Abstract
Multipotent mesenchymal stem cells (MSCs) are recently found to alter the tumor condition. However their exact role in tumor development is not yet fully unraveled. MSCs were established to perform many of their actions through paracrine effect. Thus investigation of MSC secretome interaction with tumor cells may provide important information for scientists who are attempting to apply stem cells in the treatment of the disease. In this study we investigated the effect of human Wharton's jelly derived MSC (WJ-MSCs) secretome on proliferation, apoptotic potential of A549 lung cancer cells, and their response to the chemotherapeutic agent doxorubicin. WJ-MSCs were isolated from human umbilical cord and then characterized according to the International Society for Cellular Therapy criteria and WJ-MSC secretome was collected. BrdU cell proliferation assay and Annexin V-PI staining were used for the evaluation of cytotoxic and proapoptotic effects of WJ-MSC secretome on A549 cells. WJ-MSC secretome neither induced proliferation of lung cancer cells nor affected the apoptotic potential of the tumor cells. We also studied the combinatorial effect of WJ-MSC secretome and the anticancer drug doxorubicinwhich showed no induction of drug resistance when A549 cells was treated with combination of WJ-MSC secretome and doxorubicin. Although MSCs did not show antitumor properties, our in vitro results showed that MSC secretome was not tumorigenic and also did not make lung cancer cells resistant to doxorubicin. Thus MSC secretome could be considered safe for other medical purposes such as cardiovascular, neurodegenerative, and autoimmune diseases which may exist or occur in cancer patients.
Keywords: A549 lung cancer cells, Mesenchymal stem cells, Secretome
INTRODUCTION
Stem cells play important roles in tumor microenvironment. Recently, multipotent mesenchymal stem cells (MSCs) found to home and reside within the tumor microenvironment with great affinity (tumor tropism) altering the tumor condition (1). Numerous studies have investigated the effect of mesenchymal stem cells on different cancer types. However, the results are controversial and no consistent conclusion has been presented till now. To shed the light on these controversies, further investigations are imperative in this field. Previous studies have shown that diverse results can be obtained depending on the tumor type, MSC source, MSC dose, time of injection, study design, and the animal model (2). Bone marrow (BM) has been the most common source for isolation of MSCs; however, BM sampling is an invasive process and is available in a very limited amount. Thus, MSCs from other sources should be investigated in particular.
Umbilical cord mesenchymal stem cells (UC-MSCs) are one of the best alternatives to BM-MSCs. UC-MSCs are categorized as peri-natal stem cells which are neither embryonic stem cells nor somatic (adult) stem cells; they represent a bridge between embryonic and adult stem cells. They display standard properties and criteria including cell surface marker expression, plastic adherence, and differentiation into the cells of mesenchymal lineage (i.e., fat, bone, muscle, and cartilage) under appropriate conditions to be accepted as mesenchymal stem cell. However, UC derived MSCs have the advantages of both embryonic and adult stem cells as they possess pluripotency properties and multipotent tissue maintenance. On the other hand, they do not encompass similar ethical challenges as embryonic stem cells (when proper institutional review board approval and/or patient consent are obtained). However, the problem of relative limited proliferative potential (i.e. difficulty of expanding ex vivo.) as observed with adult stem cells or invasive and high risk tissue sampling from patients are not challenging for peri-natal sources of stem cells (3).
Considering the fact that MSCs are proposed for many therapeutic goals (4), their interaction with tumor cells should be determined. MSCs may inhibit or potentiate cancer tumor cell growth. Thus their use in patients with cancers may be contraindicated. In addition, application of MSCs in cancer patients is not safe if they make tumor cells resistant to the chemotherapeutic agents. Therefore, we considered these possibilities in our study design.
Chemical factors secreted from different cell types in the tumor microenvironmet play important roles in the tumor development. In the other words, the bidirectional signaling between malignant cells and their secreted cytokines with non-malignant cells affects the establishment, progression, and metastatic dissemination of cancer cells significantly (5). MSCs as a member of tumor micro-environment and a cell with high paracrine relationship action (6,7,8) have the potential to release substances which affect tumor cells (9). However, their interaction with different tumor types is diverse and should be investigated in each cancer type.
Lung cancer is one of the most malignant tumors and represents a major threat to the human health. Although a great deal of investigations is in progress (10,11,12) and the treatment of the lung cancer has been improved, the mortality in the lung cancer patients remains high and it is the leading cause of the death among diverse cancer types (13). In order to decrease cancer mortality, studies should focus on further prevention of the cancers and new treatment strategies.
The objective of the current study was to investigate the effect of UC-MSC secretome on the human lung cancer cells (A549).
MATERIALS AND METHODS
Materials
High glucose dulbecco's modified eagle's medium (DMEM), Roswell Park Memorial Institute (RPMI) 1640, Trypsin-EDTA solution, fetal bovine serum (FBS), antibiotic solution containing streptomycin (10 mg/ml) and penicillin (10000 IU/ml) and amphotericin B (25 μg/ml), dimethyl sulfoxide solution (all from PAA Laboratories GmbH, Austria), anti CD34, anti CD45, anti CD73, anti CD90, anti CD105 conjugated mouse anti-human antibodies and their isotypes (eBioscience, Austria), STEMPRO osteogenesis and adipogenesis and chondrogenesis differentia-tion kits (Gibco, USA), Oil Red O (Sigma Aldrich, USA), Alizarin Red S (Fluka, USA), Alcian Blue (Sigma Aldrich, USA), IFNγ (Exir, Iran), Liposomal doxorubicin (Exir, Iran), Apoptosis detection kit (Roche, Germany), BrdU cell proliferation kit (Roche, Germany), 10 cm petri dish, T25 and T75 tissue culture flasks (Orange, Belgium), 6, 12, 24, and 96 well plates (Orange, Belgium).
Isolation and expansion of human Wharton's jelly MSCs
Umbilical cord was collected from healthy donor after her first term delivery (cesarean section) and immediately transferred to the laboratory (after informed patient consent). The umbilical cord was cut into 1.5-cm length pieces under laminar flow hood. Each piece was then cut open lengthwise and the umbilical blood vessels were removed. The gelatinous tissue surrounding the vessels was excised and plated on a sterile 6-cm plate (12-14 pieces in each plate), then covered completely with culture medium (Low glucose DMEM supplemented with 10% FBS and 1% pen/strep), plates were incubated at 37 °C in humidified incubator under 5% CO2. The media were replaced every 2-4 days until cells migrated out of tissue fragments and proliferated to form confluent monolayer. Then cells were subcultured and used for further experiments (14).
MSC characterization
Surface marker detection
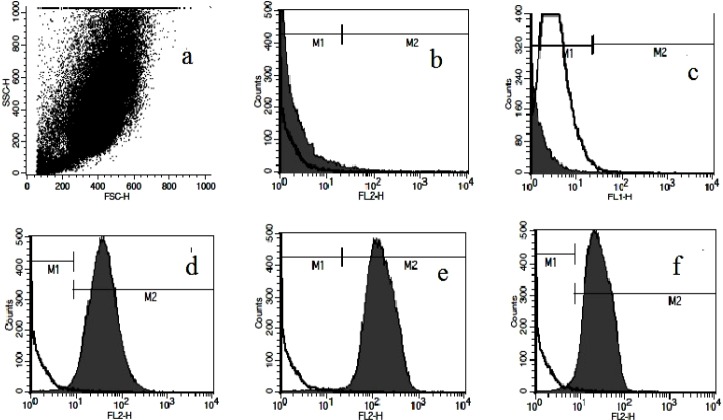
Antihuman antibodies which mark the following cell surface markers were used: CD34, CD45, CD73, CD90, and CD105. Cells incubated with identical concentrations of FITC-, PE-, conjugated mouse IgG isotype antibodies provided as negative controls. At least 10,000 events were acquired on BD FACSCalibur® flowcytometer (BD biosciences, USA), and the results were analyzed using CellQuest™ Pro Software (Version 5.1).
Mesodermal lineage differentiation
WJ-MSCs at passage 6-7 were used for investigation of their differentiation potential to osteocytes, adipocytes, and chondrocytes. Differentiation experiments were performed using STEMPRO osteogenesis and adipogenesis and chondrogenesis differentiation kits as described by manufacturer.
Osteogenic differentiation was evaluated by Alizarin Red-S staining. Oil Red O solution was used for differentiated adipocyte staining. Paraffin blocks were prepared from differentiated chondrogenic mass, and then cut into 5 μm sections onto slides and sections were stained with Alcian Blue solution.
Lung cancer cell preparation and culture expansion
A549 cells were obtained from Pasture Institute of Iran and grown in RPMI 1640 medium supplemented with 10% FBS and 1% Pen/Strep. Cells were incubated under 5% CO2 and 37 °C condition. The media were changed every 3 days and cells were subcultured when confluence obtained, until enough number of cells produced.
Preparation of WJ-MSC secretome before and after IFNγ stimulation
Early passaged hWJ-MSCs (P4) were first grown to 60% confluence in DMEM medium. Cells were divided into two groups: intact MSCs and IFNγ stimulated MSCs. Culture medium was removed and then MSCs of the second group was treated with IFNγ1b (20 ng/ml) (15).
After 48 h the medium was discarded and monolayers were washed twice with PBS to remove any residual medium and the cells grown in DMEM for 24 h after which the MSC secretome (MSC sec) and IFNγ stimulated MSC secretome (stMSC sec) was separated.
The secretome was centrifuged in 1800 rpm for 5 min to remove cell debries and then filtered using 0.22 μm filters and stored at -80 °C until experiment was performed. Total protein concentration of the secretome was measured by nanodrop spectrometer (Eppendorf, USA).
Cytotoxicity assay of WJ-MSC secretome effect on cancer cells
A549 cells were seeded in 96 well plates at density of 5000 cells/ml. After 24 h cells were treated with 50% MSC sec, or 50% MSC sec + 0.2 μg/ml liposomal doxorubicin, or 50% stMSC sec, or 50% stMSC sec + 0.2 μg/ml liposomal doxorubicin for a duration of 72 h. Then BrdU proliferation assay was performed according to the manufacturer's instruction. Briefly; cells were incubated with BrdU labeling solution for 2 h, then fixed and DNA was denatured. Cells were incubated with peroxidase conjugated anti-BrdU antibody. Color reaction was occurred after addition of the substrate and colorimetric assay was performed.
Apoptosis assay using Annexin V- PI staining
A549 cells were seeded in 6 well plates at a density of 6000 cells/ml. After 24 h cells were treated with 50% MSC sec, or 50% MSC sec + 0.2 μg/ml liposomal doxorubicin, or 50% stMSC sec, or 50% stMSC sec + 0.2 μg/ml liposomal doxorubicin for a duration of 72 h. Then cells were harvested for co-staining with FITC conjugated Annexin V and propidium iodide using Apoptosis Detection Kit. Apoptosis assay was performed according to the manufacturer using flowcytometer.
Statistical analysis
Data are expressed as mean ± SD (standard deviation). One-way or two-way analysis of variance (ANOVA) was applied to the means to determine statistical differences between experimental groups. Post hoc comparisons were performed by Tukey's test. Differences between mean values were considered significant when P<0.05.
RESULTS
Isolation and collection of WJ-MSC secretome
MSCs were isolated successfully by explant culture of Wharton's jelly pieces (Fig. 1). The cells were passaged up to P6-7 and surface markers were identified by flowcytometry. Cells were prominently (>98%) negative for hematopoietic stem cell markers CD34 and CD45 and positive for CD73, CD90 and CD105 (Fig. 2). Differentiation potential to mesodermal lineages was also tested and WJ-MSCs differentiated to osteocytes, adipocytes and chondrocytes successfully (Fig. 3).
Fig. 1.
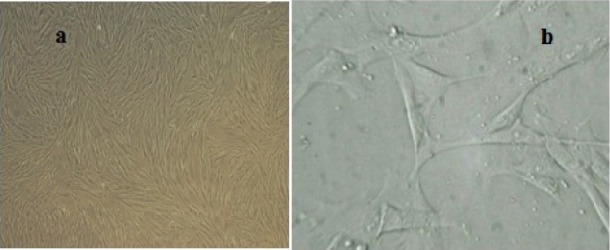
Morphological characteristics of WJ-MSCs. a; Confluent growth of mesenchymal stem cells in passage 4, b; Fibroblast like morphology of WJ-MSCs.
Fig. 2.
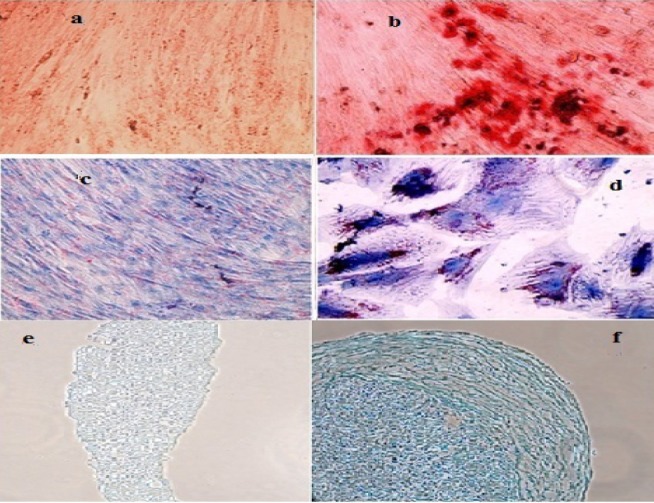
Differentiation of WJ-MSCs to mesodermal lineages. b; Alizarin Red S staining shows successful differentiation into osteocytes and a; its undifferentiated negative control, d; Oil Red O staining shows successful differentiation into adipocytes and c; its undifferentiated negative control, f; Alcian Blue staining shows successful differentiation into chondrocytes and e; its undifferentiated negative control.
Fig. 3.
Surface marker detection of WJ-MSCs. a; dot plot representation of cell population, b; Flowcytometric analyses showed lack of CD34, c; CD45 antigens, d; positive expression of CD73, e; CD90 and f; CD105 on WJ derived cells.
WJ-MSCs in P4 were divided into two groups; one group was treated with IFNγ and groups; one group was treated with IFNγ and the other remained untreated. The secretome was collected after 24 h. Total protein concentration in MSC sec was 20.8 ng/ml and in stMSC sec was 24.6 ng/ml.
proliferation of A549 lung cancer cells with WJ-MSC secretome
A549 cells were treated with WJ-MSC secretome for 72 h, and cell proliferation was evaluated by BrdU incorporation assay. Results showed that WJ-MSC secretome did not proliferate differently from untreated cells (P>0.05). Cancer cells growth which were treated with MSC sec was 104.59% ± 0.044 compared to untreated cells (Fig. 4a).
Fig. 4.
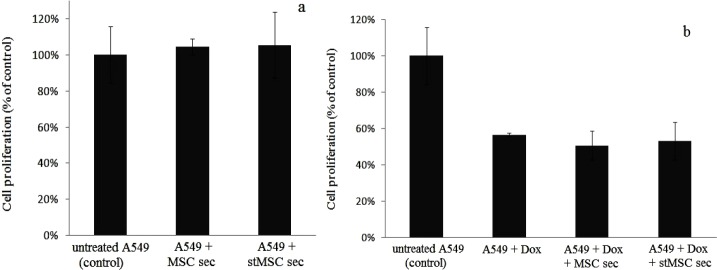
BrdU proliferation assay. a; A549 cell proliferation did not change significantly before and after treatment with hWJ-MSC secretome (obtained from intact MSCs or IFNγ stimulated MSCs), b; WJ-MSC secretome did not induce resistance to doxorubicin in A549 cells because A549 cell proliferation did not change significantly when treated by doxorubicin alone or doxorubicin + hWJ-MSC secretom or doxorubicin + IFNγ stimulated hWJMSC secretome.
Induction of apoptosis in A549 lung cancer cells with WJ-MSC secretome
Proapototic effect of WJ-MSC secretome on A549 cells was tested by Annexin V-PI staining. Our results showed that there was no significant distinction between treated and untreated groups (Fig. 5). Percent of live cells (lower left in Fig. 5a, b and c) were 98.54% ± 0.56 and 98.89% ± 0.43 in untreated and MSC sec treated groups respectively.
Fig. 5.
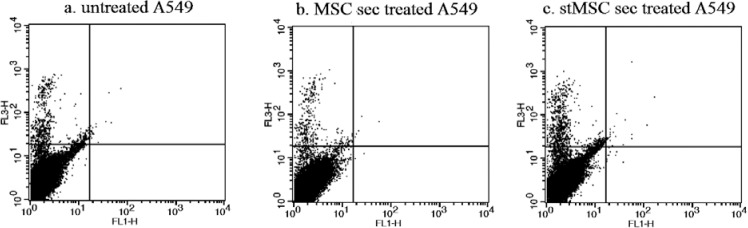
AnnexinV-PI apoptosis assay on A549 cell line treated with hWJ-MSC seretome. There is no significant difference between a; untreated A549, b; A549 treated with hWJ-MSC secretome and c; A549 treated with IFNγ stimulated hWJ-MSC secretome.
Induction of chemo resistance in A549 lung cancer cells by WJ-MSC secretome
To analyze if WJ-MSC secretome causes chemo resistance to doxorubicin, A549 cells were exposed to both MSC secretome and liposomal doxorubicin concomitantly. The IC50 of the drug was first determined and then doxorubicin was added to MSC secretome. Proliferation and apoptosis assay results showed that incubation of lung cancer cells with doxorubicin in the presence of MSC secretome did not change proliferation potential of cells or their apoptotic events. A549 cell proliferation in Dox treated group and MSC sec + Dox treated group was 56.66% ± 0.009 and 50.67 ± 0.079, respectively (Fig. 4a).
Effect of WJ-MSC secretome on A549 lung cancer cells following IFNγ treatment
Twenty ng/ml IFNγ was applied for MSC stimulation in order to change secretome composition and to increase its anticancer properties. However the effect was not detectable on A549 cancer cells and lung cancer cell proliferation or apoptosis did not differ significantly when MSC sec or stMSC sec were applied. Cell proliferation rate in cancer cells that treated with MSC sec and stMSC sec was 104.59% ± 0.044 and 105.31% ± 0.18 of untreated cells respectively (Fig. 4a). In addition A549 proliferation in MSC + Dox treated group and stMSC + Dox treated group were 50.67% ± 0.079 and 53.17% ± 0.105 respectively (Fig. 4b).
DISCUSSION
In the present study we evaluated the paracrine effect of human Wharton's jelly MSC secretome on A549 lung cancer cell line. Our results showed that WJ-MSC secretome did not induce either tumor cell growth or apoptosis. WJ-MSC secretome also didn’t cause resistance to liposomal doxorubicin when used in combination with the chemotherapeutic agent.
Recently considerable attention has been given to MSCs as an effective cell therapy candidate (16). Their biological properties, such as their potential capacity to replace degenerated cells in various organs and their potential use as immune-suppressors in conditions such as graft-versus-host disease attracted clinicians to apply this cell type in clinical setting (17). Their homing ability to tumor site makes them promising candidates for cancer therapy. However, the application of MSCs in cancer treatment has been associated with a general concern related to their biosafety. This concern has been potentiated by various reports in which concluded that MSCs could be involved in cancer initiation in vivo (18,19) and also by the implication that MSCs may transform into malignant cells in vitro (20,21).
It is clear that MSCs secrete a number of paracrine factors that may influence tumor growth (22); in this regard MSC secretome may affect tumor cells and potentiate or inhibit their development. However in this study human umbilical cord MSC secretome did not promote A549 cell growth. This result display safety of Wharton's jelly MSC secretome use in lung cancer of A549 cell type.
MSC secretome can be advantageous to MSC itself because of safety issues. A recent report has also shown fusion between MSCs and gastrointestinal epithelial cells, suggesting the generation of a more cancer prone cell type (23). When MSC secretome was used, cell-cell interactions did not occur.
Previous studies in which A549-MSC interaction was investigated, used bone marrow or adipose derived mesenchymal stem cells. The effect of Wharton's jelly MSCs on A549 cells has not been previously investigated. Results of Ohkouchi and coworkers suggested that stanniocalcin-1 secreted by bone marrow MSC-like cells in tumor stroma plays a critical role in enhancing the Warburg effect and making tumors resistant to the reactive oxygen intermediates (24). Hsu and colleagues also showed that A549 or CL1-5 lung cancer cells co-cultured with MSCs can lead to an increased sphere formation, drug resistance and overexpression of pluripotency markers through activation of the IL-6/JAK2/STAT3 pathway (25). On the other hand, Li and coworkers demonstrated that hBM-MSCs could inhibit proliferation of SK-MES-1 and A549 cells, and induce apoptosis of tumor cells in vitro via some soluble factors.
An animal study also confirmed suppression of tumorigenesis and tumor angiogenesis by treating preliminarily tumor cells with the hMSC secreted factors (26). Another investigation on BM-MSCs also found that, MSCs stimulated A549 and H446 cell lines in vivo, while inhibited their proliferation in vitro. Researchers concluded that these discrepancies may be due to the differential effects of MSCs on heterogeneous cell populations especially cancer stem cells and more developed tumor cells within the two lung cancer cells (27).
In all above mentioned studies, MSCs affected by A549 cancer cells and it is probable that MSCs produce tumorigenic substances when they are in contact with tumor cells. However, when they are not affected by tumor cells (either by direct cell-cell contact or indirect paracrine release) their secretome composition is different and do not contain tumorigenic substances which may not induce tumor progression. Do and colleagues showed that adipose tissue derived MSCs converted to tumor associated fibroblasts when exposed to A549 secreted materials (28). Shin and coworkers also displayed that Lysophosphatidic acid (LPA) which is enriched with serum and malignant effusion of cancer patients, can induce adipose tissue derived MSCs to plays a key role in tumorigenesis by stimulating the adhesion and proliferation of cancer cells (29). Therefore, a possible way is the use of intact MSC secretome instead of MSC itself.
We also investigated whether modifications can induce tumor inhibitory function in WJ-MSCs against A549 cell line. Genetically engineered MSCs for generating antitumor effect are investigated in many studies (30,31,32,33). TRAIL-loaded MSCs induced apoptosis in A549 cells and reduced tumor growth in A549 xenograft mouse model and also glioma-bearing mice (30,33). However genetic transformation may produce some challenges. One of the possible dangers of transgenic cell therapy is that the insertion disrupts a genomic locus that is responsible for tumor suppression (insertional mutagenesis) (34). Therefore we preferred to stimulate MSCs by addition of IFNγ to their culture media.
IFNγ as an inflammatory cytokine plays important roles in cancer. It is secreted from Th1 cells within the anti-tumoral defense reaction and acts in paracrine manner alone or in combination with other cytokines to suppress tumor cell growth (35) by stimulation of several anti-proliferative and thus tumoricidal biochemical pathways in macrophages and other cells and also in tumor cell lines. (36). IFNγ signaling enhances infiltration of mononuclear cells into tumor tissue which secrete TNFα and nitric oxide (37).
On the other hand evidences proved that IFNγ alter mesenchymal stem cell functions (38). Therefore we also hypothesized that it may induce antitumor activity of mesenchymal stem cells. However the effect of secretome obtained from IFNγ stimulated WJ-MSCs on A549 cells did not differ from intact MSCs. This result do not confirm that IFNγ stimulated cells produces same secretome as unstimulated cells, but probable changes in secretome can not alter WJ-MSC effect on lung cancer cells and actually this modification cannot generate antitumoral effect in WJ-MSCs against A549 cells. We also investigated combinatorial effect of doxorubicin and WJ-MSC secretome on A549 cells. We found that UC-MSC secretome did not induce resistance in A549 lung cancer cells to liposomal doxorubicin. This result also confirmed safety of WJ-MSC secretome in lung cancer of A549 cell type.
CONCLUSION
Human WJ-MSC secreted substances did not inhibit A549 lung cancer cell proliferation, nor promote it. MSC secretome also did not affect apoptosis events in A549 cells. Concomitant exposure of A549 cells to MSC secretome and chemotherapeutic agent doxorubicin did not lead to any significant alterations in doxorubicin cytotoxic effects. Our results indicated that WJ-MSCs neither showed anticancer properties, nor promoted cancer cell growth and resistance to doxorubicin. These results can be useful in the safety considerations of MSC therapy for cardiovascular or neurodegenerative complications in cancer patients.
ACKNOWLEDGMENTS
We thank, Mrs. Dana and Miss. Naji, the staff of Applied Physiology Research Center for their excellent and compassionate cooperation. We are also thankful to Mrs. Moazen and Mrs. Mirian from Pharmaceutical Biotechnology Department for their kind technical assistance. The content of this paper is extracted from the Ph.D thesis NO. 391439 submitted by F. Hendijani which was financially supported by the Research Department of Isfahan University of Medical Sciences, Isfahan, I.R. Iran.
REFERENCES
- 1.Torsvik A, Bjerkvig R. Mesenchymal stem cell signaling in cancer progression. Cancer Treat Rev. 2013;39:180–188. doi: 10.1016/j.ctrv.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Klopp AH, Gupta A, Spaeth E, Andreeff M, Marini F., 3rd Concise review: Dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem Cells. 2011;29:11–9. doi: 10.1002/stem.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taghizadeh RR, Cetrulo KJ, Cetrulo CL. Wharton's Jelly stem cells: future clinical applications. Placenta. 2011;32:S311–S315. doi: 10.1016/j.placenta.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Fan CG, Zhang QJ, Zhou JR. Therapeutic potentials of mesenchymal stem cells derived from human umbilical cord. Stem Cell Rev. 2011;7:195–207. doi: 10.1007/s12015-010-9168-8. [DOI] [PubMed] [Google Scholar]
- 5.Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 6.Bassi EJ, de Almeida DC, Moraes-Vieira PM, Camara NO. Exploring the role of soluble factors associated with immune regulatory properties of mesenchymal stem cells. Stem Cell Rev. 2012;8:329–342. doi: 10.1007/s12015-011-9311-1. [DOI] [PubMed] [Google Scholar]
- 7.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mardani A, Varshosaz J, Hassanzadeh F, Rostami M. Preparation and characterization of inhalable and targeted nanocomposite particles of doxorubicin for treatment of lung cancer. Res Pharm Sci. 2012;7:S289. [Google Scholar]
- 11.Soltanzad F, Samadishams S, Barar J, Nazemyieh H, Omidi Y. Evaluation of cytotoxicity and anti-cancer effect of Ferula szowitsiana methanolic extract on lung cancer A549 cell-lines. Res Pharm Sci. 2012;7:S103. [Google Scholar]
- 12.Samarghandian S, Boskabady M. Caspase-dependent pathway in apoptosis induced by Safranal in alveolar human lung cancer cell line. Res Pharm Sci. 2012;7:S743. [Google Scholar]
- 13.R, Naishadham D, Jemal A. Cancer statistics, 2012. CA-CANCER J CLIN. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 14.Hendijani F, Sadeghi-Aliabadi H, Haghjooy Javanmard S. Comparison of human mesenchymal stem cells isolated by explant culture method from entire umbilical cord and Wharton's jelly matrix. Cell Tissue Bank. 2014 doi: 10.1007/s10561-014-9425-1. DOI 10.1007/s10561-014-9425-1. [DOI] [PubMed] [Google Scholar]
- 15.Du J, Zhou L, Chen X, Yan S, Ke M, Lu X, et al. IFN-gamma-primed human bone marrow mesenchymal stem cells induce tumor cell apoptosis in vitro via tumor necrosis factor-related apoptosis-inducing ligand. Int J Biochem Cell Biol. 2012;44:1305–1314. doi: 10.1016/j.biocel.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 16.Bongso A, Fong CY. The therapeutic potential, challenges and future clinical directions of stem cells from the Wharton's Jelly of the human umbilical cord. Stem Cell Rev. 2013;9:226–240. doi: 10.1007/s12015-012-9418-z. [DOI] [PubMed] [Google Scholar]
- 17.Eggenhofer E, Steinmann JF, Renner P, Slowik P, Piso P, Geissler EK, et al. Mesenchymal stem cells together with mycophenolate mofetil inhibit antigen presenting cell and T cell infiltration into allogeneic heart grafts. Transpl Immunol. 2011;24:157–163. doi: 10.1016/j.trim.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 19.Mohseny AB, Szuhai K, Romeo S, Buddingh EP, Briaire-de Bruijn I, de Jong D, et al. Osteosarcoma originates from mesenchymal stem cells in consequence of aneuploidization and genomic loss of Cdkn2. J Pathol. 2009;219:294–305. doi: 10.1002/path.2603. [DOI] [PubMed] [Google Scholar]
- 20.Spaeth EL, Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B, et al. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One. 2009;4:e4992. doi: 10.1371/journal.pone.0004992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren Z, Wang J, Zhu W, Guan Y, Zou C, Chen Z, et al. Spontaneous transformation of adult mesenchymal stem cells from cynomolgus macaques in vitro. Exp Cell Res. 2011;317:2950–2957. doi: 10.1016/j.yexcr.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Estrada R, Li N, Sarojini H, An J, Lee MJ, Wang E. Secretome from mesenchymal stem cells induces angiogenesis via Cyr61. J Cell Physiol. 2009;219:563–571. doi: 10.1002/jcp.21701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrand J, Noel D, Lehours P, Prochazkova-Carlotti M, Chambonnier L, Menard A, et al. Human bone marrow-derived stem cells acquire epithelial characteristics through fusion with gastrointestinal epithelial cells. PLoS One. 2011;6:e19569. doi: 10.1371/journal.pone.0019569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohkouchi S, Block GJ, Katsha AM, Kanehira M, Ebina M, Kikuchi T, et al. Mesenchymal stromal cells protect cancer cells from ROS-induced apoptosis and enhance the Warburg effect by secreting STC1. Mol Ther. 2012;20:417–423. doi: 10.1038/mt.2011.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu HS, Lin JH, Hsu TW, Su K, Wang CW, Yang KY, et al. Mesenchymal stem cells enhance lung cancer initiation through activation of IL-6/JAK2/STAT3 pathway. Lung Cancer. 2012;75:167–177. doi: 10.1016/j.lungcan.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Li L, Tian H, Chen Z, Yue W, Li S, Li W. Inhibition of lung cancer cell proliferation mediated by human mesenchymal stem cells. Acta Biochim Biophys Sin (Shanghai) 2011;43:143–148. doi: 10.1093/abbs/gmq118. [DOI] [PubMed] [Google Scholar]
- 27.Luo D, Yan X, Liu D, Zhou X, Liu G. Differential effects of mesenchymal stem cells on a heterogeneous cell population within lung cancer cell lines. Mol Cell Biochem. 2013;378:107–116. doi: 10.1007/s11010-013-1600-3. [DOI] [PubMed] [Google Scholar]
- 28.Do EK, Kim YM, Heo SC, Kwon YW, Shin SH, Suh DS, et al. Lysophosphatidic acid-induced ADAM12 expression mediates human adipose tissue-derived mesenchymal stem cell-stimulated tumor growth. Int J Biochem Cell Biol. 2012;44:2069–2076. doi: 10.1016/j.biocel.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 29.Shin SH, Kim J, Heo SC, Kwon YW, Kim YM, Kim IS, et al. Proteomic identification of betaig-h3 as a lysophosphatidic acid-induced secreted protein of human mesenchymal stem cells: paracrine activation of A549 lung adenocarcinoma cells by betaig-h3. Mol cell proteomics. 2012;11 doi: 10.1074/mcp.M111.012385. M111 012385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim SM, Lim JY, Park SI, Jeong CH, Oh JH, Jeong M, et al. Gene therapy using TRAIL-secreting human umbilical cord blood-derived mesenchymal stem cells against intracranial glioma. Cancer Res. 2008;68:9614–9623. doi: 10.1158/0008-5472.CAN-08-0451. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Zhang L, Xu W, Qian H, Ye S, Zhu W, et al. Experimental therapy for lung cancer: umbilical cord-derived mesenchymal stem cell-mediated interleukin-24 delivery. Curr cancer drug targets. 2013;13:92–102. [PubMed] [Google Scholar]
- 32.Zhao WH, Cheng JX, Shi PF, Huang JY. Human umbilical cord mesenchymal stem cells with adenovirus-mediated interleukin 12 gene transduction inhibits the growth of ovarian carcinoma cells both in vitro and in vivo. Nan fang yi ke da xue xue bao. 2011;31:903–907. [PubMed] [Google Scholar]
- 33.Mohr A, Lyons M, Deedigan L, Harte T, Shaw G, Howard L, et al. Mesenchymal stem cells expressing TRAIL lead to tumour growth inhibition in an experimental lung cancer model. J Cell Mol Med. 2008;12:2628–2643. doi: 10.1111/j.1582-4934.2008.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Momin EN, Vela G, Zaidi HA, Quinones-Hinojosa A. The Oncogenic potential of mesenchymal stem cells in the treatment of cancer: directions for future research. Curr Immunol Rev. 2010;6:137–148. doi: 10.2174/157339510791111718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto M, Kamigaki T, Yamashita K, Hori Y, Hasegawa H, Kuroda D, et al. Enhancement of anti-tumor immunity by high levels of Th1 and Th17 with a combination of dendritic cell fusion hybrids and regulatory T cell depletion in pancreatic cancer. Oncol Rep. 2009;22:337–343. [PubMed] [Google Scholar]
- 36.Brandacher G, Winkler C, Schroecksnadel K, Margreiter R, Fuchs D. Antitumoral activity of interferon-gamma involved in impaired immune function in cancer patients. Curr Drug Metab. 2006;7:599–612. doi: 10.2174/138920006778017768. [DOI] [PubMed] [Google Scholar]
- 37.Beatty GL, Paterson Y. Regulation of tumor growth by IFN-gamma in cancer immunotherapy. Immunol Res. 2001;24:201–210. doi: 10.1385/IR:24:2:201. [DOI] [PubMed] [Google Scholar]
- 38.Croitoru-Lamoury J, Lamoury FM, Caristo M, Suzuki K, Walker D, Takikawa O, et al. Interferon-gamma regulates the proliferation and differentiation of mesenchymal stem cells via activation of indoleamine 2,3 dioxygenase (IDO) PLoS One. 2011;6:e14698. doi: 10.1371/journal.pone.0014698. [DOI] [PMC free article] [PubMed] [Google Scholar]