Abstract
1. Harmonia axyridis was first recorded in Britain in 2004. Two subsequent earlier records were received from 2003.
2. The UK Ladybird Survey, a citizen science initiative involving online recording, was launched in 2005 to encourage people across Britain to track the spread of H. axyridis. Tens of thousands of people have provided records of H. axyridis and other species of ladybirds, creating an invaluable dataset for large-scale and long-term research. Declines in the distribution of seven (of eight assessed) native species of ladybird have been demonstrated, and correlated with the arrival of H. axyridis, using the records collated through the UK Ladybird Survey.
3. Experimental research and field surveys have also contributed to our understanding of the ecology of H. axyridis and particularly the process of invasion. Harmonia axyridis arrived in Britain through dispersal and introduction events from regions in which it was deliberately released as a biological control agent. The rapid spread of this species has been attributed to its high natural dispersal capability by means of both flight and anthropogenic transport. A number of factors have contributed to the successful establishment and indeed dominance of this polymorphic species within aphidophagous guilds, including high reproductive capacity, intra-guild predation, eurytopic nature, high resistance to natural enemies within the invaded range, and potentially phenotypic plasticity.
4. The global invasion by H. axyridis and subsequent research on this species has contributed to the general understanding of biological invasions.
Keywords: Biological invasions, citizen science, intra-guild predation, invasive species, monitoring and surveillance, non-native species
Introduction
The first British record of Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae), the harlequin ladybird, was from Sible Hedingham, Essex, England, in 2004 (Majerus et al., 2006). Widely introduced across continental Europe as a biological control agent of aphids, it had never been intentionally introduced into Britain (Brown et al., 2008a). However, it was perhaps inevitable that individuals from the introduced populations in Europe would arrive in Britain because H. axyridis has excellent dispersal abilities (Brown et al., 2008b; Jeffries et al., 2013).
The first record of H. axyridis within Britain, alongside the rapid spread of this species elsewhere in Europe, triggered a number of responses:
Rapid development of online recording and the launch of the UK Ladybird Survey including the Harlequin Ladybird Survey [encompassing the Biological Records Centre (BRC) hosted Coccinellidae Recording Scheme].
Research collaborations across Europe through the establishment of a working group ‘Risks and benefits of exotic biological control agents’ within the International Organisation for Biological and Integrated Control (IOBC).
Publication of a review in Ecological Entomology outlining potential impacts of the arrival of H. axyridis (Majerus et al., 2006).
The review published in Ecological Entomology (Majerus et al., 2006) outlined a number of predictions (Table 1) and provided a framework for research specifically within Britain but with relevance across Europe and beyond. Indeed, H. axyridis was noted as providing ‘entomologists with a unique and exciting opportunity to monitor the spread and impacts of an invasive alien insect in British environments that might prove a timely model study for future ecological impact assessments’. The long history of invasion of H. axyridis in America was highlighted in the review and it was recognised that there was much to be gained from comparative studies building on the research findings available from America (Koch & Galvan, 2008).
Table 1.
Predictions following the arrival of Harmonia axyridis in Britain (Majerus et al., 2006) alongside a summary of recent evidence, supporting references, and overall conclusions, based on current understanding, with respect to the importance of factors in determining success of invasion by this species
| Prediction | Evidence | References | Conclusion |
|---|---|---|---|
| Eurytopic nature of H. axyridis will contribute to rapid spread | The range of host plant associations and widespread distribution of H. axyridis in Britain reflect the eurytopic nature of this species, although coniferous woodlands may negatively affect the spread of H. axyridis. | Brown et al. (2008b, 2011a) | + |
| Habitat breadth is an important factor contributing to the invasion success of H. axyridis. | |||
| Climatic adaptability of H. axyridis will give it a competitive advantage over some of the more niche-specific native ladybirds | Climatic conditions have not been a barrier to the colonisation and spread of H. axyridis in southern Britain, but are speculated to have limited its abundance in northern England and in Scotland. | Comont et al. (2012) and Purse et al. (2014) | +/? |
| There are clear discrepancies between the observed and predicted (climate model) distributions of H. axyridis, and it is apparent that climate is an important factor in determining the spread of this species but alongside other interacting biotic and abiotic factors. | |||
| Maritime climate of Britain will allow H. axyridis to breed throughout the summer, with no requirement for a summer dormancy | Continual breeding of this species is apparent and at least two generations of H. axyridis have been observed each year since arrival. | Brown et al. (2008b) and Roy et al. (2011a) | + |
| Multivoltinism contributes to the rapid rate of population growth of H. axyridis each year and, consequently, to spread. | |||
| Phenotypic plasticity will allow H. axyridis to successfully and regularly extend its breeding season to September, October, and even into November | Phenotypic plasticity displayed by H. axyridis enables local adaptation at temporal and spatial scales; increase in autumnal melanisation may have accelerated the spread of H. axyridis. | Michie et al. (2010) and Purse et al. (2014) | ? |
| Further work is required to elucidate the importance of phenotypic plasticity in the invasion success of H. axyridis. | |||
| H. axyridis will spread across the entire British mainland by 2008 | The first record of H. axyridis in Scotland was in 2007. However, there are relatively few records in Scotland and its distribution and breeding there are limited. | Brown et al. (2008a,2008b, 2011b) and Roy et al. (2011a) | + |
| High dispersal ability of this species has clearly been demonstrated in most of England and Wales. | |||
| Spread and increase of H. axyridis in Britain may therefore prove to be beneficial to crop systems by restricting aphid numbers below economically damaging levels and so reduce the use of chemical pesticides | Recent research highlights the importance of H. axyridis as an aphid predator in crop systems in the UK. | Wells (2011) | ? |
| Further work is required to explore the ecosystem-level impact of H. axyridis on pest insects and particularly the ecosystem service provided by this alien predator. | |||
| Harmonia axyridis is likely to have a negative effect on other aphidophages in three ways: resource competition, intra-guild predation, and intraspecific competition | There is considerable evidence of intra-guild predation from laboratory and field observations. | Ware and Majerus (2008), Ware et al. (2009), Wells et al. (2010), Brown et al. (2011a), Wells (2011), Roy et al. (2012) and Brown et al. (2014) | + |
| Observations from the UK Ladybird Survey highlight a strong correlation between the presence of H. axyridis and declines in the distribution of native ladybird species. | |||
| Further work is required on competitive interactions, although recent research in laboratory mesocosms suggests that high aphid density does not reduce intra-guild predation. | |||
| There is considerable evidence of negative effects of H. axyridis on other species, but effects on ecosystem function require further work. | |||
| Efficient chemical defence and relatively large size would provide H. axyridis with a significant reproductive advantage over many native British species | A few studies indicate the importance of chemical defence and body size in intra-guild interactions. | Bezzerides et al. and (2007) and Ware et al. (2008) | +/? |
| The importance of chemical defence and large size in contributing to reproductive advantage of H. axyridis over native species requires further investigation. | |||
| H. axyridis will become a nuisance to humans | There have been many reports of H. axyridis forming large aggregations in domestic dwellings, and in some cases people have reported this species as a nuisance. | Roy et al. (2011a) | − |
| There is some evidence of negative effects on humans. |
+, important factor; –, unimportant; ?, undecided.
Harmonia axyridis has been the inspiration and focus of research across the globe (Sloggett, 2005, 2012). Indeed, 19 papers were published in a special issue of the journal BioControl as a result of the collaboration through the IOBC working group ‘Risks and benefits of exotic biological control agents’ (Roy & Wajnberg, 2008). These publications have been widely cited and demonstrate the collaborative approach to research on H. axyridis. Here we provide an overview of research findings, particularly in Britain, over the last 10 years. We highlight the contributions made through research on H. axyridis to the field of invasion biology, focusing on predictions from Majerus et al. (2006); the manuscript has been structured to align with the review (Majerus et al., 2006).
Factors affecting the population demography of Harmonia axyridis in Britain
The records of H. axyridis received through the UK Ladybird Survey have enabled the spread of this invader to be documented from early in the invasion process (Brown et al., 2008b). The considerable media attention in response to immediate notification of the arrival of H. axyridis in England led to approximately 100 verified records of the species from September to December 2004. These records were mainly from the south-east of England, with many from coastal areas, and only three outlying 10-km squares recorded in northern England (Brown et al., 2008b). Harmonia axyridis spread west and north within Britain, with the northerly spread rate from 2004 to 2008 calculated as 105 km year–1 (Brown et al., 2008b) and by 2009 was recorded in 1022 10-km squares encompassing all regions of England and Wales, with approximately 75% of 10-km squares within the invaded range having verified records (Fig. 1).
Fig. 1.
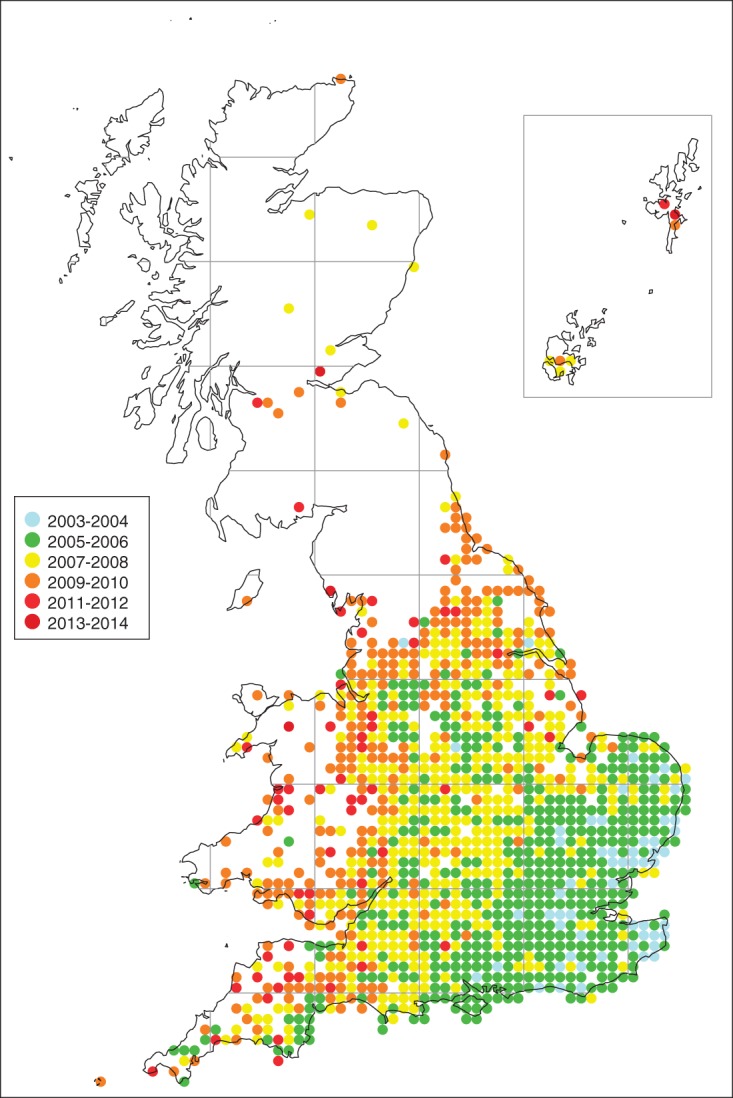
Harmonia axyridis occurrence in 10-km squares in Britain from 2004 to 2014. Where a square has been recorded in more than 1 year, occurrence in the earliest year is shown (blue, 2003–2004; green, 2005–2006; yellow, 2007–2008; orange, 2009–2010; red, 2011–2012; burgundy, 2013–2014).
Eurytopic nature
Harmonia axyridis is highly eurytopic in Britain, thriving in a wide range of habitats, as predicted by Majerus et al. (2006). It is particularly successful in urban localities, spreading faster into areas containing a high proportion of urban land (Purse et al., 2014). The species also thrives in rural locations; based on UK Ladybird Survey data, 19% of the 1-km squares with H. axyridis records were predominantly arable or horticultural land (Brown, 2010). The population dynamics of H. axyridis in crop systems (wheat, corn, broad bean, and potato crops) have been studied in Belgium and indicate that H. axyridis arrives 7–8 days after the dominant native coccinellids (Jansen & Hautier, 2008; Vandereycken et al., 2013). A 1-year study (2008) involving field observations in wheat and bean crops in southern England reported an absence of H. axyridis in wheat (aphid abundance was reported as low) but the presence of H. axyridis co-occurring with other coccinellids in bean crops (Wells, 2011). Harmonia axyridis was the most common aphidophagous species in bean crops, and the presence of this species was correlated with aphid abundance (Wells, 2011).
In Britain, 5% of H. axyridis records were from 1-km squares dominated by woodland (mostly broadleaved or mixed). The percentages of records submitted to the UK Ladybird Survey from various vegetation types were: deciduous trees and shrubs, 56%; herbaceous plants, 29%; evergreen trees and shrubs, 11%; grasses and others, 4% (Brown, 2010). Indeed, H. axyridis has been recorded from more than 75 plant families, dominated by Aceraceae (14% of records with associated plant data), Rosaceae (13%), and Malvaceae (10%) (Brown, 2010). Larvae of the species were recorded from about 50 of these families (Brown et al., 2011a), notably Aceraceae (22% of records with associated plant data), Malvaceae (18%), Rosaceae (10%) and Urticaceae, Betulaceae, and Salicaceae (5% each) (Brown, 2010). Thus, in Britain, H. axyridis thrives on deciduous trees and shrubs such as limes, maples, birches, and roses, as well as a variety of herbaceous plants, including stinging nettle. Similar patterns of host plant association have been observed in other regions of Europe (Roy et al., 2012; Panigaj et al., 2014). Records of H. axyridis from coniferous trees in Britain are quite limited, unlike in parts of its native range (Brown et al., 2011a).
The widespread distribution of H. axyridis in the UK reflects the eurytopic nature of this species. Indeed, the ability of H. axyridis to thrive in association with a diverse range of host plants undoubtedly explains the observed breadth of habitat types occupied by H. axyridis. A recent study on the spread of H. axyridis, including consideration of landscape factors, suggested that coniferous woodland, after correcting for bias in recording intensity, might negatively affect the spread of this species (Purse et al., 2014). Currently there is limited information within the UK Ladybird Survey database on specific plant associations, and what is there is mainly found in comment fields and so requires considerable work to extract (Brown, 2010). Further developments of the UK Ladybird Survey will include capturing information on plant associations as defined data fields within the online recording forms to enable future research on ecological networks.
Climate
Climatic conditions have not been a barrier to the colonisation and spread of H. axyridis in southern Britain, but are speculated to have limited its abundance in northern England and in Scotland (Brown et al., 2008b). In these northern areas, records of successful breeding by H. axyridis are very limited. Climatic modelling studies have indicated that nearly all of mainland Britain is suitable for H. axyridis, with the exception of northern Scotland (Poutsma et al., 2008). The model proposed by Poutsma et al. (2008) has proved to be a good predictor of the expanding distribution of H. axyridis in Europe (Brown et al., 2011b), but the parameters used may need slight refinement to include observations on distribution from the UK Ladybird Survey. The combination of lower temperatures and higher precipitation in Scotland than in England appears to restrict H. axyridis to warm urban localities in Scotland, especially in terms of successful reproduction. The Orkney Islands and Shetland Islands (off the north-east coast of Scotland) are indicated as climatically unsuitable for H. axyridis (Poutsma et al., 2008); whilst there have been isolated records of individual adults from these northern islands, these ladybirds seem to have arrived on produce imported from the mainland (Ribbands et al., 2009) and there are no records here of juveniles of H. axyridis, or indeed of any other coccinellid species.
The most northerly record of H. axyridis in Europe is from Trondheim, Norway (Saethre et al., 2010), substantially further north than the Shetland Islands, but Oslo appears to be the most northerly location where H. axyridis has become established. Majerus et al. (2006) predicted that climate change may provide H. axyridis with a further competitive advantage over native British coccinellids. Predictions from modelling approaches suggest that H. axyridis may indeed benefit from climate warming through further northward expansion (Purse et al., 2014) and increased voltinism is also possible. As in parts of its native range, such as Japan (Osawa, 2011), H. axyridis is multivoltine in Britain and usually completes two generations per year (Brown et al., 2008b) (Fig. 2). Larval peaks are in June and October and there is the potential for three generations in particularly favourable years. Records of larvae in late December (winter) are not unusual. The resultant high population is presumed to encourage higher rates of dispersal for the species compared with native, univoltine species.
Fig. 2.
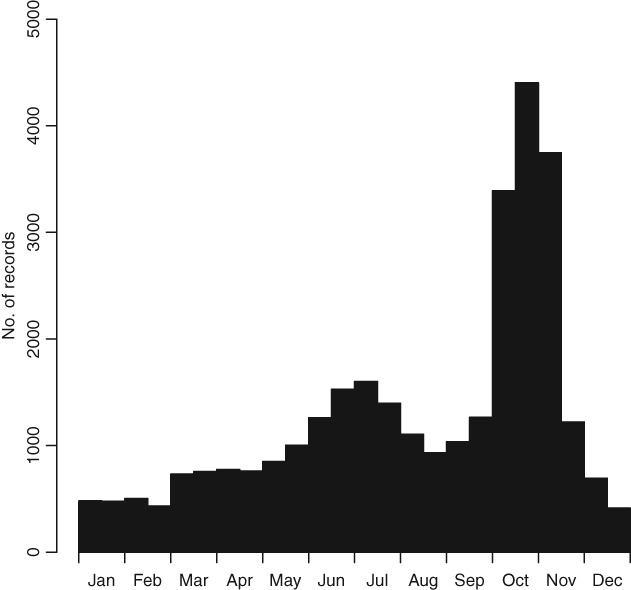
Harmonia axyridis phenogram displaying number of H. axyridis records in the UK Ladybird Survey database as monthly counts.
While climate models in part explain the distribution of H. axyridis within the invaded range, there are clear discrepancies between the observed and predicted distributions of H. axyridis. There are, of course, many factors that influence the invasion process and the distribution of species over time. Indeed, the interactions among landscape factors, climate, and species traits (such as polymorphism) in determining the distribution of ladybirds are complex (Comont et al., 2014a).
Summarising, the apparent lack of success of H. axyridis in Scotland could be attributed to a number of factors:
Biogeographic features such as mountain ranges may act as barriers to species dispersal and invasion (Wilson et al., 2009). Such barriers to H. axyridis in Britain include the Cambrian mountains (Wales) and particularly the Pennine mountains (northern England) (Brown et al., 2011b). The reasons why mountains may block dispersal are effectively encompassed within habitat and climatic limitations (see later).
Habitat factors such as soil type, land use, and vegetation type have a direct effect on ladybird prey species and therefore an indirect effect on ladybird populations (Comont et al., 2014a). Eurytopic ladybirds such as H. axyridis tend to thrive in habitats (such as arable and urban) with high prey abundance. In less favourable habitats such as those at higher altitudes (principally moorland and heathland in Britain), H. axyridis is found at lower densities, if at all.
The cooler and wetter climate typical of northern and upland regions of Britain is less favourable to many predatory insect species and their prey. Whilst the native range of H. axyridis includes southern Siberia, with very cold winter temperatures [e.g. mean January temperatures of –13 °C (daily high) to –18 °C (daily low) in Novosibirsk], it may be the combination of wet conditions with cold temperatures that is particularly unfavourable for H. axyridis in Scotland. Native ladybirds tend to be much lower in both species number and abundance in Scotland than in England; indeed, in Scotland, 25 of the 46 resident British coccinellid species are either absent or occur very rarely (Roy et al., 2011a).
Lower human population density in Scotland than in England and Wales could partially explain the low number of records in Scotland. This places a potential bias on our dataset, as clearly we would expect to receive fewer records from less populated areas. However, there are many 10-km squares in Scotland with high numbers of records of other species of ladybird, suggesting that the level of recording intensity is sufficient to derive a robust assessment of the distribution of H. axyridis throughout Britain.
There is still much to reveal about the spread of H. axyridis and it is important to recognise that invasions are dynamic processes. The UK Ladybird Survey dataset has been used in many studies exploring the interactions between abiotic and biotic factors in determining the distribution of ladybirds (Brown et al., 2008b; Brown, 2010; Purse et al., 2014; Comont et al., 2014a) and demonstrates the huge value of such citizen science initiatives for continued analysis of large-scale and long-term ecological processes.
Phenotypic adaptability
There have been some intriguing insights into the phenotypic adaptability of H. axyridis over the past 10 years. Perhaps the most compelling evidence of phenotypic adaptability is in relation to colour pattern polymorphism. Harmonia axyridis is a polymorphic species for both the pattern and colour of the pronota and elytra (Majerus et al., 2006). Three main colour morphs have been reported in Britain: f. succinea, f. spectabilis, and f. conspicua. Additionally, there are a few records of f. equicolor and f. aulica; the nominate form – f. axyridis – has not been reported in Britain. The non-melanic f. succinea is the most abundant colour form and comprises approximately 80% of records (Brown et al., 2008b; Purse et al., 2014). The influence of temperature on f. succinea is intriguing; individual H. axyridis f. succinea (non-melanic) eclosing from pupae late in the year have larger spots than those eclosing in spring and early summer (Michie et al., 2010). Recent research has indicated that the phenotypic plasticity displayed by H. axyridis enables local adaptation at temporal and spatial scales (Michie et al., 2010), whereby melanism, which may be important in thermoregulation (Brakefield & de Jong, 2011), is considered costly in summer and beneficial in winter (Michie et al., 2010). Michie et al. (2010) proposed that melanisation may have accelerated the spread of H. axyridis.
Modelling approaches using the UK Ladybird Survey dataset have explored the prediction that melanic colour forms have a thermal regulatory advantage and consequently spread more rapidly than the non-melanic colour form (Purse et al., 2014). It was apparent that while increased sunshine significantly enhanced the spread of the non-melanic form (f. succinea), the spread was more rapid within hectads containing a high proportion of urban land cover and marginally slower in hectads containing high conifer cover (Purse et al., 2014). Additional recent research suggests that the colour pattern polymorphism of H. axyridis and variation in other life-history traits could contribute to the invasion success of this species (Majerus et al., 2006; Michie et al., 2010; Purse et al., 2014).
There have been a number of studies exploring the influence of life-history traits on the distribution of coccinellids including H. axyridis (Comont et al., 2012, 2014a). The traits database compiled for these studies provides a rare opportunity to explore variation in life-history traits between species. Indeed, it has been insightful to include information on species traits alongside the distribution data from the UK Ladybird Survey to explain trends in the distribution patterns of ladybirds in Britain (Comont et al., 2014a). Climate and habitat datasets available for Britain have added further value to these analyses (Comont et al., 2014a). It would be fascinating to extend this research beyond Britain to consider life-history traits in a biogeographic context. Additionally, modelling approaches enable eloquent exploration of large-scale and long-term datasets to test predictions and ultimately construct further hypotheses. Detailed empirical approaches are required to examine the mechanisms at play.
Dispersal potential
The rapid spread of H. axyridis has been a consequence of both natural dispersal by flight and anthropogenic processes. Recent research using innovative research tools, namely vertical-looking entomological radar, have provided intriguing insights into the flight patterns of H. axyridis. Harmonia axyridis and C. septempunctata were detected at 1100 m above ground level moving at 60 km h−1 and sustaining flight for up to 2 h, indicating a high capacity for long-distance dispersal.
Much of Britain is densely populated and has an elaborate transport network, and there are many reports of H. axyridis being transported accidentally in or on vehicles. Inadvertent movement with people and goods has undoubtedly facilitated the spread of the species in Britain. For example, in 2007, H. axyridis was first recorded from Scotland (Holroyd et al., 2008) as a result of the ladybird being transported in a suitcase; the first record from the Orkney islands (northern Scotland, 2008; Ribbands et al., 2009) and from Northern Ireland (2007; Murchie et al., 2008) involved the ladybirds being transported with vegetables from mainland Britain. The most noticeable record in this regard is that of the population that was initiated by transport of ladybirds to a supermarket in Derby (north-central England) in 2004. The beetles spread rapidly from there and are likely to have accelerated the northerly spread of the species from 2005.
Natural enemy interactions
Natural enemy escape provides an appealing hypothesis for explaining the success of an invader (Roy et al., 2011b). The Enemy Release Hypothesis (ERH) (Elton, 1958; Torchin et al., 2003) predicts that an alien species will be less affected by specialised natural enemies (predators, parasites and pathogens) than will native species. Thus, the alien gains a competitive advantage and may rapidly increase in abundance and distribution (Elton, 1958; Torchin et al., 2003; Colautti et al., 2004). The premise of this theory is that natural enemies are important in regulating populations (Roy & Lawson Handley, 2012), but the empirical evidence for this, and consequently the ERH, is limited (Roy & Cottrell, 2008; Roy et al., 2011b). There have been some advances in understanding the role of natural enemies in the H. axyridis invasion over the last 10 years (Kenis et al., 2008; Roy et al., 2013; Comont et al., 2014b).
Arguably the most important natural enemies of ladybirds in Britain are pathogens (such as Beauveria bassiana) and several species of endoparasitic Hymenoptera and Diptera (Roy et al., 2011a, 2013; Ceryngier et al., 2012; Comont et al., 2014b). There were early indications that some of the ladybird's natural enemies native to Britain would attack H. axyridis (Hall et al., 2009; Ware et al., 2010), but laboratory studies indicated the low susceptibility of this invader in comparison to native species (Koyama & Majerus, 2008; Roy et al., 2008b). Laboratory research indicates that H. axyridis is an unfavourable host for Dinocampus coccinellae (Schrank) (Hymenoptera: Braconidae) (Hoogendoorn & Heimpel, 2002; Berkvens et al., 2010b). The exact mechanism involved in the resistance of H. axyridis to D. coccinellae is unclear, but teratocyte cells produced by D. coccinellae (involved in both immunosuppression of the host and nutrition of the parasitoid) follow an abnormal pattern of growth within H. axyridis, which could explain the impeded development of D. coccinellae within this invader (Firlej, 2012). The fungal pathogen B. bassiana commonly infects native species of ladybird (such as C. septempunctata), but again H. axyridis appears to be highly resistant (Roy et al., 2008b). A recent study also demonstrated that H. axyridis individuals contain high numbers of obligate parasitic microsporidia, which appear to have no adverse affects on H. axyridis but cause high mortality when artificially injected into C. septempunctata (Vilcinskas et al., 2013). The ecological relevance of this study requires further investigation because injection is an artificial process and far removed from the natural mechanisms involved in microsporidia transmission.
Clearly there is a need to extend studies of natural enemies to the field in order to ensure ecological relevance. One recent study from Britain confirmed low rates of parasitism of H. axyridis, particularly in comparison to the native C. septempunctata (Comont et al., 2014b). Indeed, pupae of H. axyridis were parasitised, primarily by Phalacrotophora fasciata (Fallén) and Phalacrotophora berolinensis Schmitz (Diptera: Phoridae), at an exceptionally low level (1.73%) and adults were not found to be parasitised at all in this study. In contrast, parasitism of the co-occurring C. septempunctata was high (20.91% pupae, 5.67% adults). This provides evidence in support of the ERH, i.e. success of the invader may result from a reduction or absence of natural enemies (Elton, 1958; Torchin et al., 2001). However, further work is required to elucidate population-level effects of this difference in parasitism rates between the alien and native species. There is no doubt that H. axyridis represents an excellent opportunity to explore natural enemy interactions and their role in the invasion process.
Impacts
Benefits as a pest control agent
There has been little focus in Britain on the role of H. axyridis as a beneficial pest control agent (Wells, 2011). The effects of H. axyridis on aphid populations in British crop systems are unknown and are worthy of further investigation, particularly with respect to ecosystem services and resilience (Koch & Galvan, 2008; Vilà et al., 2009).
Negative effects on pest and non-pest herbivorous insects
Harmonia axyridis has a wide diet breadth (reviewed by Hodek & Evans, 2012) and, in the absence of aphids, can complete development on a combination of other foods including coccids, adelgids, psyllids and many other insects, including conspecifics (Tedders & Schaefer, 1994; Koch, 2003; Flowers et al., 2005; Onofre Soares et al., 2005; Majerus et al., 2006; Hodek & Evans, 2012), but also Ephestia kuehniella (Lepidoptera: Pyralidae) eggs (Berkvens et al., 2010a) and pollen (Berkvens et al., 2008, 2010a). Therefore, H. axyridis is predicted to pose a threat to many species. However, there have been few studies exploring the population-level effects of H. axyridis on non-target herbivorous insects. One study recognised the potential for H. axyridis to negatively affect monarch butterflies, Danaus plexippus (L.) (Lepidoptera: Nymphalidae), in the US (Koch et al., 2003). In the UK Ladybird Survey database, there are records of H. axyridis predating lepidopterans (such as the eggs of noctuid moths) in Britain, but the extent of such predation is unknown. Further research is required to examine the population dynamics of these interacting species. It is also important to note that H. axyridis is not unique amongst the coccinellids in having a wide diet breadth (Sloggett, 2012).
Negative effects on other aphidophages
Harmonia axyridis is widely recognised as a top predator within aphidophagous guilds (Pell et al., 2008). However, as highlighted by Majerus et al. (2006), the negative effects of H. axyridis are likely to be the result of a complex range of interactions, with H. axyridis having a competitive edge through resource competition, intra-guild predation (IGP), and a more plastic phenotype than other aphidophagous species. There have been many published studies exploring such interactions, particularly IGP, and to a lesser extent, competition (Phoofolo & Obrycki, 1998; Ware et al., 2009). Initially these were mostly small-scale laboratory studies of ladybird interactions within Petri dishes, which demonstrated strong asymmetric IGP in favour of H. axyridis over native ladybirds (Pell et al., 2008; Ware & Majerus, 2008; Ware et al., 2008; Roy et al., 2008a). In contrast, it is apparent that in such Petri dish experiments Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae) is an intra-guild predator of H. axyridis (Nedvěd et al., 2013), but in mesocosm experiments IGP was in favour of H. axyridis over C. carnea (Wells, 2011).
Understanding of intra-guild interactions has progressed by increasing the scale with the use of more realistic experimental arenas than Petri dishes. Mesocosm studies have included interactions between coccinellids and non-coccinellid aphidophages such as neuropterans (Wells et al., 2010; Wells, 2011) and syrphids (Ingels & De Clercq, 2011). Such approaches to exploring IGP are critical for informing risk assessment by enabling rapid assessment of interactions for a range of potential prey species and different life stages of both the intra-guild predator and prey. Additionally, assessing the effects of aphid density on IGP provides further context to the experiments (Ware et al., 2009; Wells, 2011), but results so far suggest that the prevalence of IGP is not reduced by increased aphid density (Wells, 2011). However, extrapolating findings from laboratory studies to the field is challenging and many questions remain with respect to the ecological relevance of IGP. Molecular tools provide exciting opportunities for investigating community interactions in the field (Roy & Lawson Handley, 2012). Analyses using the polymerase chain reaction have been employed to detect prey DNA from the guts of field-collected H. axyridis samples. Initial work in Britain assessed larval gut contents for two intra-guild prey – Adalia bipunctata (L.) (Coleoptera: Coccinellidae) and Adalia decempunctata (L.) (Coleoptera: Coccinellidae) – and both were detected within H. axyridis (Thomas et al., 2013). This work was extended to investigate predation of neuropterans and syrphids by H. axyridis with testing of samples from five European countries (Brown et al., 2014). Through this study it was apparent that while syrphids were detected in the gut of H. axyridis, neuropterans were not. Gas chromatography-mass spectrometry has been used in mainland Europe for the detection of ladybird IGP and revealed similar results (Hautier et al., 2011).
The taxonomic breadth of studies demonstrating IGP by H. axyridis supports the contention that H. axyridis is an aggressive coccinellid with a tendency for intra-guild predation that could seriously affect the abundance of native coccinellids and dramatically reduce their available niches in the predator complex (Elliott et al., 1996). Furthermore, observations from the UK Ladybird Survey (Roy et al., 2012) highlight the potential for H. axyridis to dramatically disrupt native guilds in Britain (Majerus et al., 2006). However, further understanding of the implications of IGP by H. axyridis on ecological resilience and function should be prioritised. Recent research from America found no evidence that H. axyridis consumed coccinellid eggs in the field, but suggested that exploitative and apparent competition might explain declines of native species in the presence of H. axyridis (Smith & Gardiner, 2013). There is an urgent need for detailed field studies to quantitatively document the interactions between invaders and other species within the community. Ecological network analysis provides exciting opportunities for detailed exploration of the complex interactions across the aphidophagous community (Roy & Lawson Handley, 2012). It will be particularly intriguing to explore the concept of ecological resilience and extend research on ecological networks to consideration of other invaded systems (Romanuk et al., 2009).
Negative effects on humans
Overwintering aggregations of H. axyridis have undoubtedly been one of the most notable aspects of invasion by this species. Many people report sightings from their houses during autumn and winter to the UK Ladybird Survey, with the annual peak of records generally being in late October and early November. Many people have reported problems associated with overwintering aggregations of H. axyridis, specifically staining of soft furnishings and unpleasant smell associated with the secretion of reflex blood. There have been observations of thousands of individuals inside houses and in the bell towers and porches of churches (Roy et al., 2011a). However, the impacts on people, beyond a minor nuisance, are limited in the British context. Allergic reactions to H. axyridis are rare (Goetz, 2008) but there have been a few reports of such reactions in Britain.
In wine-growing regions of North America, H. axyridis has attained the status of a pest (Koch et al., 2004; Koch & Galvan, 2008). This is not the case for all vineyard owners, some of whom have looked on its appearance in their (British) vineyards favourably (DeCourcy, 2009), despite concerns elsewhere over negative effects on wine production (Galvan et al., 2008). There are no known reports of negative impacts in vineyards in Britain, where grape-growing is rare. Harmonia axyridis has a tendency to aggregate on soft fruits, including grapes, and exacerbates damage through feeding, but also contaminates the crop because it is difficult to separate the beetles at harvest. The tainting caused by H. axyridis crushed with the grapes is problematic. However, recently concerns have been raised that it is not just H. axyridis that causes such problems in North America, but also C. septempunctata, native to Britain but an alien species in North America (Botezatu et al., 2013). Both H. axyridis and C. septempunctata contribute alkyl methoxypyrazines, and particularly isopropyl methoxypyrazine, to wine at concentrations that are considered to have a negative impact on wine quality (Botezatu et al., 2013). Although there are no effective and recommended control strategies available for H. axyridis (Kenis et al., 2008), there are indications that sulphur dioxide (in the form of potassium metabisulphite), a commonly used antimicrobial and antioxidant in wine production, repels H. axyridis from grapevines (Glemser et al., 2012).
Potential control strategies
Methods for controlling the spread of H. axyridis have been proposed (Kenis et al., 2008). Harmonia axyridis produces an aggregation pheromone to attract other individuals to overwinterwing habitats (Verheggen et al., 2007). The use of the aggegration pheromone within a network of traps has been proposed and could potentially work at a local scale (such as in a vineyard, where preventing H. axyridis from aggregating within bunches of grapes would be advantageous). However, at a large scale there would be practical implications that would render this approach unfeasible; a very large number of traps would be needed and the costs involved in managing the traps would be prohibitively high.
There are a number of natural enemies of H. axyridis that could potentially exert control, but population-level effects of natural enemies on the regulation of ladybirds are poorly understood (Roy et al., 2011c; Comont et al., 2014b). Additionally, as outlined earlier, studies on the interactions between H. axyridis and natural enemies strongly indicate that H. axyridis is less susceptible to attack by native parasitoids and pathogens than are native ladybirds, although this may change in the future. The ectoparasitic mite Coccipolipus hippodamiae (McDaniel & Morrill) (Acarina: Podapolipidae) naturally occurs in Europe and causes sterility in female H. axyridis (Rhule et al., 2010). Therefore, this mite has been considered as a biological control candidate (Rhule et al., 2010). However, some native ladybird species are also susceptible to it. Whilst H. axyridis may be more susceptible because of the nature of its life cycle, rigorous risk assessments would be needed before any artificial releases of the mite are considered (Rhule et al., 2010), and in our opinion the mite represents a control strategy that is too risky.
Implications for invasion biology
Harmonia axyridis was speculated as a model species for understanding invasion (Roy & Wajnberg, 2008). The unified framework for invasion biology proposed by Blackburn et al. (2011) recognises that the invasion process can be considered as discrete stages and that there are barriers a species must overcome to establish and subsequently spread. There are many ways in which research on invasion by H. axyridis has provided evidence to underpin mechanisms of invasion (Table 2). From the transport of this invader beyond the limits of its native geographic range to the dramatic spread of this species within the invaded range, there has been extensive ecological research documenting the processes and exploring the underlying mechanisms of invasion. However, there are still many knowledge gaps and opportunities for studies on H. axyridis contributing to our understanding of invasion biology.
Table 2.
Examples of studies on Harmonia axyridis over the last 10 years that have provided evidence to underpin understanding of the invasion process (Blackburn et al., 2011)
| Stage of invasion | Barrier | Evidence |
|---|---|---|
| Transport | Geography | The Altai mountains provide a biogeographic barrier to spread from the native range, but introduction as a biological control agent enabled the global spread of H. axyridis (Brown et al., 2011b). |
| Species that has been transported beyond the limits of its native geographic range and that has established a population in an area where it was not known to occur previously. | Accidental transport from continental Europe to Britain alongside natural dispersal contributed to the arrival of H. axyridis in 2004 (Brown et al., 2008b). | |
| Introduction | Captivation or cultivation | Introductions of H. axyridis in continental Europe were predominantly in glasshouses for the control of aphids, but individuals could have escaped into the wider countryside (Adachi-Hagimori et al., 2011). |
| Species can be prevented from becoming an invader by a human-imposed barrier. Many animal and plant species exist in captivity and/or cultivation beyond the limits of their native ranges, but fail to cross the physical barriers of a fence or hedge. This barrier is probably lower for species in cultivation than for those in captivity | Many widespread invasions arise from successful invasive populations rather than directly from the native range (invasive bridgehead effect) and this has been demonstrated for H. axyridis. An invasive population in eastern North America appears to have been the source that invaded the European, South American, and African continents, with some admixture with a biological control strain in Europe (Lombaert et al., 2010). | |
| Establishment | Survival | Successful overwintering in Britain since 2004–2005 (Brown et al., 2008b) |
| Introduced population can fail to establish because individuals in the population fail to survive. Failure to establish can result from factors associated with the species (e.g. reproductive rate or specialism), the location (e.g. presence of enemies or mutualists), apparently stochastic features of the individual introduction event (especially propagule pressure) or, often, their interaction (e.g. species location, such as climate matching); these factors can act on survival or reproduction, or both. | Harmonia axyridis is climatically matched with most regions of the world, including mainland Britain (Poutsma et al., 2008). | |
| Low susceptibility to natural enemies within the invaded range (Roy et al., 2008b, 2011b,2011c; Berkvens et al., 2010b; Comont et al., 2014b). | ||
| Reproduction | Successful breeding in Britain since 2005 (Brown et al., 2008b) | |
| An introduced population can fail to establish because individuals in the population either fail to survive, or survive but fail to reproduce. | Multivoltine species (Brown et al., 2008b) | |
| Spread | Dispersal | Ability to exploit resources in a wide range of habitats has ensured spread across Britain but limited spread north of Pennine and west of Cambrian mountains (Brown et al., 2011b). |
| A spreading population essentially faces multiple, sequential establishment events, under an ever greater range of environmental conditions. | Low susceptibility to natural enemies within the invaded range (Koyama & Majerus, 2008; Roy et al., 2008b; Berkvens et al., 2010b; Comont et al., 2014b). | |
| Harmonia axyridis are able to travel 18 km in a ‘typical’ high-altitude flight, but up to 120 km if flying at higher altitudes, indicating a high capacity for long-distance dispersal (Jeffries et al., 2013). | ||
| Environmental | Exploitation of buildings as favourable overwintering location (Brown et al., 2008a) | |
| The invasive range is determined by the extent of suitable environment, and the environmental barrier sets the limits to this. | Exploitation of wide range of habitats, especially anthropogenic ones, including urban and crop systems (Brown et al., 2011b). |
The stage of invasion and barrier (with extracts of the relevant text provided in italics) are defined by Blackburn et al. (2011), and selected evidence derived from research on H. axyridis is outlined.
Future directions: the next 10 years
The arrival of H. axyridis in Britain was met with trepidation; indeed, in the press release announcing the arrival of H. axyridis, Professor Michael Majerus described this species as ‘the most invasive ladybird on Earth’. The dramatic spread of H. axyridis suggests that it is one of the fastest-spreading invaders worldwide and is worthy of this description. However, H. axyridis has successfully been used as a model invasive alien species and has been the inspiration for global collaborations; the last decade of research is indicative of the enthusiasm and commitment of many biologists. Nevertheless, there is scope to expand the collaborations, particularly to increase the breadth of parallel studies conducted in the native and invaded regions. A recent symposium on H. axyridis in China (International Congress on Biological Invasions, Qingdao, 23–27 October 2013) highlighted the willingness for such global collaboration and the insights that can be gained from scientists working across Asia.
There have been an impressive number of studies on H. axyridis over the last 10 years that have provided mechanistic evidence (Table 1) alongside models explaining large-scale patterns and processes. The potential of IGP as an important force structuring aphidophagous communities has been highlighted, but understanding of the ecological relevance of IGP across complex networks of species is lacking. Additionally, the relative importance of competition and IGP should be assessed; indeed, it is thought that competitive interactions might be more important than IGP in driving declines of native species (Smith & Gardiner, 2013). The numerical dominance of H. axyridis in many habitats across Britain is evident, but the effects of the species on ecosystem function are unclear. There are clear indications that H. axyridis is escaping natural enemies within the invaded range but over the next 10 years it would seem plausible that the natural enemies will begin to adapt. Indeed, H. axyridis represents an abundant resource for parasites and pathogens.
Harmonia axyridis has provided unique and detailed insights into invasion biology over the decades, and the demand for scientific evidence to underpin invasion biology will undoubtedly be high over the next 10 years. In recent years, the European Commission (EC) has intensified its commitment to providing a comprehensive and manageable solution to invasive alien species in Europe. A European Union (EU) Regulation (http://ec.europa.eu/environment/nature/invasivealien/index_en.htm) has recently been adopted. Scientifically robust risk assessments, as laid down in the Regulation, will be essential. The number of records of H. axyridis received by the UK Ladybird Survey demonstrates the critical role that people can play in alien species surveillance. Such surveillance is critical to strategies for early-warning and rapid response. A recent horizon-scanning exercise has highlighted the species most likely to arrive, establish, and threaten biodiversity within the next 10 years, and the top 30 species include six terrestrial invertebrates (Roy et al., 2014).
Acknowledgments
The late Professor Michael Majerus was instrumental in the establishment of the Harlequin Ladybird Survey and encouraged many people around the world in their entomological research. We are extremely grateful to the thousands of people who have shared their ladybird observations over the years and contributed so richly to our ecological understanding. The UK Ladybird Survey is hosted by the Biological Record Centre, which receives support from both the Natural Environment Research Council and the Joint Nature Conservation Committee. The IOBC Working Group ‘Risks and Benefits of Exotic Biological Control Agents’ and the COST Action TD1209 ‘Alien Challenge’ have facilitated discussions and collaborations on H. axyridis. We thank the editor and anonymous reviewers for their helpful suggestions. Finally, we thank Ecological Entomology for publishing ‘The potential impacts of the arrival of the harlequin ladybird, Harmonia axyridis (Pallas) (Coleoptera : Coccinellidae), in Britain’ (Majerus et al., 2006) which inspired this review.
References
- Adachi-Hagimori T, Shibao M, Tanaka H, Seko T, Miura K. Control of Myzus persicae and Lipaphis erysimi (Hemiptera: Aphididae) by adults and larvae of a flightless strain of Harmonia axyridis (Coleoptera: Coccinellidae) on non-heading Brassica cultivars in the greenhouse. BioControl. 2011;56:207–213. [Google Scholar]
- Berkvens N, Bonte J, Berkvens D, Deforce K, Tirry L, De Clercq P. From Biological Control to Invasion: The Ladybird Harmonia Axyridis as a Model Species. Springer-Verlag, Amsterdam, the Netherlands; 2008. Pollen as an alternative food for Harmonia axyridis. 201–210. [Google Scholar]
- Berkvens N, Landuyt C, Deforce K, Berkvens D, Tirry L, De Clercq P. Alternative foods for the multicoloured Asian lady beetle Harmonia axyridis (Coleoptera: Coccinellidae) European Journal of Entomology. 2010a;107:189–195. [Google Scholar]
- Berkvens N, Moensa J, Berkvens D, Samihc MA, Tirry L, De Clercq P. Dinocampus coccinellae as a parasitoid of the invasive ladybird Harmonia axyridis in Europe. Biological Control. 2010b;53:92–99. [Google Scholar]
- Bezzerides AL, McGraw KJ, Parker RS, Husseini J. Elytra color as a signal of chemical defense in the Asian ladybird beetle Harmonia axyridis. Behavioral Ecology and Sociobiology. 2007;61:1401–1408. [Google Scholar]
- Blackburn TM, Pyšek P, Bacher S, Carlton JT, Duncan RP, Jarošík V, et al. A proposed unified framework for biological invasions. Trends in Ecology & Evolution. 2011;26:333–339. doi: 10.1016/j.tree.2011.03.023. [DOI] [PubMed] [Google Scholar]
- Botezatu AI, Kotseridis Y, Inglis D, Pickering GJ. Occurrence and contribution of alkyl methoxypyrazines in wine tainted by Harmonia axyridis and Coccinella septempunctata. Journal of the Science of Food and Agriculture. 2013;93:803–810. doi: 10.1002/jsfa.5800. [DOI] [PubMed] [Google Scholar]
- Brakefield PM, De Jong PW. A steep cline in ladybird melanism has decayed over 25 years: a genetic response to climate change? Heredity. 2011;107:574–578. doi: 10.1038/hdy.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PMJ. 2010. The spread of the harlequin ladybird Harmonia axyridis (Coleoptera: Coccinellidae) in Europe and its effects on native ladybirds. PhD thesis, Anglia Ruskin University, Cambridge, U.K.
- Brown PMJ, Adriaens T, Bathon H, Cuppen J, Goldarazena A, Hagg T, et al. Harmonia axyridis in Europe: spread and distribution of a non-native coccinellid. BioControl. 2008a;53:5–21. [Google Scholar]
- Brown PMJ, Roy HE, Rothery P, Roy DB, Ware RL, Majerus MEN. Harmonia axyridis in Great Britain: analysis of the spread and distribution of a non-native coccinellid. BioControl. 2008b;53:55–67. [Google Scholar]
- Brown PMJ, Frost R, Doberski J, Sparks T, Harrington R, Roy HE. Decline in native ladybirds in response to the arrival of Harmonia axyridis: early evidence from England. Ecological Entomology. 2011a;36:231–240. [Google Scholar]
- Brown PMJ, Thomas CE, Lombaert E, Jeffries DL, Estoup A, Handley L-JL. The global spread of Harmonia axyridis (Coleoptera: Coccinellidae): distribution, dispersal and routes of invasion. BioControl. 2011b;56:623–641. [Google Scholar]
- Brown PMJ, Ingels B, Wheatley A, Rhule EL, De Clercq P, van Leeuwen T, et al. Intraguild predation by Harmonia axyridis (Coleoptera: Coccinellidae) on native insects in Europe: molecular detection from field samples. Entomological Science. 2014;18:130–133. [Google Scholar]
- Ceryngier P, Roy HE, Poland RL. 2012. Natural enemies of ladybird beetles. 375–443. Wiley-Blackwell, Chichester, U.K. [Google Scholar]
- Colautti RI, Ricciardi A, Grigorovich IA, Macisaac HJ. Is invasion success explained by the enemy release hypothesis? Ecology Letters. 2004;7:721–733. [Google Scholar]
- Comont RF, Roy HE, Lewis OT, Harrington R, Shortall CR, Purse BV. Using biological traits to explain ladybird distribution patterns. Journal of Biogeography. 2012;39:1772–1781. [Google Scholar]
- Comont R, Roy H, Harrington R, Shortall C, Purse B. Ecological correlates of local extinction and colonisation in the British ladybird beetles (Coleoptera: Coccinellidae) Biological Invasions. 2014a;16:1805–1817. [Google Scholar]
- Comont RF, Purse BV, Phillips W, Kunin WE, Hanson M, Lewis OT, et al. Escape from parasitism by the invasive alien ladybird, Harmonia axyridis. Insect Conservation and Diversity. 2014b;7:334–342. [Google Scholar]
- Decourcy G. 2009. Long term implications of the arrival in the British Isles of Harmonia axyridis, the harlequin ladybird.
- Elliott N, Kieckhefer R, Kauffman W. Effects of an invading coccinellid on native coccinellids in an agricultural landscape. Oecologia. 1996;105:537–544. doi: 10.1007/BF00330017. [DOI] [PubMed] [Google Scholar]
- Elton CS. The Ecology of Invasions by Animals and Plants. London, U.K: Methuen; 1958. [Google Scholar]
- Firlej I. Teratocytes growth pattern reflects host suitability in a host–parasitoid assemblage. Annals of the Entomological Society of America. 2012;105:328–338. [Google Scholar]
- Flowers R, Salom S, Kok L. Competitive interactions among two specialist predators and a generalist predator of hemlock woolly adelgid, Adelges tsugae (Homoptera: Adelgidae), in the laboratory. Environmental Entomology. 2005;34:664–675. [Google Scholar]
- Galvan TL, Koch RL, Hutchison WD. Impact of fruit feeding on overwintering survival of the multicolored Asian lady beetle, and the ability of this insect and paper wasps to injure wine grape berries. Entomologia Experimentalis et Applicata. 2008;128:429–436. [Google Scholar]
- Glemser EJ, Dowling L, Inglis D, Pickering GJ, McFadden-Smith W, Sears MK, et al. A novel method for controlling multicolored Asian lady beetle (Coleoptera: Coccinellidae) in vineyards. Environmental Entomology. 2012;41:1169–1176. doi: 10.1603/EN11151. [DOI] [PubMed] [Google Scholar]
- Goetz DW. Harmonia axyridis ladybug invasion and allergy. Allergy and Asthma Proceedings. 2008:123–129. doi: 10.2500/aap.2008.29.3092. [DOI] [PubMed] [Google Scholar]
- Hall R, Ware R, Michie LJ. First record of field parasitism of immature stages of the Harlequin Ladybird Harmonia axyridis (Pallas) (Col.: Coccinellidae) by the braconid wasp Dinocampus coccinellae (Shrank) (Hym.: Braconidae) Entomologist's Record and Journal of Variation. 2009;121:57–58. [Google Scholar]
- Hautier L, San Martin G, Callier P, De Biseau J-C, Grégoire J-C. Alkaloids provide evidence of intraguild predation on native coccinellids by Harmonia axyridis in the field. Biological Invasions. 2011;13:1805–1814. [Google Scholar]
- Hodek I. Food relationships. In: Hodek I, van Emden HF, Honek A, Evans EW, editors. Ecology and Behaviour of the Ladybird Beetles (Coccinellidae) Wiley-Blackwell, Chichester, U.K; 2012. 141–274. [Google Scholar]
- Holroyd O, Brown PMJ, Roy HE, Majerus MEN. The harlequin ladybird, Harmonia axyridis, reaches Scotland. Entomologist's Record and Journal of Variation. 2008;120:42–43. [Google Scholar]
- Hoogendoorn A, Heimpel G. Indirect interactions between an introduced and a native ladybird beetle species mediated by a shared parasitoid. Biological Control. 2002;25:224–230. [Google Scholar]
- Ingels B, De Clercq P. Effect of size, extraguild prey and habitat complexity on intraguild interactions: a case study with the invasive ladybird Harmonia axyridis and the hoverfly Episyrphus balteatus. BioControl. 2011;56:871–882. [Google Scholar]
- Jansen JP, Hautier L. Ladybird population dynamics in potato: comparison of native species with an invasive species, Harmonia axyridis. BioControl. 2008;53:223–233. [Google Scholar]
- Jeffries DL, Chapman J, Roy HE, Humphries S, Harrington R, Brown PMJ, et al. Characteristics and drivers of high-altitude ladybird flight: insights from vertical-looking entomological radar. PLoS ONE. 2013;8:e82278. doi: 10.1371/journal.pone.0082278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenis M, Roy HE, Zindel R, Majerus MEN. Current and potential management strategies against Harmonia axyridis. BioControl. 2008;53:235–252. [Google Scholar]
- Koch RL. The multicolored Asian lady beetle, Harmonia axyridis: a review of its biology, uses in biological control, and non-target impacts. Journal of Insect Science. 2003;3:32. doi: 10.1093/jis/3.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch RL, Galvan TL. Bad side of a good beetle: the North American experience with Harmonia axyridis. BioControl. 2008;53:23–35. [Google Scholar]
- Koch RL, Hutchison WD, Venette RC, Heimpel GE. Susceptibility of immature monarch butterfly, Danaus plexippus (Lepidoptera: Nymphalidae: Danainae), to predation by Harmonia axyridis (Coleoptera: Coccinellidae) Biological Control. 2003;28:265–270. [Google Scholar]
- Koch R, Burkness E, Burkness SJW, Hutchison W. Phytophagous preferences of the multicolored Asian lady beetle (Coleoptera: Coccinellidae) for autumn-ripening fruit. Journal of Economic Entomology. 2004;97:539–544. doi: 10.1093/jee/97.2.539. [DOI] [PubMed] [Google Scholar]
- Koyama S, Majerus MEN. Interactions between the parasitoid wasp Dinocampus coccinellae and two species of coccinellid from Japan and Britain. BioControl. 2008;53:253–264. [Google Scholar]
- Lombaert E, Guillemaud T, Cornuet J-M, Malausa T, Facon B, Estoup A. Bridgehead effect in the worldwide invasion of the biocontrol harlequin ladybird. PLoS ONE. 2010;5:e9743. doi: 10.1371/journal.pone.0009743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerus M, Strawson V, Roy H. The potential impacts of the arrival of the harlequin ladybird, Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae), in Britain. Ecological Entomology. 2006;31:207–215. [Google Scholar]
- Michie LJ, Mallard F, Majerus MEN, Jiggins FM. Melanic through nature or nurture: genetic polymorphism and phenotypic plasticity in Harmonia axyridis. Journal of Evolutionary Biology. 2010;23:1699–1707. doi: 10.1111/j.1420-9101.2010.02043.x. [DOI] [PubMed] [Google Scholar]
- Murchie AK, Moore JP, Moore GA, Roy HE. The harlequin ladybird (Harmonia axyridis (Pallas)) (Coleoptera: Coccinellidae), found in Ireland. Irish Naturalists' Journal. 2008;29:25–26. [Google Scholar]
- Nedvěd O, Xenia F, Ungerova D, Kalushkov P. Alien vs. Predator–the native lacewing Chrysoperla carnea is the superior intraguild predator in trials against the invasive ladybird Harmonia axyridis. Bulletin of Insectology. 2013;66:73–78. [Google Scholar]
- Onofre Soares A, Coderre D, Schanderl H. Influence of prey quality on the fitness of two phenotypes of Harmonia axyridis adults. Entomologia Experimentalis et Applicata. 2005;114:227–232. [Google Scholar]
- Osawa N. Ecology of Harmonia axyridis in natural habitats within its native range. BioControl. 2011;56:613–621. [Google Scholar]
- Panigaj L, Zach P, Honěk A, Nedvěd O, Kulfan J, Martinková Z, et al. The invasion history, distribution and colour pattern forms of the harlequin ladybird beetle Harmonia axyridis (Pall.)(Coleoptera, Coccinellidae) in Slovakia, Central Europe. ZooKeys. 2014;412:89–102. doi: 10.3897/zookeys.412.6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pell JK, Baverstock J, Roy HE, Ware RL, Majerus MEN. Intraguild predation involving Harmonia axyridis: a review of current knowledge and future perspectives. BioControl. 2008;53:147–168. [Google Scholar]
- Phoofolo MW, Obrycki JJ. Potential for intraguild predation and competition among predatory Coccinellidae and Chrysopidae. Entomologia Experimentalis et Applicata. 1998;89:47–55. [Google Scholar]
- Poutsma J, Loomans AJM, Aukema B, Heijerman T. Predicting the potential geographical distribution of the harlequin ladybird, Harmonia axyridis, using the CLIMEX model. BioControl. 2008;53:103–125. [Google Scholar]
- Purse BV, Comont R, Butler A, Brown PMJ, Kessel C, Roy HE. Landscape and climate determine patterns of spread for all colour morphs of the alien ladybird Harmonia axyridis. Journal of Biogeography. 2014;42:575–588. [Google Scholar]
- Rhule EL, Majerus ME, Jiggins FM, Ware RL. Potential role of the sexually transmitted mite Coccipolipus hippodamiae in controlling populations of the invasive ladybird Harmonia axyridis. Biological Control. 2010;53:243–247. [Google Scholar]
- Ribbands B, Brown PMJ, Roy HE, Majerus MEN. The most northerly record of the Harlequin ladybird (Col., Coccinellidae) in the British Isles. Entomologist's Monthly Magazine. 2009;145:43–44. [Google Scholar]
- Romanuk TN, Zhou Y, Brose U, Berlow EL, Williams RJ, Martinez ND. Predicting invasion success in complex ecological networks. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences. 2009;364:1743–1754. doi: 10.1098/rstb.2008.0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy HE, Cottrell TE. Forgotten natural enemies: interactions between coccinellids and insect-parasitic fungi. European Journal of Entomology. 2008;105:391–398. [Google Scholar]
- Roy HE, Lawson Handley L-J. Networking: a community approach to invaders and their parasites. Functional Ecology. 2012;26:1238–1248. [Google Scholar]
- Roy HE, Wajnberg E. From biological control to invasion: the ladybird Harmonia axyridis as a model species. BioControl. 2008;53:1–4. [Google Scholar]
- Roy HE, Baverstock J, Ware RL, Clark SJ, Majerus MEN, Baverstock KE, et al. Intraguild predation of the aphid pathogenic fungus Pandora neoaphidis by the invasive coccinellid Harmonia axyridis. Ecological Entomology. 2008a;33:175–182. [Google Scholar]
- Roy HE, Brown PMJ, Rothery P, Ware RL, Majerus MEN. Interactions between the fungal pathogen Beauveria bassiana and three species of coccinellid: Harmonia axyridisCoccinella septempunctata and Adalia bipunctata. BioControl. 2008b;53:265–276. [Google Scholar]
- Roy HE, Brown PMJ, Frost R, Poland RL. Atlas of the Ladybirds (Coccinellidae) of Britain and Ireland. Wallingford, U.K: Biological Records Centre; 2011a. [Google Scholar]
- Roy HE, Handley LJL, Schoenrogge K, Poland RL, Purse BV. Can the enemy release hypothesis explain the success of invasive alien predators and parasitoids? BioControl. 2011b;56:451–468. [Google Scholar]
- Roy HE, Rhule E, Harding S, Handley L-JL, Poland RL, Riddick EW, et al. Living with the enemy: parasites and pathogens of the ladybird Harmonia axyridis. BioControl. 2011c;56:663–679. [Google Scholar]
- Roy HE, Adriaens T, Isaac NJB, Kenis M, Onkelinx T, San Martin G, et al. Invasive alien predator causes rapid declines of native European ladybirds. Diversity and Distributions. 2012;18:717–725. [Google Scholar]
- Roy HE, Brown PMJ, Comont RF, Poland RL, Sloggett JJ, Majerus M, et al. Naturalists' Handbook 10: Ladybirds. Exeter, U.K: Pelagic Publishing; 2013. [Google Scholar]
- Roy HE, Peyton J, Aldridge DC, Bantock T, Blackburn TM, Britton R, et al. Horizon scanning for invasive alien species with the potential to threaten biodiversity in Great Britain. Global Change Biology. 2014;20:3859–3871. doi: 10.1111/gcb.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saethre MG, Staverløkk A, Hofsvang T. The history of Harmonia axyridis (Pallas 1773) in Norway. IOBC/WPRS Bulletin. 2010;58:97–104. (Working Group “Benefits and Risks of exotic biological Control Agents”) [Google Scholar]
- Sloggett JJ. Are we studying too few taxa? Insights from aphidophagous ladybird beetles (Coleoptera: Coccinellidae) European Journal of Entomology. 2005;102:391–398. [Google Scholar]
- Sloggett JJ. Harmonia axyridis invasions: deducing evolutionary causes and consequences. Entomological Science. 2012;15:261–273. [Google Scholar]
- Smith CA, Gardiner MM. Biodiversity loss following the introduction of exotic competitors: does intraguild predation explain the decline of native lady beetles? PLoS ONE. 2013;8:e84448. doi: 10.1371/journal.pone.0084448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedders WL, Schaefer PW. Release and establishment of Harmonia axyridis (Coleoptera, Coccinellidae) in the southeastern United States. Entomological News. 1994;105:228–243. [Google Scholar]
- Thomas AP, Trotman J, Wheatley A, Aebi A, Zindel R, Brown PMJ. Predation of native coccinellids by the invasive alien Harmonia axyridis (Coleoptera: Coccinellidae): detection in Britain by PCR-based gut analysis. Insect Conservation and Diversity. 2013;6:20–27. [Google Scholar]
- Torchin M, Lafferty K, Kuris A. Release from parasites as natural enemies: increased performance of a globally introduced marine crab. Biological Invasions. 2001;3:333–345. [Google Scholar]
- Torchin ME, Lafferty KD, Dobson AP, McKenzie VJ, Kuris AM. Introduced species and their missing parasites. Nature. 2003;421:628–630. doi: 10.1038/nature01346. [DOI] [PubMed] [Google Scholar]
- Vandereycken A, Brostaux Y, Joie E, Haubruge E, Verheggen FJ. Occurrence of Harmonia axyridis (Coleoptera: Coccinellidae) in field crops. European Journal of Entomology. 2013;110:285–292. [Google Scholar]
- Verheggen FJ, Fagel Q, Heuskin S, Lognay G, Francis F, Haubruge E. Electrophysiological and behavioral responses of the multicolored Asian lady beetle, Harmonia axyridis Pallas, to sesquiterpene semiochemicals. Journal of Chemical Ecology. 2007;33:2148–2155. doi: 10.1007/s10886-007-9370-6. [DOI] [PubMed] [Google Scholar]
- Vilà M, Basnou C, Pyšek P, Josefsson M, Genovesi P, Gollasch S, et al. How well do we understand the impacts of alien species on ecosystem services? A pan-European, cross-taxa assessment. Frontiers in Ecology and the Environment. 2009;8:135–144. [Google Scholar]
- Vilcinskas A, Stoecker K, Schmidtberg H, Rohrich CR, Vogel H. Invasive harlequin ladybird carries biological weapons against native competitors. Science. 2013;340:862. doi: 10.1126/science.1234032. [DOI] [PubMed] [Google Scholar]
- Ware RL, Majerus MEN. Intraguild predation of immature stages of British and Japanese coccinellids by the invasive ladybird Harmonia axyridis. BioControl. 2008;53:169–188. [Google Scholar]
- Ware RL, Ramon-Portugal F, Magro A, Ducamp C, Hemptinne JL, Majerus MEN. Chemical protection of Calvia quatuordecimguttata eggs against intraguild predation by the invasive ladybird Harmonia axyridis. BioControl. 2008;53:189–200. [Google Scholar]
- Ware RL, Yguel B, Majerus MEN. Effects of competition, cannibalism and intra-guild predation on larval development of the European coccinellid Adalia bipunctata and the invasive species Harmonia axyridis. Ecological Entomology. 2009;34:12–19. [Google Scholar]
- Ware R, Michie L, Otani T, Rhule E, Hall RJ. Adaptation of native parasitoids to a novel host: the invasive coccinellid Harmonia axyridis. IOBC Bulletin. 2010;58:175–182. [Google Scholar]
- Wells PM. 2011. Intraguild predation by Harmonia axyridis: effects on native enemies and aphid suppression. PhD thesis, University of Cambridge.
- Wells PM, Baverstock J, Majerus MEN, Jiggins FM, Roy HE, Pell JK. Intraguild predation of non-coccinellid aphid natural enemies by Harmonia axyridis: prey range and factors influencing intraguild predation. IOBC/WPRS Bulletin. 2010;58:185–196. (Working Group “Benefits and Risks of exotic biological Control Agents”) [Google Scholar]
- Wilson JRU, Dormontt EE, Prentis PJ, Lowe AJ, Richardson DM. Something in the way you move: dispersal pathways affect invasion success. Trends in Ecology & Evolution. 2009;24:136–144. doi: 10.1016/j.tree.2008.10.007. [DOI] [PubMed] [Google Scholar]


