Abstract
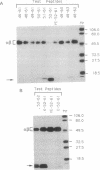
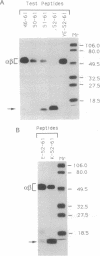
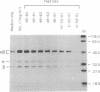
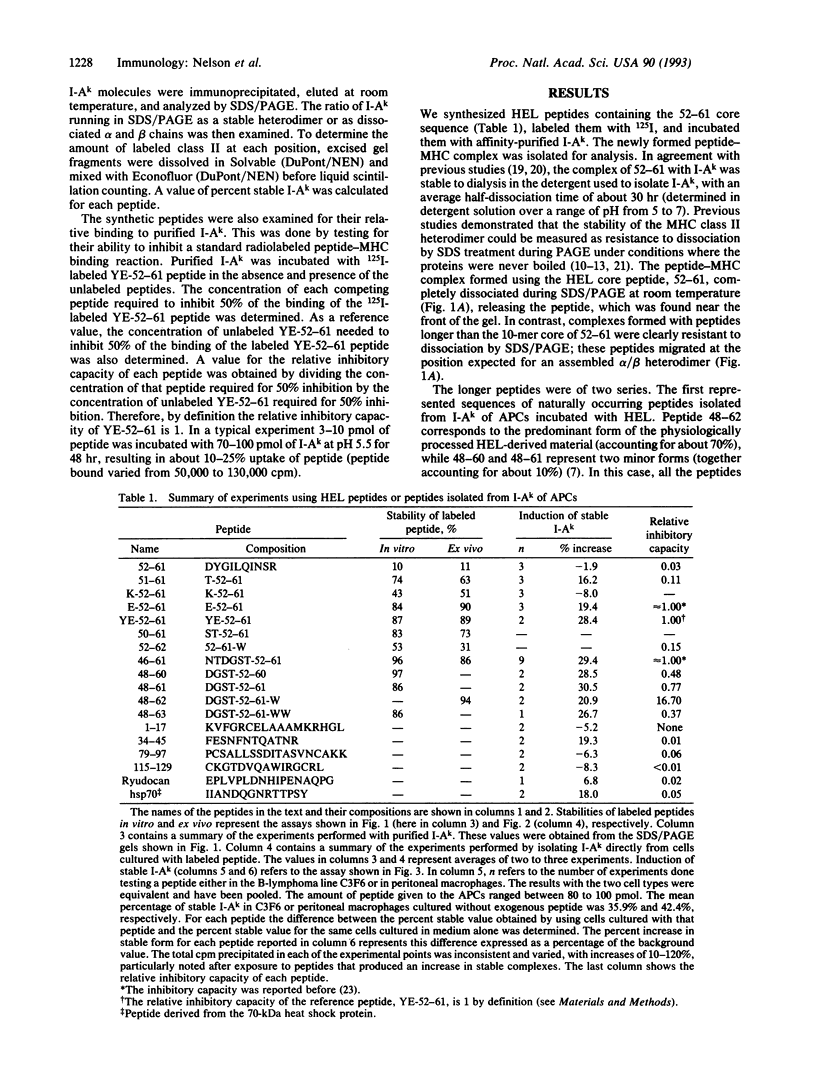
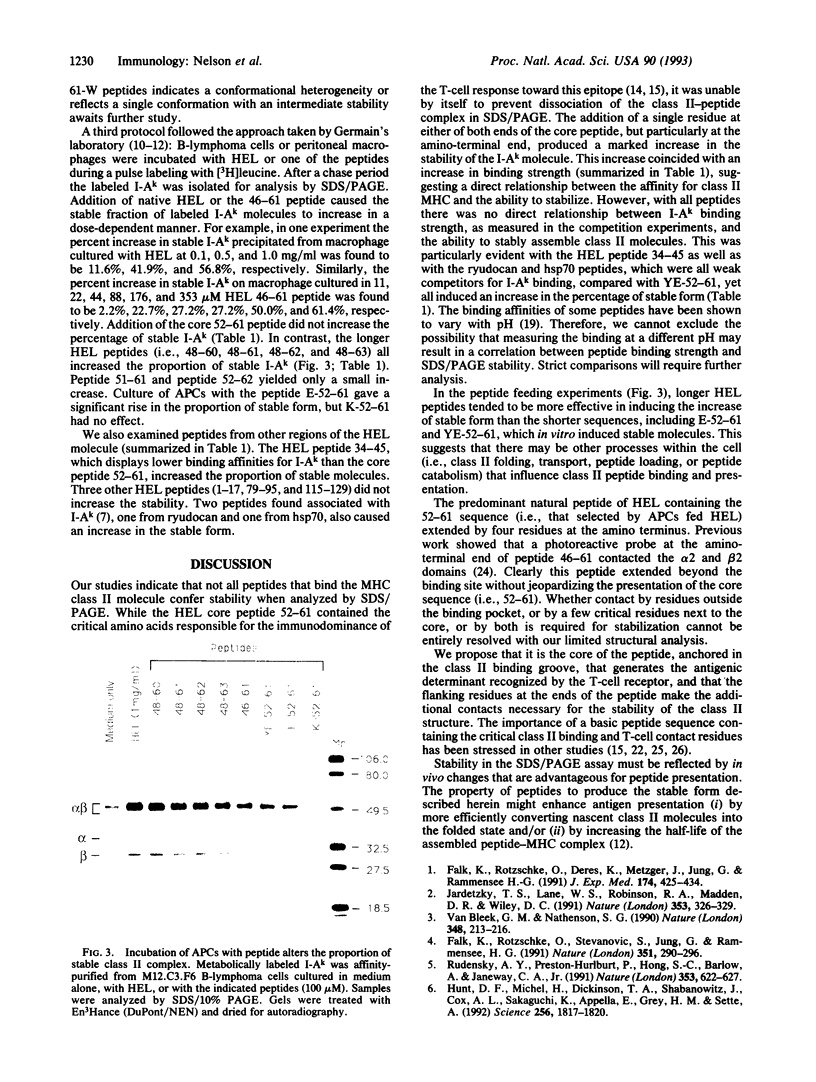
We have examined the interactions of various peptides with the mouse class II major histocompatibility complex molecule I-Ak. The peptides were derived from the model protein hen egg white lysozyme (HEL). The immunodominant peptide of HEL is a 10-mer, residues 52-61. Our previous work established that this sequence contains the key residues for binding and presentation to T cells. Now we show that the binding of this 10-mer sequence resulted in complexes of I-Ak and peptide that, in SDS/PAGE (without boiling the protein), rapidly dissociated from the component alpha and beta chains. The binding interactions were studied in vitro, by incubating purified I-Ak and radiolabeled peptide, or ex vivo, by using antigen-presenting cells incubated with peptides. Peptides with additional residues at either the amino or carboxyl terminus behaved dramatically differently. Complexes of I-Ak with the longer peptides were stable to SDS/PAGE. Very few amino acid additions result in the change from unstable to stable complexes. The important issue here is that when cultured with HEL, antigen-presenting cells selected the HEL peptides containing the 52-61 sequences that favored stability [Nelson, C. A., Roof, R. W., McCourt, D. W. & Unanue, E. R. (1992) Proc. Natl., Acad. Sci. USA 89, 7380-7383]. Also, from other studies, such sequences correlate with a high immunogenicity of the peptide. We conclude that there are structural features of peptides that change the stability of the class II molecule and that are independent of the "core" peptide seen by the T cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. M., Matsueda G. R., Adams S., Freeman J., Roof R. W., Lambert L., Unanue E. R. Enhanced immunogenicity of a T cell immunogenic peptide by modifications of its N and C termini. Int Immunol. 1989;1(2):141–150. doi: 10.1093/intimm/1.2.141. [DOI] [PubMed] [Google Scholar]
- Allen P. M., Matsueda G. R., Evans R. J., Dunbar J. B., Jr, Marshall G. R., Unanue E. R. Identification of the T-cell and Ia contact residues of a T-cell antigenic epitope. 1987 Jun 25-Jul 1Nature. 327(6124):713–715. doi: 10.1038/327713a0. [DOI] [PubMed] [Google Scholar]
- Allen P. M., Strydom D. J., Unanue E. R. Processing of lysozyme by macrophages: identification of the determinant recognized by two T-cell hybridomas. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2489–2493. doi: 10.1073/pnas.81.8.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Jenis D. M., Grey H. M. Isolation and characterization of antigen-Ia complexes involved in T cell recognition. Cell. 1986 Dec 26;47(6):1071–1077. doi: 10.1016/0092-8674(86)90822-6. [DOI] [PubMed] [Google Scholar]
- Dornmair K., McConnell H. M. Refolding and reassembly of separate alpha and beta chains of class II molecules of the major histocompatibility complex leads to increased peptide-binding capacity. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4134–4138. doi: 10.1073/pnas.87.11.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Deres K., Metzger J., Jung G., Rammensee H. G. Identification of naturally processed viral nonapeptides allows their quantification in infected cells and suggests an allele-specific T cell epitope forecast. J Exp Med. 1991 Aug 1;174(2):425–434. doi: 10.1084/jem.174.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Stevanović S., Jung G., Rammensee H. G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991 May 23;351(6324):290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- Germain R. N., Hendrix L. R. MHC class II structure, occupancy and surface expression determined by post-endoplasmic reticulum antigen binding. Nature. 1991 Sep 12;353(6340):134–139. doi: 10.1038/353134a0. [DOI] [PubMed] [Google Scholar]
- Harding C. V., Roof R. W., Allen P. M., Unanue E. R. Effects of pH and polysaccharides on peptide binding to class II major histocompatibility complex molecules. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2740–2744. doi: 10.1073/pnas.88.7.2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughton G., Arnold L. W., Bishop G. A., Mercolino T. J. The CH series of murine B cell lymphomas: neoplastic analogues of Ly-1+ normal B cells. Immunol Rev. 1986 Oct;93:35–51. doi: 10.1111/j.1600-065x.1986.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Hunt D. F., Michel H., Dickinson T. A., Shabanowitz J., Cox A. L., Sakaguchi K., Appella E., Grey H. M., Sette A. Peptides presented to the immune system by the murine class II major histocompatibility complex molecule I-Ad. Science. 1992 Jun 26;256(5065):1817–1820. doi: 10.1126/science.1319610. [DOI] [PubMed] [Google Scholar]
- Jardetzky T. S., Lane W. S., Robinson R. A., Madden D. R., Wiley D. C. Identification of self peptides bound to purified HLA-B27. Nature. 1991 Sep 26;353(6342):326–329. doi: 10.1038/353326a0. [DOI] [PubMed] [Google Scholar]
- Krieger J. I., Karr R. W., Grey H. M., Yu W. Y., O'Sullivan D., Batovsky L., Zheng Z. L., Colón S. M., Gaeta F. C., Sidney J. Single amino acid changes in DR and antigen define residues critical for peptide-MHC binding and T cell recognition. J Immunol. 1991 Apr 1;146(7):2331–2340. [PubMed] [Google Scholar]
- Ljunggren H. G., Stam N. J., Ohlén C., Neefjes J. J., Höglund P., Heemels M. T., Bastin J., Schumacher T. N., Townsend A., Kärre K. Empty MHC class I molecules come out in the cold. Nature. 1990 Aug 2;346(6283):476–480. doi: 10.1038/346476a0. [DOI] [PubMed] [Google Scholar]
- Luescher I. F., Crimmins D. L., Schwartz B. D., Unanue E. R. The sites in the I-Ak histocompatibility molecule photoaffinity labeled by an immunogenic lysozyme peptide. J Biol Chem. 1990 Jul 5;265(19):11177–11184. [PubMed] [Google Scholar]
- Luescher I. F., Unanue E. R. Purification and photoaffinity labeling of the I-Ak histocompatibility molecule. J Immunol Methods. 1990 Dec 31;135(1-2):233–245. doi: 10.1016/0022-1759(90)90277-3. [DOI] [PubMed] [Google Scholar]
- Nabavi N., Ghogawala Z., Myer A., Griffith I. J., Wade W. F., Chen Z. Z., McKean D. J., Glimcher L. H. Antigen presentation abrogated in cells expressing truncated Ia molecules. J Immunol. 1989 Mar 1;142(5):1444–1447. [PubMed] [Google Scholar]
- Nelson C. A., Roof R. W., McCourt D. W., Unanue E. R. Identification of the naturally processed form of hen egg white lysozyme bound to the murine major histocompatibility complex class II molecule I-Ak. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7380–7383. doi: 10.1073/pnas.89.16.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Rothbard J. B., Gefter M. L. Interactions between immunogenic peptides and MHC proteins. Annu Rev Immunol. 1991;9:527–565. doi: 10.1146/annurev.iy.09.040191.002523. [DOI] [PubMed] [Google Scholar]
- Rudensky AYu, Preston-Hurlburt P., Hong S. C., Barlow A., Janeway C. A., Jr Sequence analysis of peptides bound to MHC class II molecules. Nature. 1991 Oct 17;353(6345):622–627. doi: 10.1038/353622a0. [DOI] [PubMed] [Google Scholar]
- Sadegh-Nasseri S., Germain R. N. A role for peptide in determining MHC class II structure. Nature. 1991 Sep 12;353(6340):167–170. doi: 10.1038/353167a0. [DOI] [PubMed] [Google Scholar]
- Sadegh-Nasseri S., Germain R. N. How MHC class II molecules work: peptide-dependent completion of protein folding. Immunol Today. 1992 Feb;13(2):43–46. doi: 10.1016/0167-5699(92)90131-P. [DOI] [PubMed] [Google Scholar]
- Stern L. J., Wiley D. C. The human class II MHC protein HLA-DR1 assembles as empty alpha beta heterodimers in the absence of antigenic peptide. Cell. 1992 Feb 7;68(3):465–477. doi: 10.1016/0092-8674(92)90184-e. [DOI] [PubMed] [Google Scholar]
- Townsend A., Ohlén C., Bastin J., Ljunggren H. G., Foster L., Kärre K. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature. 1989 Aug 10;340(6233):443–448. doi: 10.1038/340443a0. [DOI] [PubMed] [Google Scholar]
- Van Bleek G. M., Nathenson S. G. Isolation of an endogenously processed immunodominant viral peptide from the class I H-2Kb molecule. Nature. 1990 Nov 15;348(6298):213–216. doi: 10.1038/348213a0. [DOI] [PubMed] [Google Scholar]