected from Chadegan (in Isfahan province- Iran) and identified at the Botany Department of the Faculty of Sciences, University of Isfahan. A voucher specimen (1519) was deposited at the herbarium of the School of Pharmacy and Pharmaceutical Sciences of Isfahan University of Medical Sciences.
The plant materials were dried in shadow and grained to powder using electrical miller (Moulinex, France). The essential oil was prepared by hydrodistillation according to European Pharmacopoeia (21). Gas chromatography (GC) analysis of the essential oil was carried out on a Perkin-Elmer 8500 gas chromatograph with FID detector and a BP-1 capillary column as previously described (12).
The mass spectra (MS) were recorded on a Hewlett-Packard 5983A mass selective detector coupled with a Hewlett-Packard 6890 gas chromatograph, equipped with a HP-5MS capillary column as previously described (12).
In vitro contractility assessment
All animals were handled in accordance with the internationally accepted principles for laboratory animal use and care, as recommended by university authority (Committee for the Update of the Guide for the Care and Use of Laboratory Animals, 2010) (22).
On the day of experiment, male Wistar rats (180–220 g, bred in School of Pharmacy animal house, Isfahan, Iran), were killed by a blow on the head followed by exsanguination. The abdomen was opened and a piece of ileum was dissected and placed in oxygenated Tyrode's solution at room temperature. Longitudinal strips of ileum, 2-3 cm long, were then prepared and mounted under 1 g resting force in an organ bath (Harvard, England) filled with Tyrode's solution at 37 °C and continuously bubbled with O2. The tissues were washed several times with fresh Tyrode's solution and allowed to relax to a stable baseline. Contractions were induced by KCl (80 mM), acetylcholine (ACh, 500 nM, 30s contact) or electrical field stimulation (EFS, 6 V and 50 Hz for 1 s duration in 10 min intervals) and recorded on a Harvard Universal Oscillograph (England) pen recorder device as described before (12). After reproducible contractions were established the essential oil, limonene or α-terpineol were added directly into organ bath at 10 min intervals. Initially a number of pilot experiments were carried out for determination of effective concentration ranges of limonene, α-terpineol and the essential oil of D. kotschyi. Then full concentration response curves were obtained for each drug using 6-9 different concentration of drugs.
In case of KCl, drugs were added into the bath in a cumulatively manner while for ACh and EFS non-cumulative method was used. After maximum inhibitory effect was achieved, the tissues were washed with fresh Tyrode's solution and tested to see if the inhibition was reversible. All experiments were performed alongside time-matched vehicle treated controls.
Drugs and solutions
Tyrode's solution composed of (mM): NaCl; 136.9, KCl; 2.68, CaCl2;1.8, MgCl2;1.05, NaHCO3; 11.9, NaH2PO4; 0.42 and glucose; 5.55, were made up in distilled water. Unless stated, all chemicals and drugs were from Merck (Darmstadt, Germany). The following drugs were used in this research: α-terpineol, limonene (Roth, Germany), and essential oil of D. kotschyi, acetylcholine hydrochloride (Sigma, Germany). The essential oil, limonene and α-terpineol were made up as 10 μl/ml stock solution in dimethyl sulphoxide (DMSO), diluted in distilled water (1 μl/ml & 100 nl/ml). KCl (2 M) stock solutions were prepared in distilled water. ACh was made up as 100 mM stock solution and acidified by 1% acetic acid, and further serial dilutions were made in distilled water.
Measurements and statistical analysis
Contractile response to KCl, ACh and EFS were measured as maximum amplitude from the baseline, just before addition of next concentration of the drugs and expressed as the percentage of the initial response in the absence of drugs for each tissue. All the values are quoted as mean ± standard error of the mean (SEM). Statistical significance were assessed using a one-way analysis of variance (ANOVA) for repeated measures and when appropriate were compared with the control groups using unpaired Student's t-test. Differences were considered statistically significant for P<0.05.
Whenever appropriate, the IC50 value (drug concentration causing 50% of maximum response), was calculated. SigmaPlot (version 11) computer program was used for statistical analysis and drawing the graphs for calculation of IC50 values.
RESULTS
Plant materials analysis
The yield of essential oil of D. kotschyi was about 66% with a mild pleasant smell. At least 49 compounds were identified in the essential oil of D. kotschyi. The results of GC-MS analysis of the D. kotschyi essential oil are shown in Table 1. Fifteen compounds accounts for 80% of the total oil. The rest were accounted for less than 1% of total constituents. The main components were α-pinene (10%), neral (11%), geraniol (10%), α-citral (12%) and limonene (9%).
Table 1.
Constituents of essential oil of aerial part of Dracocephalum kotschyi collected from Chadegan district. The compounds are listed in order of their percent (%) in the essential oil. RT shows the retention time on the HP-5 MS. From 49 identified only compounds which accounted for more than 1% of the essential oil are listed here.

In vitro contractility assessment
Rat isolated ileum suspended in the fresh Tyrode's solution gradually relaxed to a stable baseline over 10-20 min. Rat ileum contracted rapidly to EFS, reaching a peak followed by partial relaxation which was then followed by a second peak and then relaxed towards the baseline as described before (12,23). ACh only caused a single contraction in rat ileum during 30 s of the contact time. KCl (80 mM) caused tonic contraction which maintained for the duration of study.
D. kotschyi essential oil, limonene and α-terpineol (8 nl/ml -128 nl/ml) concentration dependently reduced contractile responses to KCl. At its highest used concentration the contractile response to KCl was totally removed (Fig. 1). At bath concentration of 64 nl/ml limonene removed the contractile response to KCl while the essential oil and α-terpineol only inhibited the response by 60% and 79% respectively. The IC50 values are given in Table 1 for comparison. After washing the tissue with fresh Tyrode's solution, the contractile response to KCl was gradually restored. There was no statistically difference in the vehicle treated time match control tissues over the course of studies (ANOVA).
Fig. 1.
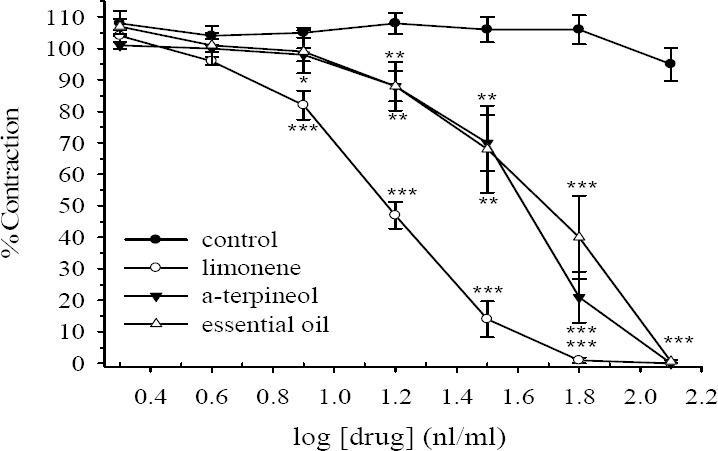
Cumulative effect of limonene, α-terpineol and Dracocephalum kotschyi essential oil on tension development to potassium chloride (KCl, 80 mM) in isolated ileum of rats. Ordinate scale: ileum contraction expressed as % of initial control response. Abscissa scale: log10 concentration of drugs (limonene, α-terpineol or D. kotschyi essential oil). Lines drawn through the points, using two fold increments in concentration. The points are mean and the vertical bars show the SEM (n=6). The oscillation in the control group is not statistically significant (ANOVA). Stars shows statistical differences between each drug concentration with its corresponding vehicle treated control. Keys: *P<0.05, **P<0.01, ***P<0.001 (Student's t-test). Maximum concentration of the vehicle (DMSO) in the bath was 0.64%.
Limonene, α-terpineol and the essential oil of D. kotschyi (1-128 nl/ml) concentration dependently inhibited ileum contraction induced by ACh (0.5 μM, Fig. 2). The order of potencies were limonene > essential oil > α-terpineol. The essential oil and limonene at 32 potencies were limonene > essential oil > α-terpineol. The essential oil and limonene at 32 nl/ml bath concentration almost vanished the contractile response to ACh, while α-terpineol only reduced the ACh response by about 4%. The IC50 values are compared in Table 2. There was some increase in responses of ACh in the tissues treated with the vehicle (DMSO + water) but these changes were not statistically significant (Fig. 2). The inhibitory effect of the essential oil, limonene and α-terpineol on ACh responses was reversible following washing the tissue with fresh Tyrode's solution. Relaxant effect of limonene, α-terpineol and the essential oil of D. kotschyi were also examined on biphasic contractions induced by EFS. All three compounds in a concentration dependent manner inhibited ileum contractions induced by EFS (Fig. 3). The ranges and order of inhibition are similar to that of ACh. The IC50 value of limonene, α-terpineol and the essential oil of D. kotschyi on the initial and the secondary contractile response are shown in Table 2. Inhibitory effect of these compounds on the both initial and the secondary contraction induced by EFS were more or less the same (Figs. 3a, 3b). Following washing the tissue with fresh Tyrode's solution, the inhibitory effect of these compounds was reversible. The isolation in responses of vehicle treated time-matched control tissues were not statistically significant (ANOVA).
Fig. 2.
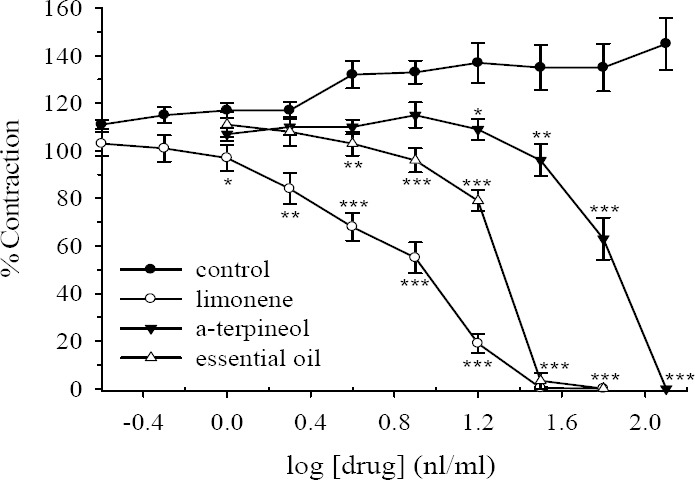
Effect of limonene, α-terpineol and Dracocephalum kotschyi essential oil on tension development to acetylcholine (ACh, 0.5 μM) in isolated ileum of rats. Ordinate scale: ileum contractions expressed as % of initial control response. Abscissa scale: log10 concentration of drugs (limonene, α-terpineol or D. kotschyi essential oil). Lines drawn through the points, using two fold increments in concentration. The points are mean and the vertical bars show the SEM (n=6). The increase in the response of vehicle treated control tissues is not statistically significant (ANOVA). Stars shows statistical differences between each drug concentration with its corresponding vehicle treated control. Keys: *P<0.05, **P<0.01, ***P<0.001 (Student's t-test). Maximum concentration of vehicle (DMSO) in the bath was 1.28%.
Table 2.
IC50 Values (inhibitory concentration causing 50% of maximum response) of Dracocephalum kotschyi essential oil, limonene and α-terpineol on rat ileum contractions induced by KCl, acetylcholine and initial and secondary contractile response to electrical field stimulation.

Fig. 3.
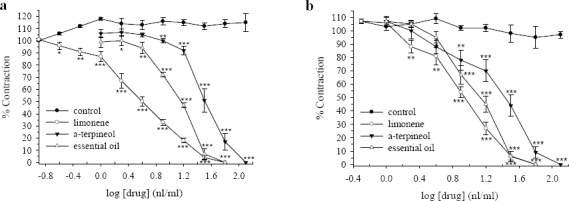
Effect of limonene, α-terpineol and Dracocephalum kotschyi essential oil on tension development to a; first and b; second contractile responses to electrical field stimulation (EFS, 6 V, 50 Hz, 1 s duration) in isolated ileum of rats. Ordinate scale: ileum contractions expressed as % of initial control response. Abscissa scale: log10 concentration of drugs (limonene, α-terpineol or D. kotschyi essential oil). Lines drawn through the points, using two fold increments in concentration. The points are mean and the vertical bars show the SEM (n=6). The changes in the response of vehicle treated control tissues is not statistically significant (ANOVA). Stars shows statistical differences between each drug concentration with its corresponding vehicle treated control. Keys: *P<0.05, **P<0.01, ***P<0.001 (Student's t-test). Maximum concentration of vehicle (DMSO) in the bath was 1.28%.
DISCUSSION
Dracocephalum species have traditionally been used for the treatment of a number of aliments including GI tract disorders (24,25,26). It is believed that this plant has astringent and carminative properties (26). Among these species D. kotschyi has widely been used for its antispasmodic and analgesic effect in Iranian Folk Medicine (3). However, so far there is no pharmacological report on the antispasmodic activity of this plant. In this research we have studied antispasmodic of essential oil of D. kotschyi and two of its major constituents limonene and α-terpineol on isolated ileum. Although all these compounds had relaxant effect on ileum contractions induced by KCl, ACh and EFS but they had different potencies. Limonene was the most potent component, while the potency of α-terpineol was similar to that of the essential oil on KCl responses. The potency of α-terpineol was less than limonene in all cases. Furthermore, the potency of α-terpineol on KCl induced contraction was similar to the essential oil but it was weaker on inhibiting contraction induced by ACh or EFS. For example limonene is about 8 times more potent than the essential oil while essential oil is 4 times more potent than the α-terpineol in inhibiting ACh responses.
Therefore, it is very likely that limonene is the most active constituent which is responsible for inhibitory action of the essential oil. Alfa-terpineol on the other hand, is either equipotent or less potent than the essential oil and furthermore accounts for less than 2% composition of the essential oil therefore, it can not be responsible for the antispasmodic activity of the essential oil. However, α-terpineol has been reported to be responsible for the analgesic effect of the essential oil (6). Other identified constituents of the essential oil are geraniol, α-pinene and β-pinene, linalool and citral. Ciral which is a mixture of two monoterpenes (geranial and neral), has antispasmodic activity on rat ileum contractions induced by KCl, ACh or 5-HT (18). α-pinene and β-pinene also have shown to inhibit rat ileum contraction induced by KCl and ACh (16). Geraniol is another component of the essential oil which has also reported to reduce contractions induced by KCl, ACh or EFS in rat ileum (12). Linalool, another constituent, is reported to reduce the ACh evoked release in neuromuscular junction (27). From identified components in the essential oil, geraniol (10%), limonene (9%), α-pinene (10%) and citral (12.5%) are the major components with antispasmodic effect on rat ileum and therefore, they ought to have major contribution in the inhibitory effect seen with the essential oil.
In smooth muscles, contraction is produced either by Ca2+ entry through voltage gated or ligand gated calcium channels, or by inositol triphosphate (IP3) medicated Ca2+ release from intracellular stores (28). KCl cause membrane depolarization of smooth muscle and activation of voltage gated Ca2+ channels, while ACh by activation of M3 muscarinic receptors which is G-protein coupled receptors, releases the Ca2+ ions from intracellular stores (29,30). Therefore, in this research two different spasmogens with two different mechanism of action was used. For instance, muscarinic antagonist at concentrations which totally remove the response to ACh had no effect on contraction induced by KCl (12,15). On the other hand, nifedipine totally removed contractile response to KCl, while only partially remove the response to ACh (15).
Application of EFS is more closely related to natural stimulation of ileum, because in this way the contractile activity is due to the release of neurotrasmitters form entric nervous system (ENS). The ENS consists of neurons whose cell bodies lie in the intramural plexes in the wall of the intestine (31). The ENS is a complex network of neurons involving many neuropeptides (including substance P, vasoactive intestinal peptide and neuropeptide Y) and other neurotransmitters (such as ACh, 5-HT, nitric oxide and ATP) (28,31).
Opioids like loperamide and muscarinic receptor antagonists such atropine are two known groups of antispasmodic drugs with clinical use (30,32,33,34,35). In comparison, the inhibitory effect of D. kotschyi essential oil is more similar to loperamide than atropine. Therefore, mechanism effect of the essential oil is somehow different from pure muscarinc antagonists or calcium channel blockers because it removed both responses to KCl and ACh. Inhibition of ileum contraction by these three spasmogens (ACh, KCl and EFS) indicated that a more general mechanism of action may be involved probably at myosin light chain level.
CONCLUSION
The essential oil of D. kotschyi had an inhibitory effect on contractions induced by KCl, ACh and EFS on rat ileum. The main components which cause a decrease in GI motility are limonene, geraniol, α-pinene and citral. Other constituents including α-terpineol may have minor contribution in inhibiting ileum contraction. Investigation of exact mechanism of action of each component is recommended for further drug development.
ACKNOWLEDGMENTS
The content of this paper is extracted from the Pharm.D thesis NO. 391481 submitted by F. Kasiri which was financially supported by the Research Department of Isfahan University of Medical Sciences, Isfahan, I.R. Iran.
REFERENCES
- 1.Rechinger KH. Flora Iranica. Akademische Druck-u Verlagsanstalt Graz. 1982:218. [Google Scholar]
- 2.Naghibi F, Mosaddegh M, Mohammadi Motamed M, Ghorbani A. Labiatae family in folk medicine in Iran from ethnobotany to pharmacology. Iranian J Pharm Res. 2005;2:63–79. [Google Scholar]
- 3.Zargari A. Tehran: Tehran University Publication; 1990. Medicinl plants; pp. 82–83. [Google Scholar]
- 4.Yaghmai M, Taffazoli R. The essential oil of Dracocephalum kotschyi Boiss. Flavour and Frag J. 1988;3:33–36. [Google Scholar]
- 5.Saeidnia S, Gohari AR, Hadjiakhoondi A, Shafiee A. Bioactive compounds of the volatile oil of Dracocephalum kotschyi. J Biosci. 2007;62:793–796. doi: 10.1515/znc-2007-11-1203. [DOI] [PubMed] [Google Scholar]
- 6.Golshani S, Karamkhani F, Monsef-Esfehani HR, Abdollahi M. Antinociceptive effects of the essential oil of Dracocephalum kotschyi in the mouse writhing test. J Pharm Pharmaceut Sci. 2004;7:76–79. [PubMed] [Google Scholar]
- 7.Faham N, Javidnia K, Bahmani M, Amirghofran Z. Calycopterin, an immunoinhibitory compound from the extract of Dracocephalum kotschyi. Phytother Res. 2008;22:1154–1158. doi: 10.1002/ptr.2382. [DOI] [PubMed] [Google Scholar]
- 8.Amirghofran Z, Azadbakht M, Karimi MH. Evaluation of the immunomodulatory effect of five herbal plants. J Ethanopharmacol. 2000;72:167–172. doi: 10.1016/s0378-8741(00)00234-8. [DOI] [PubMed] [Google Scholar]
- 9.Sajjadi SE, Movahedian Atar AM, Yektaian A. Antihyperlipidemic effect of hydroalcoholic extract and polyphenolic fraction from Dracocephalum kotschyi Boiss. Pharm Acta Helv. 1998;73:167–170. doi: 10.1016/s0031-6865(98)00016-8. [DOI] [PubMed] [Google Scholar]
- 10.Jahaniani F, Ebrahimi SA, Rahbar-Roshandel N, Mahmoudian M. Xanthomicrol is the main cytotoxic component of Dracocephalum kotschyii and a potential anti-cancer agent. Phytochem. 2005;66:1581–1592. doi: 10.1016/j.phytochem.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 11.Hajhashemi V, Sadraei H, Ghannadi AR, Mohseni M. Antispasmodic and anti-diarrhoeal effect of Satureja hortensis L. essential oil. J Ethnopharmacol. 2000;71:187–192. doi: 10.1016/s0378-8741(99)00209-3. [DOI] [PubMed] [Google Scholar]
- 12.Sadraei H, Asghari G, Emami S. Inhibitory effect of Rosa damascena Mill. Flower essential oil, geraniol and citronellol on rat ileum contraction. Res Pharm Sci. 2013;8:17–23. [PMC free article] [PubMed] [Google Scholar]
- 13.Sadraei H, Asghari G, Naddafi A. Relaxant effect of essential oil and hydro-alcoholic extract of Pycnocycla spinosa Decne. ex Boiss. On ileum contractions. Phytother Res. 2003;17:645–649. doi: 10.1002/ptr.1217. [DOI] [PubMed] [Google Scholar]
- 14.Sadraei H, Asghari G, Hekmatti AA. Antispasmodic effect of three fraction of hydroalcoholic extract of Pycnocycla spinosa. J Ethnopharmacol. 2003;86:187–190. doi: 10.1016/s0378-8741(03)00077-1. [DOI] [PubMed] [Google Scholar]
- 15.Sadraei H, Shokoohinia Y, Sajjadi SE, Mozafari M. Antispasmodic effect of Prangos ferulacea acetone extract and its main component osthole on ileum contraction. Res Pharm Sci. 2010;5:29–39. [PMC free article] [PubMed] [Google Scholar]
- 16.Sadraei H, Asghari G, Hajhashemi V, Kolagar A, Ebrahimi M. Spasmolytic activity of essential oil and variousn extract of Ferula gummosa Boiss on ileum contractions. Phytomed. 2001;8:370–376. doi: 10.1078/0944-7113-00052. [DOI] [PubMed] [Google Scholar]
- 17.Sadraei H, Ghannadi AR, Takei-bavani M. Effects of Zataria multiflora and Carum carvi essential oils and hydroalcoholic extracts of Passiflora incarnata, Berberis integerrima and Crocus sativus on rat isolated uterus contractions. Inter J Aromother. 2003;13:121–128. [Google Scholar]
- 18.Sadraei H, Ghannadi AR, Malekshahi K. Relaxant effect of essential oil of Melissa officinalis and citral on rat ileum contraction. Fitoterapia. 2003;74:445–452. doi: 10.1016/s0367-326x(03)00109-6. [DOI] [PubMed] [Google Scholar]
- 19.Sadraei H, Hajhashemi V, Ghannadi AR, Mohseni M. Antispasmodic effect of aerial part of Teucurium poliume L. essential oil on rat isolated ileum in vitro. Med J Islam Repub Iran. 2001;14:355–358. [Google Scholar]
- 20.Sadraei H, Ghannadi AR, Malekshahi K. Composition of the essential oil of asa-foetida and its spasmolytic action. Saudi Pharm J. 2003;11:136–140. [Google Scholar]
- 21.Samuelsson G. Sweden: Pharmaceutical Press; 1999. Drugs of natural origin, Stockholm, Swedish; pp. 48–49. [Google Scholar]
- 22.Committee for the update of the guide for the care and use of laboratory animals National Research Council. Washington DC: The National Academies Press; 2010. Guide for the Care and use of Laboratory animals; pp. 11–37. [Google Scholar]
- 23.Ekblad E, Sundler F. Motor responses in rat ileum evoked by nitric oxide donors vs. field stimulation: Modulation by pituitary adenylate cyclase activating peptide forskolin and guanylate cyclase inhibitors. J Pharmacol Exp Ther. 1997;283:23–28. [PubMed] [Google Scholar]
- 24.Budantsev AL, Shavarda AL. Chemical composition and useful properties of dracocephalum species of U.S.S.R Flora:I Contents and composition of essential oils. Rastit Resur. 1989;22:550–561. [Google Scholar]
- 25.Amin G. Tehran: Ministry of Health Publications; 1991. Popular Medicinal Plants of Iran; p. 41. [Google Scholar]
- 26.Chopra RN, Nayar SL, Chopra IC. New Delhi: CSIR; 1950. Glossary of Indian Medicinal Plants; p. 18. [Google Scholar]
- 27.Re L, Barocci S, Sonnino S, Mencarelli A, Vivani C, Paolucci G, et al. Linalool modifies the nicotinic receptor-ion channel kinetics at the mouse neuromuscular junction. Pharmacol Res. 2000;42:82–177. doi: 10.1006/phrs.2000.0671. [DOI] [PubMed] [Google Scholar]
- 28.Rang HP, Dale MM, Ritter JM, Flower RJ. 6th edi. Chapter 9. London: Churchill Livingstone; 2007. Rang and Dale's Pharmacology; pp. 131–167. [Google Scholar]
- 29.Ratz PH, Berg KM, Urban NH, Miner AS. Regulation of smooth muscle calcium sensitivity: KCl as calcium-sensitizing stimulus. Am J Physiol Cell Physiol. 2005;288:769–783. doi: 10.1152/ajpcell.00529.2004. [DOI] [PubMed] [Google Scholar]
- 30.Goyal RK. Identification localization and classification of muscarinic receptor subtypes in the gut. Life Sci. 1988;43:2209–2220. doi: 10.1016/0024-3205(88)90414-6. [DOI] [PubMed] [Google Scholar]
- 31.Goyal RK, Kirano I. The enteric nervous system. N Eng J Med. 1996;334:1106–1115. doi: 10.1056/NEJM199604253341707. [DOI] [PubMed] [Google Scholar]
- 32.Pasricha PJ. Treatment of disorders of bowel motility and water flux, antiemetics, agents used in biliary and pancreatic disease. In: Hardman J.G., Limbird L.E., editors. Goodman & Gilmans The Pharmacological Basis of Therapeutic. Vol. 11. New York: McGraw-Hill; 2006. pp. 983–1008. [Google Scholar]
- 33.Daugherty LM. Loperamide hydrochloride. Am Pharm. 1990:45–48. doi: 10.1016/s0160-3450(15)31396-9. [DOI] [PubMed] [Google Scholar]
- 34.Kromer W. Endogenous and exogenous opioid in the control of gastrointestinal motility and secretion. Pharmacol Rev. 1988;40:121–162. [PubMed] [Google Scholar]
- 35.Reynolds IJ, Gould RJ, Snyder SH. Loperamide: Blocked of calcium channels as a mechanism for antidiarrheal effect. J Pharmacol Exp Ther. 1984;231:628–632. [PubMed] [Google Scholar]


