Abstract
Descurainia sophia is a plant widely distributed and used as folk medicine throughout the world. Different extracts of aerial parts and seeds of this plant have been shown to inhibit the growth of different cancer cell lines in vitro. In this study, cytotoxic activity of D. sophia seed volatile oil was evaluated. D. sophia seed powder was mixed with distilled water and left at 25 °C for 17 h (E1), 23 h (E2) and 28 h (E3) to autolyse. Then, the volatile fractions of E1, E2, and E3 were collected after steam distillation for 3 h. Cytotoxic effects of the volatile oils alone or in combination with doxorubicin (mixture of E1 or E2 at 50 μg/ml or E1 at 100 μg/ml with doxorubicin at 0.1, 1, 10 μM) against MCF-7 cell line were determined using MTT assay. Cytotoxic effect of E1 volatile oil was also determined on HeLa cell line. The results indicated that 1-buten-4-isothiocyanate was the major isothiocyanate found in the volatile oils. The results of cytotoxic evaluations showed that volatile constituents were more toxic on MCF-7 cells with IC50< 100 μg/ml than HeLa cells with IC50> 100 μg/ml. No significant differences were observed between cytotoxic activities of E1, E2 and E3 on MCF-7 cell line. Concomitant use of E1 and E2 (50 μg/ml) with doxurubicin (1 μM) significantly reduced the viability of MCF-7 cells compared to the negative control, doxorubicin alone, or each volatile fraction. The same result was obtained on HeLa cells, when E1 (100 μg/ml) was concurrently used with doxorubicin (1 μM).
Keywords: Descurainia Sophia, Cytotoxicity, MCF-7, HeLa
INTRODUCTION
Descurainia sophia (L.) Weeb ex Prantl (Flixweed) belonging to the family Brassicaceae (Cruciferae) is a plant which is widely distributed throughout Europe, Asia and the Middle East (1,2). The extract of aerial parts of this plant is used in folk medicines for the treatment of throat diseases, measles and smallpox. Its tincture is used as diuretic, antihelmintic and hemostatic for internal hemorrhages (3).
Seeds of Flixweed have been traditionally used to relieve cough, prevent asthma, reduce edema, promote urination and also for their cardiotonic effect (4). Seeds have also been used in the Iranian traditional medicine for diarrhea (in boiled form) and constipation (in cold water), especially for the prevention of water loss and constipation among Iranian Hajj pilgrims (5).
A new sinapoyl glycoside and a cytotoxic cardenolide glycoside from ethanolic extract of the seeds of D. sophia (6) have been reported. Biological screening of alcoholic extract of the aerial parts of D. sophia has shown analgesic, antipyretic and anti-inflammatory effects (7). Inhibitory effect of flixweed ethanolic extract on Streptomyces pyogenes has also been demonstrated (8).
Analyses of the volatile constituents of aerial parts of D. sophia by gas chromato- graphy (GC) and gas chromatography/mass spectrophotometry (GC/MS) indicated that cis-β-ocimene, menthol and neoisomenthyl acetate are the predominant fractions of the volatile oil (4). The seeds have also been shown to inhibit the growth of different cancer cell lines in vitro. For instance, n-butanol extract of the seeds significantly reduced the viability of NCI-H460, SF268 and SGC-7901 cell lines (4,9,10).
Khan and coworkers demonstrated that a flavonol glycoside, artabotryside A, isolated from the seeds of D. sophia inhibited the growth of U87 glioblastoma cells through G2/M phase arrest and induction of caspase-3-dependent apoptosis (2). Kim and collegues indicated that the ethanol extract of the seeds of D. sophia induces dose-dependent responses in A549 human non-small cell lung carcinoma cells (11).
Descurainolide A and B (new lactones), descurainin, strophanthidin and isorhamnetin-3-O-b-D-glucopyranoside were isolated from the ethanolic extract of the seeds of D. sophia, some of which have shown cytotoxicity on human stomach adenocarcinoma cell line (BGC-823) and human breast carcinoma cell line (MDA-MB-435) (12).
The hydrolyzed products of glucosinolates, namely isothiocyanates, have been shown to possess various biological activities including anti oxidative, antibacterial, anticancer and chemoprotective properties. There is a reliable correlation between dietary intake of plants containing isothiocyanates and the decrease in cancer risks (13).
Isothiocyanates may exert their cancer protection through various mechanisms including detoxification leading to decreased activation of pro-carcinogens and increased excretion of carcinogens. Detoxification enzymes are also upregulated by dietary crucifer. Furthermore, isothiocyanates may slow proliferation or increase apoptosis of cancer cells resulting in a retardation of tumor growth inhibiting CYP-dependent activation of pre-carcinogens (14).
In the present study, the cytotoxic activity of the Flixweed seed volatile oils collected after autolysis of the powder for various times were studied on the MCF-7 and HeLa cell lines.
MATERIALS AND METHODS
Materials
Seeds of D. sophia were purchased from a reliable apothecary in the city of Isfahan. The seeds were cultivated and the plant was identified as D. Sophia. A voucher specimen of the plant (No. 2834) was deposited in the herbarium of the School of Pharmacy at Isfahan University of Medical Sciences. 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) and dimethyl sulfoxide (DMSO) were purchased from the Merck, Germany. HeLa (human black cervix carcinoma, epithelioid) and MCF7 (estrogen-dependent human breast cancer) cells were procured from Pasteur Institute, Tehran, Iran. Roswell Park Memorial Institute (RPMI)-1640 culture medium (sterile liquid), fetal bovine serum (FBS), penicillin/streptomycin and trypsin-EDTA were purchased from Gibco, Scotland. Doxorubicin vial from Farmitalia (Italy) was used as the positive control.
Autolysis and collection of the volatile constituents
Seed powder of D. sophia were mixed with distilled water and left for autolysis at 25 °C for 17 h (E1), 23 h (E2) and 28 h (E3) in appropriate containers. Autolysis is a hydrolytic breakdown of glucosinolates to volatile isothiocyanates through removing the glucose moiety from glucosinolates. Then volatile constituents (E1, E2 and E3) were collected by 3 h distillation using a semi-industrial distillation apparatus and kept in a refrigerator before the use (13). The flavored water (FW) was also separated, freeze dried, and kept for subsequent cytotoxic studies.
In vitro cytotoxicity assay
Cytotoxic effects of the volatile oils alone (E1, E2, E3 at concentrations 25-250 μg/ml), FW (50-250 μg/ml)), or in combination with doxorubicin (mixture of E1 or E2 at 50 μg/ml or E1 at 100 μg/ml with doxorubicin at 0.1, 1, 10 μM) were determined against MCF-7 cell line using MTT assay. Cytotoxic effect of E1 volatile oil was also determined on HeLa cell line. In MTT assay, mitochondrial succinic dehydrogenase enzyme of viable cells would metabolically reduce the yellow soluble MTT salt into a purple insoluble formazan product. The purple solid could be dissolved in DMSO and measured spectrophotometrically at 540 nm using ELISA plate reader (15).
MCF-7 and HeLa cells were grown in RPMI 1640 medium completed with 10% FBS and 1% penicillin/streptomycin. Cell lines were maintained in a humidified atmosphere of 5% CO2 and 95% air at 37 °C (15). After 2-3 subcultures, 180 μl of cell suspension containing 2.5 × 104 and/or 5 × 104 cells per ml was seeded in 96 well microplates and incubated for 24 h (37 °C, air humidified 5% CO2). The stock solutions of E1, E2, E3, and freeze dried-FW were prepared by dissolving 10 mg of the corresponding volatile oils in 1 ml solvent composed of 60% deionized water and 40% DMSO.
These solutions were then appropriately diluted and 20 μl of each dilution was added to 96-wells microplate containing cell suspensions to reach 25, 50, 100, 120, 150, 180, 200 and 250 μg/ml final concentrations in the wells. Mixtures of E1 and E2 at concentration 50 μg/ml and doxorubicin solution at 0.1, 1, 10 μM were prepared for evaluation of synergic effects on MCF-7 cell line. Similar mixtures of E1 (100 μg/ml) and doxorubicin at 0.1, 1, 10 μM were also prepared to be evaluated on HeLa cells.
Doxorubicin was used as a positive control at 100 μM final concentration in the wells. The first column of the plate containing 180 μl of the cell suspension and 20 μl RPMI medium was regarded as negative control. The blank wells were consisted of 200 μl of the completed RPMI 1640 medium. After addition of each sample, the plates were further incubated for 48 h under the same condition.
To evaluate cell survival, each well was then incubated with 20 μl of MTT solution (5 mg/ml in phosphate buffer solution) for 3 h. Afterwards, the media in each well was gently replaced with 150 μl DMSO and pipetted to dissolve the formazan crystals. The absorbance of each well was measured at 540 nm using an ELISA plate reader (Awareness Stat Fax 2100, USA) (16). Each experiment was carried out in triplicate and repeated in three different days. In the negative control, percent cell survival was assumed as 100%. The percentage of cell viability was calculated using the following formulation:
% Cell Survival = (Mean Abs. of the test compound – Mean Abs. of the blank) / (Mean Abs. of the negative control – Mean Abs. of the blank) × 100 (1)
Statistical analysis
The results are the mean of three triplicate experiments. Analysis of variance (ANOVA) followed by LSD test using SPSS 10.0 program was used to determine the differences among various groups. The significance level was set at P<0.05.
RESULTS
The volatile constituents of the seeds of D. sophia after 17 h (E1), 23 h (E2) and 28 h (E3) autolysis was determined. 1-butene 4-isothiocyanate (3-butenyl isothiocyanate) was the main isothiocyanate detected in the volatile oils.
The results of cytotoxic evaluation of E1, E2, E3 (25-250 μg/ml) and FW (50-250 μg/ml) on MCF-7 cells are depicted in Fig. 1. As shown in Fig. 1, E1, E2, and E3 showed cytotoxic effects at concentrations ≥100 μg/ml on MCF-7 cells as compared to control group (P<0.05).
Fig. 1.
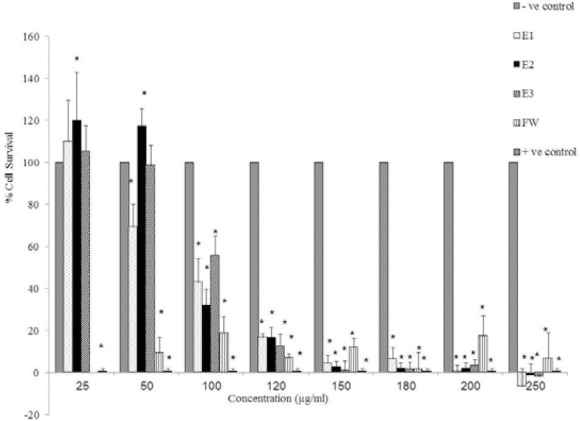
Cytotoxic effects of E1, E2, E3, and FW on MCF-7 cell line (5 × 104 cell/ml) following exposure to the concentrations between 25-250 μg/ml. Cell viability was assessed using the MTT method. Data are presented as mean ± SD, *; P<0.05 as compared to -ve control, n=3. + ve Control; doxorubicin at 100 μM.
Cell survival was diminished to less than 10% at concentrations of 150-250 μg/ml which is comparable to the cytotxic effect of the positive control, doxorubicin (100 μM). FW was cytotoxic to the MCF-7 cells at all the concentrations examined. The highest cytotoxic effect of FW was observed at 180 μg/ml which is also comparable to the cytotoxic effect of the positive control, doxorubicin.
The effect of concomitant use of E1 (50 μg/ml) with doxorubicin at various concentrations (0.1, 1, 10 μM) on MCF-7 cells (Fig. 2) indicates that the viability of MCF-7 cells was significantly reduced compared to E1 or doxorubicin alone, only when doxorubicin was used at 1 μM concentration (P<0.05), i.e. E1 (50 μg/ml) was able to induce additional 15% cell death while used with 1 μM doxorubicin. No significant synergetic effect was observed with other doxorubicin concentrations.
Fig. 2.
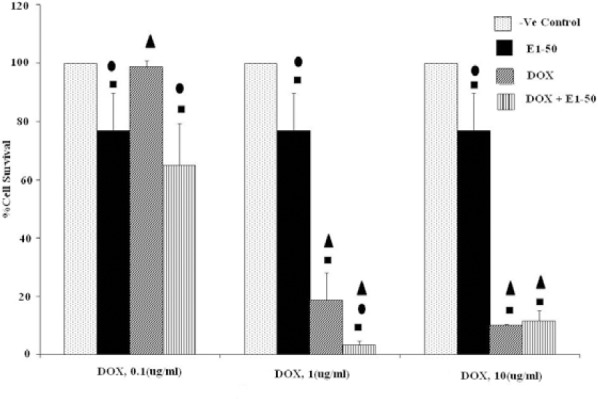
Effects of co-administration of E1 (50 μg/ml) and doxorubicin (0.1, 1 and 10 μM) on viability of MCF-7 cell line (5 × 104 cell/ml). Cell viability was assessed using the MTT method. Data are presented as mean ± SD, n=3. ■, • and ▲ representing P<0.05 comparing to the negative (-ve) control, doxorubicin and E1, respectively.
The results of concurrent administration of E2 (50 μg/ml) with doxorubicin at different concentrations (0.1, 1, 10 μM) on MCF-7 cells are shown in Fig. 3. E2 indicated synergic effect with all concentrations of doxorubicin (P<0.05). However, the mixture of E2 at 50 μg/ml and doxorubicin at1 μM was the most effective combination.
Fig. 3.
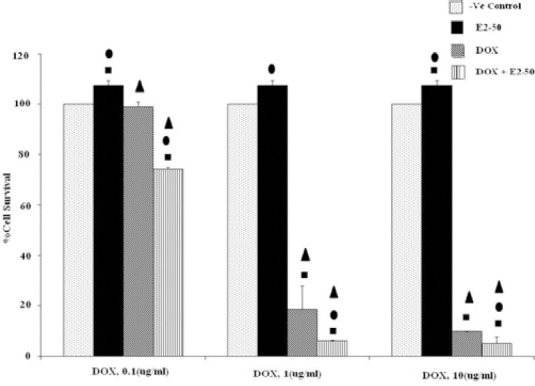
Effects of co-administration of E2 (50 μg/ml) and doxorubicin (0.1, 1 and 10 μM) on viability of MCF-7 cell line (5 × 104 cell/ml). Cell viability was assessed using the MTT method. Data are presented as mean ± SD, n=3. ■, • and ▲ representing P<0.05 comparing to the negative (-ve) control, doxorubicin and E1, respectively.
Cytotoxic effects of E1 (50-250 μg/ml) and FW (50-250 μg/ml) on HeLa cells is depicted in Fig. 4. Significant cytotoxic effects (P<0.05) was observed when E1 concentrations were between 150-250 μg/ml. On the other hand, FW was cytotoxic to HeLa cells at all tested concentrations (P<0.05).
Fig. 4.
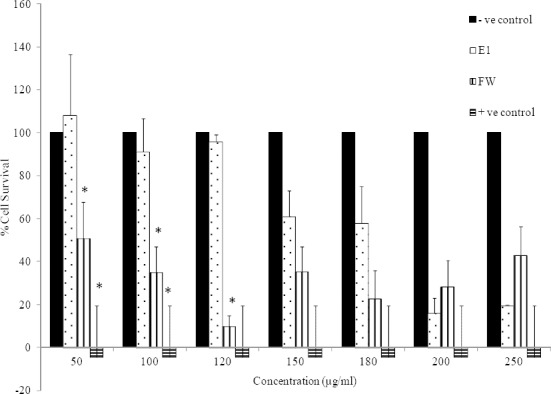
Cytotoxic effects of E1 and FW on HeLa cell line (2.5 × 104 cell/ml) following exposure to concentrations between 50-250 μg/ml. Cell viability was assessed using the MTT method. Data are presented as mean ± SD, *; P<0.05 as compared to negative (-ve) control, n=3. Positive (+ve) control was doxorubicin at 100 μM.
The results of co-administration of E1 at 100 μg/ml with different concentration of doxorubicin (0.1, 1, 10 μM) on HeLa cell line are illustrated in Fig. 5. The highest toxicity was observed when E1 was mixed with doxorubicin at 1 μM (P<0.05).
Fig. 5.
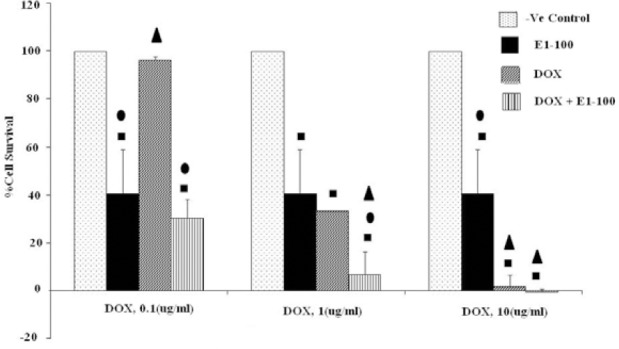
Effects of co-administration of E1 (100 μg/ml) and doxorubicin (0.1, 1 and 10 μM) on viability of HeLa cell line (5 × 104 cell/ml). Cell viability was assessed using the MTT method. Data are presented as mean ± SD, n=3. ■, • and ▲ representing P<0.05 comparing to the negative (-ve) control, doxorubicin and E1, respectively.
The IC50 values of E1, E2, E3 and FW on MCF-7 cell line were found to be close to 70, 80, 100, and 40 μg/ml, respectively. The IC50 values for E1 and FW on HeLa cells were around 180 and 70 μg/ml, respectively.
DISCUSSION
In this study the volatile oils of D. sophia seeds were collected and analysed by GC and GC/MS. The results indicated that 1-buten-4-isothiocyanate or 3-butenyl isothiocyanate was the major constituent of the volatile oils. The optimum autolysis time used in this study was similar to the previous reports (17,18,19).
Several studies have demonstrated the effectiveness of different extractions of D. sophia seeds on cancerous cell lines. For instance, n-butanol extract has shown cytotoxicity to NCI-H460, SF268 and SGC-7901 cell lines (4), while seed ethanolic extract was cytotoxic to A549 (11), BCG-823 and MDA-MB-435 cancerous cells (12). The antitumor and anticancer activities of the seed extract have been attributed to the presence of active ingredients such as kaempferol, quercetine, isorhamnetine, strophanthidin and artabotryside A (20). In the present study, the cytotoxic effects of volatile oils obtained from D. Sophia seeds, after autolysis at various times, were evaluated on MCF-7 and HeLa cell lines. Volatile oils as well as FW showed significant cell toxicity on MCF7 and HeLa cell lines. However, the results indicated that HeLa cells were less susceptible to the cytotoxic effect of the volatile oils than MCF-7. This activity could be attributed, in part, to the presence of considerable amount of 1-buten-4-isothiocianate in the volatile oils. Benzyl isothiocyanates have already been reported as apoptosis inducers (21) and cytotoxic agents which may present in the dietary regimens containing Cruciferae vegetables. The IC50 values of 5.95, 7.32 and 77.9 μM (1, 1.2 and 14.5 μg/ml) have been reported for benzyl isothiocianate, phenethyl- isothiocianate and naphthyl- isothiocianate on MCF-7 cell, respectively (22). The higher IC50 values of the volatile oils observed in the present work could be attributed to the composition of the isothiocyanates (1-buten-4-isothiocianate versus benzyl isothiocianate) and the presence of other volatile constituents like fatty acids in the volatile oils.
In the current study, the cytotoxic effects of combination of D. sophia volatile oils with doxorubicin were also investigated in order to seek any synergistic cytotoxic effects. Although doxorubicin is widely used as chemotherapeutic agent in different malignancies, multidrug resistance is one of the major concerns associated with its application. Multidrug resistance which is characterized by reduced sensitivity of the cancerous cells to a spectrum of structurally diverse chemotherapeutic agents like anthracyclines, Vinca alkaloids, epi-podophyllotoxins and their semisynthetic derivatives limits the effectiveness of chemotherapy of a variety of cancers (23). Multidrug resistance is characterized by decreased accumulation of drugs and overexpression of a highly conserved plasma membrane glycoprotein, termed P-glycoprotein (P-GP) which acts as a drug efflux pump (24). However, several diverse derivatives like tamoxifen, verapamil and cyclosporine A, known as multidrug-reversal agents (25), could enhance intracellular anticancer drug accumulation via impairing the P-GP function (26). Concurrent administration of E1and E2 with doxorubicin significantly reduced the viability of MCF-7 cell line. This observation is in accordance with the Gupta and coworkers study who reported that phenethyl-isothiocyanate has potential to enhance the cytotoxic effects of doxorubicin on MCF-7 cell line (27).
Hu and colleagues demonstrated that benzyl- and phenethyl isothiocyanates are not P-glycoprotein substrates, as their IC50 values were almost similar in MCF-7/ADR and wild type MCF-7 (28). It is also reported that butyl-, phenethyl- and naphthyl isothiocyanates inhibit the P-GP- mediated efflux of daunomycin, an antracycline anticancer agent (28,29).
As described earlier, E1 and E2 exerted synergistic cytotoxic effect on MCF-7 cells, when co-administered with doxorubicin, a drug which is a P-GP substrate. This additional cytotoxicity of doxorubicin could, in part, be attributed to the impairment of the P-GP function caused by 3-butenyl isothiocyanate, found as the major isothiocyanate of D. sophia.
Inhibition of P-GP by isothiocyanates and not being P-GP substrate at the same time makes them potential multi drug resistance (MDR) reversing and cytotoxic agent. When isothiocyanates co-administered with a P-GP substrate like doxorubicin, they could stay inside the cells longer and inhibit the efflux pump to enhance intracellular accumulation of doxorubicin. Consequently, doxorubicin dosage could be adjusted and its severe cardiotoxic side effects will be reduced.
Hermawan and coworkers reported that ethanolic extract of Moringa oleifera, a plant from Cruciferae family, consisting phenethyl-isothiocyanate and benzyl-isothiocyanate was able to increase cytotoxic effect of doxorubicin on HeLa cells (29). Our results also indicated the synergic effects of the volatile oils (50 μg/ml) with doxorubicin (1 μM) on HeLa cell line.
Papi and colleagues demonstrated that 4-methylthio-3-butenyl isothiocyanate has selective cytotoxic/apoptotic activity toward three human colon carcinoma cell lines, and very limited toxicity on normal human T-lymphocytes (30).
The results of another study conducted by Milczarek and coworkers (31) on combination treatment of sulforaphan derivatives with 5-fluorouracil revealed the antagonistic effect of isothiocyanates and 5-fluorouracil on normal cell line while other publications had proven the synergic effect of sulforaphane and doxorubicin (32) or sulporaphane and 5-fluorouracil (33) on cancer cell lines. In case of normal cells, antagonism is a beneficial interaction and considered to be even protective for the normal cells.
At last, cytotoxic activity of isothiociantes on cancerous cell lines with no cytotoxic effects on normal cells as well as their P-GP inhibitory activity and not being the P-GP substrate make them potential target to encourage new researches for cancer chemotherapy.
CONCLUSION
Cytotoxic evaluation of D. sophia volatile oils with various degrees of autolysis showed great cell toxicity on MCF-7 and HeLa cell lines. MCF-7 cells, however, were more susceptible than HeLa cell line. Cocurrent use of E1 and E2 with doxorubicin more significantly reduced the viability of MCF-7 and HeLa cells compared to each individual compound.
ACKNOWLEDGMENTS
The content of this paper is extracted from the Pharm.D thesis NO 392036 submitted by E. Khodarahmi which was financially supported by the Research Department of Isfahan University of Medical Sciences, Isfahan, I.R. Iran.
REFERENCES
- 1.Afsharypuor S, Lockwood GB. Glucosinolate degradation products, alkanes and fatty acids from plants and cell cultures of Descurainia sophia. Plant Cell Rep. 1985;4:341–344. doi: 10.1007/BF00269894. [DOI] [PubMed] [Google Scholar]
- 2.Muhammad K, Artabotryside A. a constituent from Descurainia sophia (L.) induces cell death in U87 glioma cells through apoptosis and cell cycle arrest at G2/M phase. J Med Plants Res. 2012;6:3754–3765. [Google Scholar]
- 3.Bekker NP, Chenko NT, Glushenkova AI. Lipids from Descurainia Sophia seeds. Chem Nat Compd. 2005;41:346–347. [Google Scholar]
- 4.Li J, Liu X, Dong F, Xu J, Zheng Y, Shan W. Determination of the volatile composition in essential oil of Descurainia sophia (L.) Webb ex Prantl (Flixweed) by gas chromatography/mass spectrometry (GC/MS) Molecules. 2010;15:233–240. doi: 10.3390/molecules15010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasalar M, Bagheri Lankarani K, Mehrabani D, Tolide HR, Naseri M. The effect of Descureania sophia L. and Prunus domestica L. in prevention of constipation among Iranian hajj pilgrims, Saudi Arabia. Res J Pharm Biol Chem Sci. 2013;4:1195–1204. [Google Scholar]
- 6.Lee YJ, Kim NS, Kim H, Yi JM, Oh SM, Bang OS, et al. Cytotoxic and anti-inflammatory constituents from the seeds of Descurainia sophia. Arch Pharm Res. 2013;36:536–541. doi: 10.1007/s12272-013-0066-x. [DOI] [PubMed] [Google Scholar]
- 7.Mohamed NH, Mahrous AE. Chemical constituents of Descurainia sophia L. and its biological activity. Rec Nat Prod. 2009;3:58–67. [Google Scholar]
- 8.Aghaabbasi K, Dehghan E, Baghizadeh A, Dashti H. Comparing the effect of ethanol extracts of Descurainia sophia (L.) seed and Althaea officinalis root on Streptococcus pyogenes. Zahedan J Res Med Sci. 2014;16:27–32. [Google Scholar]
- 9.Qian LW. Primary studies on anti-tumor activities and HMGR gene of Descurainia sophia (Linn.). M.Sc [thesis] China: Anhui Normal University. 2006 [Google Scholar]
- 10.Sun K, Li X, Liu JM, Wang JH, Li W, Sha Y. A novel sulphur glycoside from the seeds of Descurainia sophia (L.) J Asian Nat Prod Res. 2005;7:853–856. doi: 10.1080/1028602042000204072. [DOI] [PubMed] [Google Scholar]
- 11.Kim BY, Lee J, Park S, Bang OS, Kim N. Gene expression profile of the A549 human non-small cell lung carcinoma cell line following treatment with the seeds of Descurainia sophia, a potential anticancer drug. J Evid Based Cmpl Altern Med. 2013;2013:1–13. doi: 10.1155/2013/584604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun K, Li X, Li W, Wang J, Liu J, Sha Y. Two new lactones and one new aryl-8-oxa-bicyclo[3,2,1] oct-3-en-2-one from Descurainia sophia. Chem Pharm. Bull (Tokyo) 2004;52:1483–1486. doi: 10.1248/cpb.52.1483. [DOI] [PubMed] [Google Scholar]
- 13.Dehshahri S, Afsharypuor S, Asghari G, Mohagheghzadeh A. Determination of volatile glucosinolate degradation products in seed coat, stem and in vitro cultures of Moringa peregrina (Forssk.) Fiori. Res Pharm Sci. 2012;7:51–56. [PMC free article] [PubMed] [Google Scholar]
- 14.Gamet L, Li P, Lumeau S, Cassar G, Dupont MA, Chevolleau S, et al. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res. 2000;60:1426–1433. [PubMed] [Google Scholar]
- 15.Freshney RI. 4th ed. New York Wiley: Liss; 2000. Culture of animal cells: a manual of basic techniques; pp. 1–340. [Google Scholar]
- 16.Khodarahmi GA, Hassanzade F, Jafarian A, Chinifroosh AH, Hajseyedabutrabi AM. Cytotoxicity evaluation of some 1-[(Benzofuran-2-yl)-Phenylmethyl] Imidazols on MCF-7 and Hela Cell Lines. Res Pharm Sci. 2007;2:73–79. [Google Scholar]
- 17.Al-Gendy AA, El-gindi OD, Hafez AS, Ateya AM. Glucosinolates, volatile constituents and biological activities of Erysimum corinthium Boiss. (Brassicaceae) Food Chem. 2010;118:519–524. [Google Scholar]
- 18.Al-Gendy AA, Lockwood Artabotryside A GB. GC-MS analysis of volatile hydrolysis products from glucosinolates in Farsetia aegyptia var. ovalis. Flavour and Frag J. 2003;18:148–152. [Google Scholar]
- 19.Al-Gendy AA. Phytochemical and biological screening of glucosinolates and volatile constituents of different Brassicaceae plants growing in Egypt. Bull Fac Pharm Cairo Univ. 2008;46:235–244. [Google Scholar]
- 20.Khan M, Wang N. Descurainia sophia (L.): a weed with multiple medicinal uses. Punjab Univ J Zool. 2012;27:45–51. [Google Scholar]
- 21.Xiao D, Vogel V, Singh S. Benzyl isothiocyanate-induced apoptosis in human breast cancer cells is initiated by reactive oxygen species and regulated by Bax and Bak. Mol Cancer Ther. 2006;5:2931–2945. doi: 10.1158/1535-7163.MCT-06-0396. [DOI] [PubMed] [Google Scholar]
- 22.Tseng E, Scott-Ramsay EA, Morris ME. Dietary organic isothiocyanates are cytotoxic in human breast cancer MCF-7 and mammary epithelial MCF-12A cell lines. Exp Biol Med. 2004;229:835–842. doi: 10.1177/153537020422900817. [DOI] [PubMed] [Google Scholar]
- 23.Grant CE, Validmarsson G, Hipfner DR, Almquist KC, Cole SP, Deely RG. Overexpression of multidrug resistance-associated protein (MRP) increases resistance to natural product drugs. Cancer Res. 1994;54:357–361. [PubMed] [Google Scholar]
- 24.Bradley G, Naik M, Ling V. P-Glycoprotein expression in multidrug-resistant human ovarian carcinoma cell lines. Cancer Res. 1989;49:2790–2796. [PubMed] [Google Scholar]
- 25.Lavie Y, Cao HT, Volner A, Lucci A, Han TY, Geffen V, et al. Agents that reverse multidrug resistance, Tamoxifen, Verapamil, and Cyclosporin A, block Glycosphingolipid metabolism by inhibiting ceramide glycosylation in human cancer cells. J Biol Chem. 1997;272:1682–1687. doi: 10.1074/jbc.272.3.1682. [DOI] [PubMed] [Google Scholar]
- 26.Krishna R, Mayer LD. Multidrug resistance (MDR) in cancer mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur J Pharm Sci. 2000;11:265–283. doi: 10.1016/s0928-0987(00)00114-7. [DOI] [PubMed] [Google Scholar]
- 27.Gupta P, Srivastava SK. Antitumor activity of phenethyl isothiocyanate in HER2-positive breast cancer models. BMC Med. 2012;10:1–18. doi: 10.1186/1741-7015-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu K, Morris ME. Effects of benzyl-, phenethyl-, and alpha-naphthyl isothiocyanates on P-glycoprotein- and MRP1-mediated transport. J Pharm Sci. 2004;93:1901–1911. doi: 10.1002/jps.20101. [DOI] [PubMed] [Google Scholar]
- 29.Hermawan A, Nur KhA, Sarmoko, Dewi D, Putri P, Edy M. Ethanolic extract of Moringa oleifera increased cytotoxic effect of doxorubicin on HeLa cancer cells. J Nat Remedies. 2012;12:108–113. [Google Scholar]
- 30.Papi A, Orlandi M, Bartolini G, Barillari J, Ioro R, Paolini M, et al. Cytotoxic and antioxidant activity of 4-Methylthio-3-butenyl Isothiocyanate from Raphanus sativus L. (Kaiware Daikon) sprouts. J Agric Food Chem. 2008;56:875–883. doi: 10.1021/jf073123c. [DOI] [PubMed] [Google Scholar]
- 31.Milczarek M, Misiewicz-Krzemi I, Lubelska K, Wiktorska K. Combination treatment with 5-fluorouracil and isothiocyanates shows an antagonistic effect in Chinese hamster fibroblast cell line-V79. Acta Pol Pharm. 2011;68:331–342. [PubMed] [Google Scholar]
- 32.Wathelet J, Lori R, Leoni O, Rollin P, Quinsac A, Palmieri S. Guidelines for glucosinolates analysis in green tissues used for biofumigation. Agroindustria. 2004;3:257–266. [Google Scholar]
- 33.Pawels R, Balzarini J, Baba M, Snoeck R, Schols D. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J Virol Methods. 1988;20:390–391. doi: 10.1016/0166-0934(88)90134-6. [DOI] [PubMed] [Google Scholar]


