Abstract
In the present study possible effects of black pomegranate peel extract (PPE) on the B16F10 melanoma cells proliferation and Human Umbilical Vein Endothelial Cells (HUVECs) angiogenesis were investigated. PPE was added into the cell lines (B16F10 and HUVECs) media with different concentrations (10–450 μg/ml). After 48 h, the cell survival was measured by 3-(Dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide (MTT) assay. Angiogenesis was investigated by matrigel assay (PPE (200, 300, 400 μg/ml)); HUVECs, vascular endothelial growth factor (VEGF) mRNA expression was detected by quantitative reverse transcriptase–polymerase chain reaction (QRT-PCR) assay. VEGF concentration in culture medium of HUVECs was determined by enzyme-linked immunosorbent assay (ELISA). PPE had positive anti proliferative effect on melanoma cells in a dose-dependent manner, but not on HUVECs. The matrigel assay results indicated that PPE significantly inhibited length, size and junction of the tube like structures (P<0.05). VEGF mRNA expression and concentration levels in culture medium of PPE treated HUVECs reduced significantly in a concentration-dependent manner (P<0.05). Simultaneous inhibition of melanoma cell proliferation and angiogenesis proposed that, PPE can be a good candidate against melanoma development. Based on the results, PPE could effectively suppress angiogenesis potentially through a VEGF dependent mechanism. Further studies are needed to confirm these results.
Keywords: Pomegranate, Human Umbilical Vein Endothelial Cells, Angiogenesis, B16F10 melanoma
INTRODUCTION
Malignant melanoma is one of the most highly invasive and metastatic tumor with poor prognosis and highly resistance to treatment. Furthermore, melanoma is an increasingly common malignancy and its mortality rates have been quickly increasing in recent years (1).
Parallel with progression, melanoma acquires a rich vascular network, supporting tumor cell growth and metastases (2). Melanoma neovascularization has been correlated with poor prognosis, overall survival, ulceration, and increased rate of relapse (3).
In spite of recent advances in therapeutic approaches, the survival rate of patients with melanoma, has not improved substantially, indicating the need for novel anticancer therapies (4).
There are several anti-cancer drugs which are either natural products or natural product derivatives (5). In the present study we focus on the effect of Punica granatum peel extracts (PPE) on melanoma cancer cells and the effects on angiogenesis in vitro.
The pomegranate, P. granatum, is an ancient fruit, and the predominant member of two species comprising the Punicaceae family (6). Extracts of all parts of the fruit appear to have therapeutic effects (7). Pomegranates have great antioxidant potential due to high levels of phenolic compounds, flavonoids, anthocyanins, tannins, ascorbic acids and gallic acid present in the fruit (8,9,10). The beneficial effects of pomegranate have been reported in the prevention of various diseases such as cardiovascular disease, bacterial infection, inflammation, diabetes and skin photoaging (11,12,13,14,15).
Additionally, several studies have demonstrated the therapeutic potential of pomegranate in vivo and in vitro for the treatment of a variety of tumor types, including colorectal cancer, lung, prostate, esophagus, oral and breast cancer (16,17,18).
Pomegranate has been used in Iranian traditional medicine for a long time and for different purposes (19). Black pomegranate is one of pomegranate cultivars which bred in Iran, almost rare than others (20). It has been used as an herbal medicine for different diseases.
Previous studies have demonstrated that peel of the black pomegranate extract has the highest flavonoid content than the other pomegranate cultivar peels (19). In our previous study, we showed that black pomegranate in 10, 100, 1000 μg/ml doses can inhibit angiogenesis in vitro (21).
Moreover, it is very interesting that there are some evidences for the anti-angiogenic properties of pomegranate (6). It has been shown that some of pomegranate constituents such as estrogenic flavonoids are anti-angiogenic (7,22) or suppress angiogenesis via down regulation of factors which promote angiogenesis (23).
Since, angiogenesis is essential for melanoma tumor progression and metastatic escape, it is highly desirable to find therapeutic approaches that simultaneously inhibit melanoma tumor growth and angiogenesis.
In the present study, we have investigated the inhibitory effect of PPE on melanoma cells growth as well as endothelial cells angiogenesis, vascular endothelial growth factor (VEGF) mRNA and protein expression.
MATERIALS AND METHODS
Preparation of plant extracts
Fruit of black pomegranate obtained from Agriculture Research Center of Isfahan, Iran. The genus and species of the plant were identified by this center. The peel was separated manually, dried, and powdered before extraction. Dried pomegranate peel was extracted at room temperature for 72 h with 15 ml ethanol 70% containing 1% acetic acid and then filtered. The solvent was evaporated under vacuum using a rotary evaporator and the concentrated extracts were stored at -70 °C until the use.
Cell culture
The human umbilical vein endothelial cells (HUVECs) (C554) and melanoma cell line (B16F10) (National Cell bank of Iran affiliated to Pasteur Institute, Tehran, Iran) were cultured in dulbecco's modified eagle's medium (DMEM) supplemented with 1% antibiotics (100 units/ml penicillin and 100 μg/ml streptomycin) and 10% fetal calf serum until the third passage before performing the experiments. All cell culture materials were from Gibco, USA. Cells were grown to confluence at 37 °C in 5% CO2/air.
Cytotoxicity assay
In vitro cytotoxicity was evaluated by plating melanoma cells (1 × 104 cells/well in a 96 well plates) in 100 μl of medium per well and allowed to attach. After incubation, 100 μl various concentrations of PPE (10-450 μg/ml) were added to the cells and incubated for 48 h.
The aforementioned concentrations were adopted based on a previous study that investigated the antiproliferative and apoptotic effects of PPE on MCF-7 human breast cancer cell where each extracted concentration was added into three separated wells (24). The metabolic activity in each well was determined by 3-(Dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide (MTT) assay and compared with untreated cells as described by Okonogi and coworkers (25). Additionally, we tested for the ability of PPE to inhibit HUVECs growth using the MTT assay as described above. Plates were read by using an enzyme-linked immunosorbent assay (ELISA) plate reader (ELX 800-BioTek-USA)at 540 nm with a reference wavelength of 630 nm. The cell viability was determined by the following formula:
% Cell viability = (Mean absorbance in test wells/Mean absorbance in control wells) × 100
Angiogenesis assays
The tube formation assay was performed on 24-well plates coated with 100 μl of Matrigel Basement Membrane Matrix (Invitrogen, USA) and polymerized for 30 min at 37 °C. The cells (1 × 105) were divided in 4 groups with different treatments which include pomegranate peel extract in different concentrations (200, 300, 400 μg/ml) and dimethyl sulfoxide (DMSO) 0.1% as control group. They were plated on to a layer of Matrigel.
Matrigel cultures were incubated for 24 h at 37 °C/5% CO2 humidified atmosphere. Then the cells were stained at the end of the incubation period after the tube network has formed using a cell-permeable dye (Calcein, AM). After adding the dye, cells incubated for 30 min at 37 °C and 5% CO2 while protecting from light. The maximum dye concentration should be 2 μg/ml. Finally the center of each well was photographed with a Nikon camera attached to a Olympus BX51 fluorescent microscope(USA). Fluorescence images were quantified using the AngioQuant v1.33 software (The MathWorks, Natick, MA) to quantitate the extent of tubule formation in terms of the lengths, size, and number of junctions in each replicate well (21).
Quantitative reverse transcriptase–polymerase chain reaction
Total RNA was extracted from HUVECs who were treated or left untreated for 24 h, using RNeasy Mini plus Kit (Qiagen, Valencia, CA, USA) following the manufacturer's protocols.
The quality of RNA was verified by spectrophotometer and gel electrophoresis. cDNA was synthesized using RevertAidTM Reverse Transcriptase (Fermentas, Vilnius, Lithuania) with oligo-dT primers as described by Mowla and coworkers (26). Quantitative realtime reverse transcriptase–polymerase chain reaction (Quantitative real time RT-PCR) was performed using specific primers for VEGF and glyceraldehyde dehydrogenase (GAPDH) (as an internal control) mRNAs (Table 1) with the Maxima SYBR Green/ROX qPCR Master Mix (Fermentas, Vilnius, Lithuania) and run on the Rotor-gene 6000 (Qiagen, Hilden, Germany).
Table 1.
Specific primers for VEGF and GAPDH (as an internal control) mRNAs.

The PCR cycling conditions for the genes included an initial denaturation step at 95 °C for 10 min, followed by 45 amplification cycles consisting of denaturation at 95 °C for 15 s, annealing at 60 °C for 30 s and an extension at 72 °C for 30 s.
Determination of VEGF concentration in culture medium
The cell culture media over 24 h of the treatment were collected, centrifuged at 250 × g to remove debris, and frozen until further analysis. VEGF concentrations were determined using a quantitative ELISA (Immuno-Biological Laboratories, GmbH, Hamburg, Germany). The amount of VEGF immunoreactivity was calculated using recombinant human VEGF standards present on each microtiter plate. Optical densities were determined at 450 nm using an ELISA reader.
Statistical analysis
The experiments were performed in duplicate and replicated three times. At last, one way analysis of variance (ANOVA) and kruskal wallis analysis were performed to analyze the data using the software SPSS 15. Values of P<0.05 were considered statistically significant.
RESULTS
Inhibition of cell growth by PPE in melanoma cells but not in HUVECs
Initially, in our study, we investigated the antiproliferative effects of PPE on melanoma cell line. Therefore, using B16F10 cells, we evaluated the effect of PPE on the growth of these cells by MTT assay.
As shown in Fig. 1A, PPE treatment of HUVECs resulted in 6 and 17% decrease in cell viability at concentrations 10–450 μg/ml of PPE, respectively indicating that PPE had minimal toxic effect on these cells. PPE showed toxic effects on B16F10 cells at its lowest (1%) up to its highest (61%) concentrations dose dependently (Fig. 1B). The calculated IC50 value for the PPE against B16F10 cells was found to be 310.21 indicating that the PEE was toxic against B16F10 cells.
Fig. 1.

The effects of PPE on cell viability. As detailed in Materials and methods, A; C554 HUVECs, B; B16F10 melanoma cells were treated with PPE and the viability of cells was determined by the MTT assay. The data are expressed as the percentage of cell viability compare to control.
Inhibition of endothelial cell tube formation by black pomegranate peel extracts
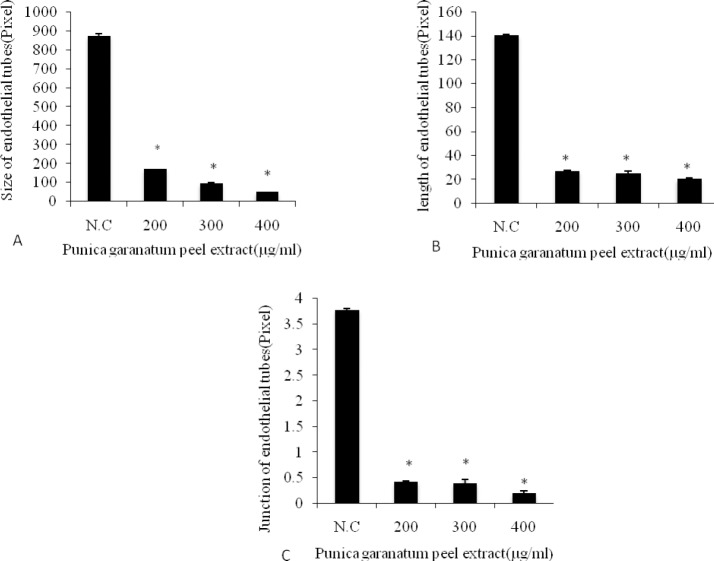
As described in earlier sections, concentrations 200, 300, and 400 μg/ml were selected to investigate the effects of PPE on angiogenesis. To ascertain the anti-angiogenic activity of PPE, the treatment started at the time of HUVEC seeding on to Matrigel. We performed the in vitro angiogenesis assay and examined the ability of endothelial cells to form tubes in the absence or presence of PPE. HUVECs tube formation was observed over a period of time. Results in Fig. 2 indicate that PPE can suppress the formation of tube-like structures at all concentrations tested in this study. Our results showed that PPE significantly reduced not only the size of the tubes (P<0.05) (Fig. 3. A) at all tested concentrations but also the length and the number of junctions of the tubes relative to the negative control group (P<0.05) (Fig.3B, 3C).
Fig. 2.

Inhibitory effect of pomegranate peel extract on tube formation of HUVECs. HUVECs were plated on the wells coated with 100 μl of Matrigel basement membrane matrix. After 24 h treatment with different doses of PPE, the cells were stained with calcein and photographed with a Nikon camera attached to a fluorescent microscope at ×10 magnification.
Fig. 3.
Effect of PPE on HUVEC tube formation in vitro (N.C:Negative Control). A; Quantitative data for size of tube are shown for 24 h of PPE treatment, B; Quantitative data for length of tube are shown for 24 h of PPE treatment, C; Quantitative data for total number of junction of tubes after 24h incubation with PPE. Data are expressed as percent inhibition compared with control and are shown as mean ± S.D. *; significantly different from control (Bonferroni modified Student's t test for multiple comparisons).
PPE inhibits expression of VEGF mRNA in HUVECS
After optimization of the QRT-PCR, expression of VEGF and GAPDH genes was determined using Quantitative real-time RT-PCR in the treated and control cells. Relative expression of VEGF gene was determined by dividing its expression amount to that of the GAPDH gene. There was a concentration-dependent relationship between PPE and its inhibitory effect on VEGF gene expression at 200, 300, and 400 μg/ml and the concentrations of 300 and 400 μg/ml significantly inhibited VEGF mRNA expression (P<0.05) (Fig. 4).
Fig. 4.

Quantitative analysis of mRNA expression of VEGF in HUVEC by Quantitative real time RT-PCR (Taqman). Total RNA were extracted from HUVEC in medium containing different treatments. The relative expression of VEGF were normalized to GAPDH levels measured in the same RNA preparation. Data shown are from three independent experiments analyzed in duplicate.
Suppression of vascular endothelial growth factor secretion by PPE
As shown in Fig. 5, treatment of HUVECs with PPE decreased the VEGF concentration in the culture medium. VEGF concentration was significantly decreased at 300 and 400 μg/ml concentrations of PPE (P<0.05) at 200 μg/ml dose of PPE, however, decreasing VEGF was not significant.
Fig. 5.

ELISA demonstrated decreased levels of VEGF in the culture medium at 300 and 400 μg/ml after 24 h incubation. Data are presented as means ± SD of three experiments. *; P<0:05.
DISCUSSION
A major microenvironmental event in melanoma tumor growth and expansion is the angiogenic switch which is a change in the balance of pro-angiogenic and anti-angiogenic molecules that results in tumor neovascularization (2). Thus, it is very favorable to find substances that simultaneously inhibit melanoma tumor growth and angiogenesis.
To the best of our knowledge, this is the first study that shows the potential anti-angiogenic effect of PPE based on the results of matrigel tube formation assays, VEGF mRNA expression, and VEGF concentration in HUVECs culture medium.
Interestingly, PPE displayed more toxicity against B16F10 melanoma than HUVECs indicating possible selectivity of PPE on tumor cells over normal cells.
Amongst the various angiogenic activators, the VEGF signaling pathway has been implicated as the key regulator of tumor neovascularization (27). Expression of VEGF mRNA is strictly associated with microvascular density (28). VEGF has been demonstrated to have a major association with initiating the process of angiogenesis through regulating proliferation, migration, and differentiation of endothelial cells (29). In the current study, PPE dose-dependently suppressed VEGF mRNA expression, suggesting that PPE inhibits angiogenesis by reducing production of VEGF at transcriptional level. Moreover PPE significantly decreased the secretion of VEGF by HUVECs in culture medium.
There is only one similar study by Toi and colleagues that investigated antiangiogenic effect of various fractions of pomegranate including pomegranate fermented juice polyphenols, pomegranate pericarp polyphenols, cold-pressed pomegranate seed oil, supercritical fluid extracted pomegranate seed oil, pomegranate seed oil polyphenols, and unsaponified pomegranate seed oil (30). The authors showed that antiangiogenic effect of pomegranate fractions was associated with strong downregulation of VEGF. These fractions further inhibited proliferation of HUVECs and angiogenesis in chicken chorioallantoic membrane model as well as myometrial and amniotic fluid fibroblasts.
Pomegranate peel is an important source of phenolics and flavonoids, such as kaempferol, luteolin, quercetin and proanthocyanidin, minerals (mainly K, N, Ca, P, Mg and Na) and complex polysaccharides. Pomegranate peels are also a rich source of hydrolysable tannins including ellagitannin, punicalagin, punicalin and pedunculagin (31). In addition, the antioxidant capacity of PPE is 10 times higher than that of pulp extract (20).
Previous studies have shown that several polyphenols, including epigallocatechin-3-gallate in green tea and resveratrol in red wine, inhibit angiogenesis. It can be hypothesized that PPE may exert anti-angiogenic effect via its polyphenols (32).
Furthermore, pomegranate juice and peels are affluent in estrogenic flavonoids like luteolin which have been shown to have anti-angiogenic effect (22,23) or to inhibit factors which promote angiogenesis, e.g, basic FGF (bFGF) (33).
In the present study, PPE exhibited antiproliferative effects on melanoma cells. It is not known which active compound (s) in PPE is responsible for its antiproliferative effects. However it can be suggested that antiproliferative effects of PPE presented in this study are largely due to its antioxidant effects and polyphenols content (34).
Suresh and coworkers in their study evaluated in vivo anticancer activity of ethanol and aqueous extracts of roots of P. granatum linn against melanoma cells in mice and in vitro inhibitory activity on MDA-MB–435 human melanoma cells (35). They showed a significant reduction in tumor growth by ethanol extract both in in vitro and in vivo models which was attributed to the collective presence of polyphenols, alkaloids and sterols.
About the antiproliferatory effect of pomegranate on the other cancer cell lines, Daiz and colleagues have shown that pomegranate extract inhibits the proliferation and viability of MMTV-Wnt-1 mouse mammary cancer stem cells in vitro (36).
In the other study Khan and coworkers showed that pomegranate fruit extract inhibited prosurvival pathways in human A549 lung carcinoma cells and tumor growth in athymic nude mice (37). Also it has been found that pomegranate juice can be used for its cancer-chemoprevention as well as cancer-chemotherapeutic effects against prostate cancer in humans (38).
CONCLUSION
inhibition of melanoma cell proliferation and angiogenesis indicates that, PPE can be a good candidate against melanoma development. Based on the results of the present study, PPE could effectively suppress angiogenesis potentially through a VEGF dependent mechanism. Further studies are needed to confirm these results.
ACKNOWLEDGMENT
This study was funded by Isfahan University of Medical Sciences (IUMS) grant number: 291188.
REFERENCES
- 1.Abe R, Fujita Y, Yamagishi S. Angiogenesis and metastasis inhibitors for the treatment of malignant melanoma. Mini Rev Med Chem. 2007;7:649–661. doi: 10.2174/138955707780859440. [DOI] [PubMed] [Google Scholar]
- 2.Mahabeleshwar GH, Byzova TV. Angiogenesis in melanoma. Semin Oncol. 2007;34:555–565. doi: 10.1053/j.seminoncol.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ribatti D, Nico B, Floris C, Mangieri D, Piras F, Ennas MG, et al. Microvascular density, vascular endothelial growth factor immunoreactivity in tumor cells, vessel diameter and intussusceptive microvascular growth in primary melanoma. Oncol Rep. 2005;14:81–84. [PubMed] [Google Scholar]
- 4.Oh SH, Woo JK, Jin Q, Kang HJ, Jeong JW, Kim KW, et al. Identification of novel antiangiogenic anticancer activities of deguelin targeting hypoxia-inducible factor-1 alpha. Int J Cancer. 2008;122:5–14. doi: 10.1002/ijc.23075. [DOI] [PubMed] [Google Scholar]
- 5.Pfisterer PH, Wolber G, Efferth T, Rollinger JM, Stuppner H. Natural products in structure-assisted design of molecular cancer therapeutics. Curr Pharm Des. 2010;16:1718–1741. doi: 10.2174/138161210791164027. [DOI] [PubMed] [Google Scholar]
- 6.Jurenka JS. Therapeutic applications of pomegranate (Punica granatum L.): a review. Altern Med Rev. 2008;13:128–144. [PubMed] [Google Scholar]
- 7.Lansky EP, Newman RA. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J Ethnopharmacol. 2007;109:177–206. doi: 10.1016/j.jep.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Mirmiran P, Fazeli MR, Asghari G, Shafiee A, Azizi F. Effect of pomegranate seed oil on hyperlipidaemic subjects: Adouble-blind placebo-controlled clinical trial. Br J Nutr. 2010;104:402–406. doi: 10.1017/S0007114510000504. [DOI] [PubMed] [Google Scholar]
- 9.Caligiani A, Bonzanini F, Palla G, Cirlini M, Bruni R. Characterization of a potential nutraceutical ingredient: Pomegranate (Punica granatum L.) seed oil unsaponifiable fraction. Plant Foods Hum Nutr. 2010;65:277–283. doi: 10.1007/s11130-010-0173-5. [DOI] [PubMed] [Google Scholar]
- 10.Sashi GK, Dobroslawa B, Muntha KR, Guoyi M, Shabana IK, Daneel F. Colon cancer chemopreventive activities of pomegranate ellagitannins and urolithins. J Agric Food Chem. 2010;58:2180–2187. doi: 10.1021/jf903762h. [DOI] [PubMed] [Google Scholar]
- 11.Basu A, Penugonda K. Pomegranate juice: a heart-healthy fruit juice. Nutr Rev. 2009;67:49–56. doi: 10.1111/j.1753-4887.2008.00133.x. [DOI] [PubMed] [Google Scholar]
- 12.Abdollahzadeh Sh, Mashouf R, Mortazavi H, Moghaddam M, Roozbahani N, Vahedi M. Antibacterial and antifungal activities of punica granatum Peel extracts against oral pathogens. J Dent (Tehran) 2011;8:1–6. [PMC free article] [PubMed] [Google Scholar]
- 13.Lee CJ, Chen LG, Liang WL, Wang CC. Anti-inflammatory effects of Punica granatum Linne in vitro and in vivo. Food Chem. 2010;118:315–322. [Google Scholar]
- 14.Parmar HS, Kar A. Antidiabetic potential of Citrus sinensis and Punica granatum peel extracts in alloxan treated male mice. Biofactors. 2007;31:17–24. doi: 10.1002/biof.5520310102. [DOI] [PubMed] [Google Scholar]
- 15.Zaid MA, Afaq F, Syed DN, Dreher M, Mukhtar H. Inhibition of UVB-mediated oxidative stress and markers of photoaging in immortalized HaCaT keratinocytes by pomegranate polyphenol extract POMx. Photochem Photobiol. 2007;83:882–888. doi: 10.1111/j.1751-1097.2007.00157.x. [DOI] [PubMed] [Google Scholar]
- 16.Adhami VM, Khan N, Mukhtar H. Cancer chemoprevention by pomegranate: laboratory and clinical evidence. Nutr Cancer. 2009;61:811–815. doi: 10.1080/01635580903285064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miguel MG, Neves MA, Antunes MD. Pomegranate (Punica granatum L.): A medicinal plant with myriad biological properties - A short review. J Med Plant Res. 2010;4:2836–2847. [Google Scholar]
- 18.Mehta R, Lanksy EP. Breast cancer chemopreventive properties of pomegranate (Punica granatum) fruitextracts in a mouse mammary organ culture. Eur J Cancer Prev. 2004;13:345–348. doi: 10.1097/01.cej.0000136571.70998.5a. [DOI] [PubMed] [Google Scholar]
- 19.Shams Ardekani MR, Hajimahmoodi M, Oveisi MR, Sadeghi N, Jannat B, Ranjbar AM, et al. Comparative antioxidant activity and total flavonoid content of Persian pomegranate (Punica granatum L.) cultivars. Iran J. 2011;10:519–524. [PMC free article] [PubMed] [Google Scholar]
- 20.Moghaddam Gh, Sharifzadeh M, Hassanzadeh Gh, Khanavi M, Hajimahmoodi M. Anti-ulcerogenic activity of the Pomegranate Peel (Punica granatum) methanol extract. Food Nutr Sci. 2013;4:43–48. [Google Scholar]
- 21.Dana N, Haghjooy Javanmard Sh, Fazilati M, Pilehvarian AA. Anti-Angiogenic effects of pomegranate Peel extract (Punica Granatum L.) on human umbilical vein endothelial cells. J Isfahan Med Sch. 2012;30:p1. [Google Scholar]
- 22.Fotsis T, Pepper MS, Montesano R, Aktas E, Breit S, Schweigerer L, et al. Phytoestrogens and inhibition of angiogenesis. Baillieres Clin Endocrinol Metab. 1998;12:649–666. doi: 10.1016/s0950-351x(98)80009-8. [DOI] [PubMed] [Google Scholar]
- 23.Le Marchand L. Cancer preventive effects of flavonoids – a review. Biomed Pharmacother. 2002;56:296–301. doi: 10.1016/s0753-3322(02)00186-5. [DOI] [PubMed] [Google Scholar]
- 24.Dikmen M, Ozturk N, Ozturk Y. The antioxidant potency of Punica granatum L. Fruit peel reduces cell proliferation and induces apoptosis on breast cancer. J Med Food. 2011;14:1638–1646. doi: 10.1089/jmf.2011.0062. [DOI] [PubMed] [Google Scholar]
- 25.Okonogi S, Duangrat C, Anuchpreeda S, Tachakittirungrod S, Chowwanapoonpohn S. Comparison of antioxidant capacities and cytotoxicities of certain fruit peels. Food Chem. 2007;103:839–846. [Google Scholar]
- 26.Mowla SJ, Emadi Bayegi M, Ziaee SAM and Nikpoor P. Evaluating expression and potential diagnostic and prognostic values of Survivin in bladder tumors: a preliminary report. Urol J. 2005;2:141–147. [PubMed] [Google Scholar]
- 27.Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, Feder J, et al. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246:1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- 28.Poon RT, Lau CP, Ho JW, Yu WC, Fan ST, Wong J. Tissue factor expression correlates with tumor angiogenesis and invasiveness in human hepatocellular carcinoma. Clin. Cancer Res. 2003;9:5339–5345. [PubMed] [Google Scholar]
- 29.Tie J, Desai J. Antiangiogenic therapies targeting the vascular endothelia growth factor signaling system. Crit Rev Oncog. 2012;17:51–67. doi: 10.1615/critrevoncog.v17.i1.50. [DOI] [PubMed] [Google Scholar]
- 30.Toi M, Bando H, Ramachandran C, Melnick SJ, Imai A, Fife RS, et al. Preliminary studies on the anti-angiogenic potential of pomegranate fractions in vitro and in vivo. Angiogenesis. 2003;6:121–128. doi: 10.1023/B:AGEN.0000011802.81320.e4. [DOI] [PubMed] [Google Scholar]
- 31.Christaki EV, Bonos EM, Florou-Paneri PC. Dietary benefits of pomegranates in humans and animals. J Food, Agric Environ. 2011;9:142–144. [Google Scholar]
- 32.Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269:199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Sartippour MR, Heber D, Zhang L, Beatty P, Elashoff D, Elashoff R, et al. Inhibition of fibroblast growth factors by green tea. Int J Oncol. 2002;21:487–491. [PubMed] [Google Scholar]
- 34.Khonkarn R, Okonogi S, Ampasavate C, Anuchapreeda S. Investigation of fruit peel extracts as sources for compounds with antioxidant and antiproliferative activities against human cell lines. Food Chem Toxicol. 2010;48:2122–2129. doi: 10.1016/j.fct.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 35.Suresh HM, Shivakumar B, Shivakumar, SI In vivo and in vitro inhibitory potential of Punica granatum Linn roots against melanoma tumor cells. RGUHS J Pharm Sci. 2011;1:216–219. [Google Scholar]
- 36.Dai Z, Nair V, Khan M, Ciolino HP. Pomegranate extract inhibits the proliferation and viability of MMTV-Wnt-1 mouse mammary cancer stem cells in vitro. Oncol Rep. 2010;24:1087–1091. [PubMed] [Google Scholar]
- 37.Khan N, Hadi N, Afaq F, Syed DN, Kweon MH, Mukhtar H. Pomegranate fruit extract inhibits prosurvival pathways in human A549 lung carcinoma cells and tumor growth in athymic nude mice. Carcinogenesis. 2007;28:163–173. doi: 10.1093/carcin/bgl145. [DOI] [PubMed] [Google Scholar]
- 38.Malik A, Afaq F, Sarfaraz S, Adhami VM, Syed DN, Mukhtar H. Pomegranate fruit juice for chemoprevention and chemotherapy of prostate cancer. Proc Natl Acad Sci U S A. 2005;102:14813–14818. doi: 10.1073/pnas.0505870102. [DOI] [PMC free article] [PubMed] [Google Scholar]