Abstract
Olfaction, the sense of smell, was a latecomer to the systematic investigation of primate sensory ecology after long years in which it was considered to be of minor importance.1 This view shifted with the growing understanding of its role in social behavior2 and the accumulation of physiological studies demonstrating that the olfactory abilities of some primates are on a par with those of olfactory-dependent mammals such as dogs and rodents.3,4 Recent years have seen a proliferation of physiological, behavioral, anatomical, and genetic investigations of primate olfaction. These investigations have begun to shed light on the importance of olfaction in the process of food acquisition. However, integration of these works has been limited. It is therefore still difficult to pinpoint large-scale evolutionary scenarios, namely the functions that the sense of smell fulfills in primates’ feeding ecology and the ecological niches that favor heavier reliance on olfaction. Here, we review available behavioral and physiological studies of primates in the field or captivity and try to elucidate how and when the sense of smell can help them acquire food.
Keywords: chemosensation, foraging, food selection, sensory ecology, sense of smell
The mammalian sense of smell is managed by several functionally distinct systems; the major ones are the main and the accessory olfactory systems.5 Although some overlap between the two exists,6 the accessory olfactory system is generally dedicated to processing intraspecific social cues and signals (for example, pheromones), whereas the main olfactory system deals with airborne chemicals from other sources.7 Thus, chemosensation via the main olfactory system is what most of us would recognize as smelling. It allows sampling, detection, and identification of volatile compounds from the environment (Fig. 1).
Figure 1.
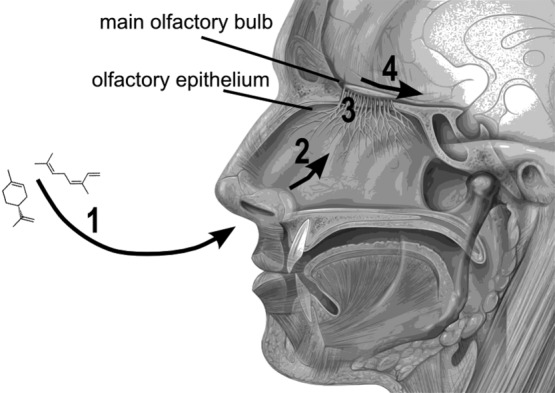
Smelling through the main olfactory system. (1) Airborne chemicals enter the nasal cavity when an individual is inhaling or actively sniffing. (2) The odorants reach the olfactory epithelium, which hosts millions of nerve cells. Each nerve cell expresses only one type of olfactory receptor (OR) and projects to the MOB, the first processing unit of the main olfactory system. (3) Odorants bind to only a few ORs and evoke action potentials that are carried to the MOB. (4) The signal from the one or more odorants are processed in the MOB, then passed to the olfactory cortex, the limbic system, and the rest of the brain.7–9 Figure adapted from an original by Patrick J. Lynch, medical illustrator; C. Carl Jaffe, MD, cardiologist; CC 2.5.
There is, by now, little argument over the notion that primates have a good sense of smell. High olfactory ability entails two major olfactory capacities: olfactory sensitivity and discrimination capacity. Olfactory sensitivity is the ability to detect odorants at relatively low concentrations. Discrimination capacity is the ability to perceive that two odors are different from one another and thus also to recognize odors. In a series of physiological studies, several primate species from different lineages have been shown to possess high olfactory sensitivity3,10 and discrimination capacity.4,11 Notably, their performance was on par with that of mammals such as dogs and rodents, which are considered to be highly olfactory-dependent. High olfactory capacities have been demonstrated to be relevant in various contexts, from social behavior12 to predator avoidance.13 Not surprisingly, another context in which the sense of smell has been shown to be useful is food acquisition. Studies in the wild have described “sniffing” behavior in foraging primates. Moreover, many experiments have shown that captive primates of several lineages can use olfactory cues to detect or choose feeding items.14,15 It thus seems clear that the sense of smell is likely to play an important role in primate feeding ecology. But beyond knowing that smelling may be important, how much do we know about what it actually does? In which contexts is it most informative? Which ecological niches favor higher reliance on olfaction and possibly select for higher olfactory sensitivity or discrimination capacity?
Previous attempts to answer some of these questions have focused on comparative analyses of anatomic and genetic components of the primate main olfactory system. Interspecific variation in several features of the main olfactory system has been documented: Species differ in the surface area of the olfactory epithelium,16 the size of the main olfactory bulb,17 and the number of functional olfactory receptor (OR) genes, which code the receptors expressed on the olfactory epithelium.18 Several studies interpreted interspecific variation in these traits as evidence that frugivorous and insectivorous diets, as well as a nocturnal activity time, are associated with superior olfactory capabilities.19–21 Others40,94 have argued that the acquisition of trichromatic vision in primates has led to a reduction in olfactory capacities (Table 1). Yet the basic assumptions here are questionable. Most importantly, it is unclear which olfactory capacities these measurements approximate and whether they are at all useful in assessing the level of reliance on olfaction in a species (Box 1, 2).
TABLE 1.
Which Ecological Niches Favor Reliance on Olfaction in Primate Feeding Ecology?
| Behavior | Anatomy (size of main olfactory bulb) | Genetics (OR repertoire; share of functional genes or proportion of pseudogenes) | ||||
|---|---|---|---|---|---|---|
| Diet | Primates rely on olfactory cues more when feeding on fruits. Olfaction is also useful in insect foraging, but probably less than in fruit foraging. No evidence of reliance on olfactory cues when feeding on leaves. | 57,67,68,77–80 | Frugivores/insectivores have a relatively larger main olfactory bulb | 19–21 | No clear difference in pseudogenization rate between species of different dietary categories (but not tested directly) | 40,94 |
| Activity time | Nocturnal owl monkeys outperform diurnal New World monkeys in olfactory-based food searching tasks | 53,54 | Nocturnal species have a relatively larger main olfactory bulb | 19–21 | No difference in pseudogenization rate between nocturnal and diurnal species (but not tested directly and based on only one species) More functional OR genes in nocturnal strepsirrhines than in diurnal haplorrhines (not tested directly) | 40,94 43 |
| Color vision | No clear differences in olfactory sensitivity or discrimination capacity between Old World routine trichromats and polymorphic New World monkeys | 10,11,82,90–92 | Not tested directly | No connection between trichromacy and loss of functional ORs | 43 | |
| Within species: dichromatic capuchins sniff more frequently when selecting figs. No difference between tri- and dichromatic spider monkeys | 64,65 | Routinely trichromatic howler monkeys have a higher proportion of OR pseudogenes than do other polymorphic New World monkeys | 40,94 | |||
Summary of the behavioral evidence for differences in the level of reliance on olfaction when feeding on different dietary categories, between diurnal and nocturnal species and between tri- and dichromats, and a comparison to the conclusions from anatomical and genetic analyses. For the issues in interpreting the variation in anatomical and genetic features of the main olfactory system, see Box 1, 2.
Box 1. Linking Brain Structures and Olfactory Performance
Two main anatomical features of the main olfactory system have been considered to be good proxies to estimate the relative importance of olfaction in different species. These features are the surface area of the olfactory epithelium, on which olfactory receptors are expressed, and the size of the main olfactory bulb (MOB), which projects to the olfactory epithelium and processes its input.7,8,16 The surface area of the olfactory epithelium can potentially be indicative of olfactory sensitivity because it could host more olfactory receptors, thus allowing detection of odorants at lower concentrations.16 However, the fact that the number of receptors per unit of area is not constant across taxa renders it an unreliable measurement.16 The MOB, on the other hand, is clearly defined in the brain and, because of its direct projection to the olfactory epithelium, may present a better approximation for the actual number of olfactory receptors and thus, olfactory sensitivity.
Volumetric data for the size of the MOB in dozens of primate species are available17 and have been used to compare different species and lineages.19–21 But how should size be measured? Primates’ brain size is strongly correlated with body mass22 and individual brain structures change allometrically with brain size.23 Thus, variation in MOB size that derives from differences in body mass presumably is not informative for any adaptationist discussion. This logic led comparative studies of primates19–21 and other taxa24 to use the relative rather than absolute size of the MOB. However, this approach has been criticized. In contrast to total brain volume, which is assumed to grow with body mass to accommodate the increase in muscle mass and sensory input from a higher surface area of the skin, olfactory information, or sensory demand, is not directly related to body size. It is therefore doubtful that a larger animal would require a larger MOB.5 In addition, the MOB is not fully constrained by total brain volume and shows some degree of independent evolution.25,26 The total number of neurons in the primate main olfactory bulb is correlated with its absolute mass.27 The size of the eyes or the primary visual cortex (V1) follows this same logic. Larger animals do not need larger eyes. The absolute size of the visual cortex is correlated with the number of neurons, and consequently with success in solving visual challenges between and within species.28 Thus, other studies have used the absolute rather than the relative size of the MOB as a proxy for olfactory sensitivity.29
Making things even more complex, when scaling aspects of the olfactory system to body or brain size, most studies log-transform the variables in order to meet the assumptions of the statistical methods employed.19–21 Although this is a standard procedure, it might alter the conclusions drawn from the same dataset (Fig. 2). For example, before log-transformations, the nocturnal owl monkeys (Aotus spp.) have the largest MOB to brain ratio of all anthropoids. After transformation, they lag behind six other diurnal taxa.17
The question of proper scaling is not unique to the main olfactory system. For example, different legitimate measurements of brain size support different hypotheses regarding encephalization in primates.30 This emphasizes that before using any measurement as a proxy for another trait, the functional and biological relations between the two must be fully understood. So while it is plausible that there is some connection between the size of the MOB in a species and its olfactory capabilities, it is difficult at this point to draw any direct line between the two.
Box 2. The Genetics of Olfaction and Comparative Studies
The Nobel-winning discovery of the olfactory receptor (OR) gene family37 introduced molecular biology to the study of olfaction and vice versa. Members of this massive gene family code the majority of the different olfactory receptors of the main olfactory system, which are expressed on the olfactory epithelium. A broader range is assumed to be associated with the ability to detect more compounds or better discriminate between odors.38,39 Since some OR genes have lost function in all primate lineages (“pseudogenization”), the remaining number of intact genes, or the proportion of pseudogenes, has been used to infer interspecific variation in olfactory discrimination capacity.18,40–43 This assumption, which is partially supported by physiological studies,44 is rather sound because animals like rodents, which are generally considered to be highly olfactory-dependent, tend to have substantially more intact ORs than do animals such as dolphins, which are considered to have little or no reliance on olfaction.45
This comes, however, with some caveats. To begin with, potentially functional ORs can be expressed in nonolfactory tissues, while pseudogenes can be expressed in the olfactory epithelium.41 Thus, the number of intact OR genes is not necessarily equivalent to the number of expressed functional receptors in the main olfactory system. Moreover, even a good estimation of the actual number of functional receptors in the main olfactory system would only be a very crude proxy for the ability to rely on olfaction to address real-life challenges. The primate OR family has gone through a birth-and-death process that included positive and purifying selection.42,46 This means that the functional genes in a given species are not simply a fraction of the ancestral stock, but a unique combination of genes that may be well adapted to species-specific ecological requirements. For example, a hypothetical dietary specialist may rely on olfaction more than a generalist does and present superior sensitivity and discrimination capacities in its respective niche, but have a smaller total number of functional OR genes because its olfactory system has to deal with less diverse stimuli. Finally, given the combinatorial nature of olfaction, even the relatively modest set of about 400 intact ORs present in humans allows the detection and discrimination of more than 400,000 different volatile compounds47 and up to 1012 different odor mixtures.48 It is therefore hard to argue that this comparatively small number of functional ORs is necessarily associated with a handicapped sense of smell.49
Thus, ignoring results from behavioral works and relying only on genetics has led to unwarranted conclusions. For example, in one of the more insightful genetic works to date, Dong and colleagues42 note that the number of functional OR genes is similar in different haplorrhine lineages and thus cannot account for “the reduced olfactory ability in apes and [Old World monkeys].” This alleged reduced ability, however, has never been demonstrated in any physiological or behavioral test. Its validity is based only on the not-fully-established assumption that a relatively smaller main olfactory bulb is associated with a reduction in olfaction (Box 1).
The genetics of olfaction clearly has a potential for going beyond low-resolution measurements such as “discrimination capacity” and telling us which species is adapted to work with which olfactory stimuli, and so to what extent a species’ main olfactory system has adapted to cope with the relevant ecological challenges. This, however, requires much more than counts of intact genes. It would not only require identifying the ecologically relevant odors, but also knowing which genetic makeup generates higher sensitivity to these stimuli and better discrimination between them. Although this approach is beginning to be established,50 we are still far from understanding the environment-olfaction interaction in such resolution for any species, let alone for primates.
Current conceptions regarding how and in what contexts olfaction plays a role in primate food acquisition are still primarily based on genetic and anatomical proxies, while behavioral works, when taken separately, often serve as no more than anecdotal support for the notion that the primate sense of smell can be useful. However, many behavioral studies have accumulated since the last reviews dealing with the roles of olfaction in primate feeding ecology.14,15 Addressing them together allows us to begin to understand how the high olfactory capacities of primates translate into success in realistic ecological tasks in the context of food acquisition.
Here, we take a step in this direction and review available behavioral studies regarding the roles of olfaction in primate feeding ecology. Rather than simply describing all instances of the use of olfaction in the context of food acquisition, we organize relevant behavioral studies in a way that can help us to depart from the general notion that olfaction is useful and, instead, elucidate what primates do with their sense of smell when addressing feeding challenges, as well when smelling is useful and when it is not. We ask two main questions: What function does the sense of smell fulfill in primate food acquisition? Which ecological niches favor higher reliance on olfaction?
The starting point for the first question is the distinction between the searching and selection phases;15 that is, the localization versus selection and quality assessment of food resources. These two processes may require different capacities and provide different kinds of information: “where might food be?” as opposed to the quality of individual items such as fruits on a tree that provides fruits with different degrees of ripeness. We then move on to the second question and examine two aspects of ecological niches that have been suggested to generate different requirements from the olfactory system: dietary strategies and the availability of visual cues. The goal is to use behavioral works and apply a qualitative comparative approach to examine whether species with different diets or those for which visual cues are less available tend to use their sense of smell more than other primates do when searching for or choosing food items. A concluding section emphasizes the difficulties presented by currently available data and offers directions for future studies.
OLFACTION: WHAT IS IT GOOD FOR?
Food acquisition is a multi-step process that includes locating food and assessing its quality.14,15 Olfaction can potentially be useful in both, over the usually longer distance to detect food items and over the shorter distances for quality assessment and selection of individual items.15 Information from these two phases does not fully overlap. For example, a high-quality fruiting tree may still have many undesirable unripe fruits. Thus, finding the tree and identifying the ripe fruits are completely different tasks that may challenge primates in different ways.
Food Detection
Olfactory guided long-distance food detection is probably the more challenging task, requiring not only the ability to detect and identify the cue, but also to follow the odor and track its source. In macroscopic scales, air movement over an odor source creates an odor plume that is similar to smoke from a chimney. Odor plumes have a weak chemical gradient across large distances and therefore maintain the integrity, or the proportion of odorants, over long distances in a rather narrow plume. This allows animals to scent-track the origin of the plume, usually by meandering in and around it.51 Humans have been shown to be able to scent-track in two-dimensional open-field conditions where the trail was restricted to the ground and movement was unconstrained.52 However, odor plumes are carried in the air and primate movement in three-dimensional space is restricted by available supports. This is in contrast to insects, which can meander around an odor plume. Thus, olfactory-based long-distance location of feeding sources is a challenge in which primates are not expected to excel.
Indeed, behavioral evidence of the ability of primates to use their sense of smell to find food over long distances is scarce. Two studies tested the ability of several wild and semi-wild New World primates to detect fruit-baited feeding platforms based on olfactory cues.53,54 Owl monkeys (Aotus spp.) and one out of two emperor tamarin (Saguinus imperator) groups were capable of detecting the baited platform above chance level using only olfactory cues. However, these were the exceptions. Titi monkeys (Callicebus cupreus), tufted capuchins (Cebus apella), saddleback tamarins (Saguinus fuscicollis), and the second emperor tamarin group failed this task. Moreover, the task was to detect baited platforms from a distance of only few meters, which is easier than the distances over which most primates search for fruits.
A more recent study reported the failure of captive owl monkeys to locate baited feeding boxes based on olfactory cues.55 The authors suggested that issues such as motivation and experimental design may have contributed to the negative results. Nevertheless, this study indicates that tracking food sources using olfactory cues is a task in which even owl monkeys, which often perform better than other New World species in similar tasks,53,54 may not excel. Apart from that, neither positive nor negative evidence of long-distance olfactory foraging is available. The absence of evidence is not necessarily evidence of absence, but the fact that the typical and easily identified scent-tracking behavior most of us would identify with dogs is practically absent from the primate literature suggests that this may simply not be a part of their normal feeding strategy.
Successful use of olfaction to detect food has been implied only in shorter distances, when tracking odor plumes is not required. Slender lorises (Loris lydekkerianus lydekkerianus) increase the level of olfactory investigation when foraging for invertebrates56 and mouse lemurs (Microcebus murinus) can detect insect prey using only their sense of smell.57 In addition, short-distance olfactory-guided foraging has been suggested to allow diademed sifakas (Propithecus diadema) to locate inflorescences hidden in leaf litter.58
Food Selection
Olfactory-based selection or assessment of individual items is probably an easier task that would usually be conducted at a very short distance in combination with other senses.15 Here, the challenge is to discriminate between the odors of, for example, ripe and unripe fruits and choose accordingly. Indeed, evidence of the importance of olfaction in primate food selection is much more abundant.
Several studies have reported an increase in sniffing behavior in situations that require quality assessment of feeding items (Fig. 3). As opposed to passive inhaling, sniffing is an active process of sampling to the main olfactory system. Repetitive sampling at differing speeds, volumes, and frequencies increases an animal's ability to assess odorant identity and concentration.59,60 Thus, elevated levels of sniffing are indicative of more thorough olfactory investigation and reliance on olfactory cues. Sniffing of feeding items from short distances has been described in captivity61 and in the wild.62,63 Since sniffing occurs after the item is located, it clearly fulfills the function of quality assessment.
Figure 3.

Sniffing of feeding items. (A) In the final stage of quality assessment, a white-faced capuchin in Área de Conservación Guanacaste, Costa Rica, sniffs a ripe fruit of Spondias purpurea after its removal (photo: Fernando Campos). (B) A chimpanzee sniffing fruits of Ficus brachylepis in Kibale National Park, Uganda (photo: Nathaniel J. Dominy). (C) A verreaux's sifaka sniffs a flower of Vanilla madagascariensis, which they occasionally consume (Andrea Springer, personal communication) in the Kirindy Forest, Madagascar. Although primates’ sniffing of flowers before ingesting them has not been systematically recorded, it is likely that olfactory cues are involved in the selection process (photo: Andrea Springer). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 2.
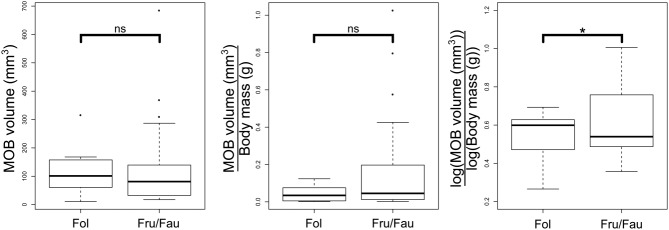
Same data, different conclusions. Differences in the size of the MOB in folivorous (Fol) and frugivorous/faunivorous (Fru/Fau) primates using three legitimate scaling methods: absolute volume of the MOB, volume of the MOB relative to body mass, and volume of the MOB relative to body mass when both variables are log-transformed. MOB volume and body mass data are from Stephan, Frahm, and Baron17 (N = 36 frugivorous/faunivorous and N = 8 folivorous species); dietary categories are from Kappeler and Heymann.31 Asterisk denotes significance at α = 0.05 from an independent contrasts linear regression model.32 Analyses were done on R 3.0.333 with package Caper,34 using an independent-rates soft-bounded constraints phylogeny from Springer and coworkers.35 The absolute size of the MOB of folivorous primates is, on average, a bit larger but at a statistically insignificant level (F = 0.53 (1, 42), p = ns). This is probably because folivorous species tend to be larger36 and thus have larger brains and MOBs. The effect is reversed when looking at relative sizes of MOBs (F = 1.05 (1, 42), p = ns) and becomes statistically significant only when data are log-transformed (F = 4.14 (1, 42), p = 0.048). This may reflect genuinely higher emphasis on olfaction in these species or merely an artifact of wrongly correcting for a larger body size in folivores.
Applying a more quantitative approach, two studies on wild spider monkeys (Ateles geoffroyi) and white-faced capuchins (Cebus capucinus) examined patterns of sniffing behavior when selecting figs.64,65 Both species increased their sniffing behavior when feeding on fig species that are visually cryptic; that is, they do not provide a reliable visual cue for ripeness. This demonstrates the importance of olfaction for fruit selection after locating them. In addition, experiments held in captivity showed that tufted capuchins increase the rate sniffing rate when unpalatable secondary compounds (pepper) are added to otherwise desired food66 and that both spider monkeys and squirrel monkeys (Saimiri sciureus) increase frequency of sniffing when facing novel items, probably in an attempt to estimate their quality.67 Thus, evidence from the wild and captivity indicates that sniffing – active, repetitive olfactory sampling – is common when examining feeding items from close proximity and increases when facing novel items or when visual cues are unavailable.
Furthermore, a few studies have examined whether primates can rely on olfactory cues to assess the quality of feeding items. In captivity, three lemur species (ruffed lemurs, Varecia variegata; Coquerel's sifakas, Propithecus coquereli; and ring-tailed lemurs, Lemur catta) were challenged to discriminate between food items under two conditions: preferred red foods (ripe fruits or young leaves) and less-preferred green foods (unripe fruits and mature leaves).68 Olfactory cues, either alone or in combination with visual cues, aided all three species to correctly choose the desired items. Our own work suggests that both cotton-top tamarins (Saguinus oedipus) and common marmosets (Callithrix jacchus) can, within one hour, learn to discriminate between random novel odors marking positive and negative rewards and use them to select desired odorless rewards (Rathke and Nevo, unpublished data). Finally, based on discrimination between positive (food-related) and negative stimuli, captive pigtailed macaques (Macaca nemestrina) learn to achieve high success rates in foraging tasks.69
Summary: The Function of Olfaction in Food Acquisition
Olfaction can potentially serve two separate functions in primate feeding ecology: food detection and selection. In fruit foraging, detection is usually done over long distances; evidence of the use of olfaction in this process is scarce to nonexistent. In contrast, there is much more evidence, from both the wild and captivity, of reliance on olfaction for quality assessment of feeding items. Olfaction can be useful for locating feeding items only when olfactory sensation takes place over very short distances that do not require tracking odor plumes. For example, insectivores may use it to determine whether tree-holes contain prey. In this case, however, the use of olfaction is functionally very similar to selection. Olfaction may also be used for quality assessment of caught invertebrates, but we are not aware of any study that has tested that.
An earlier account of primate sensory ecology14 schematically portrayed the different “sensory boundaries” of primate food perception; that is, the distances from which a foraging primate can gather information through several sensory trajectories. While acknowledging that evidence of the use of olfaction over long distances had been scarce, this article still placed the olfactory boundary far away from the foraging primate, somewhere between the visual and auditory boundaries. Given the data that have accumulated since, we think that the olfactory boundary should be pushed back and considered to be almost as small as the tactile boundary. As important as olfaction may be, current evidence suggests that it serves primates mainly at very short distances.
we think that the olfactory boundary should be pushed back and considered to be almost as small as the tactile boundary. As important as olfaction may be, current evidence suggests that it serves primates mainly at very short distances.
WHICH ECOLOGICAL NICHES FAVOR RELIANCE ON OLFACTION?
Olfaction could play an important role in primate feeding ecology even if primarily from short distances. There are examples of reliance on olfaction to acquire food in many contexts: when feeding on fruits or invertebrates, at day or night, and by representatives of all major lineages. However, there is a good reason to believe that its importance – that is, the fitness benefit from the ability to acquire olfactory information quickly and accurately – is not constant across the diverse ecological niches that primates occupy. Variation in the relative size of the main olfactory bulb and the share of functional olfactory receptor genes have been interpreted as evidence of differences in the importance of olfaction across these ecological niches (Table 1), but the validity of these proxies is questionable (Box 1, 2).
Here, we examine whether available behavioral data indicate that olfaction is more important in some ecological niches than it is in others. We look at studies that quantified sniffing behavior or directly tested success rates in foraging tasks that represent superior olfactory sensitivity or discrimination capacity. The relationship between the two is bidirectional: Ecological contexts in which olfactory cues can provide more useful information or those in which cues from other trajectories are less available are expected to increase the level of reliance on olfaction. This should manifest in increased olfactory sampling of feeding items by sniffing. Over evolutionary time, a likely scenario is that species with higher dependence on olfactory cues would be subjected to selection pressures for elaboration of olfactory sensitivity and discrimination capacity. Superior ability to exploit olfactory cues should lead, in turn, to higher allocation of time to olfactory investigation during the food-selection processes, and thus to elevated sniffing behavior.
Dietary Strategies
Olfaction may play a role in foraging for practically all primates’ dietary categories. Fleshy fruits often bear odors that are unique to their ripe phase; indeed, they may have evolved to be olfactory-conspicuous to frugivores.70,71 Further, fruit volatile compounds may be indicative of the fruits’ nutrient content.72 Mouse lemurs have been reported to consume fruit species that are more odorous than do other sympatric species.73 Olfaction may be useful in other categories as well. The volatile signatures of broken young and mature leaves are different74 and invertebrates sometimes emit odors that may allow their identification.75 However, choosing these items or searching for them over short distances also involves acquiring information from other sensory trajectories: auditory cues when catching prey, tactile cues when choosing fruits or leaves, and visual cues in practically all cases. In addition, young leaves tend to grow in predictable locations on the branch,76 which may provide a “micro-spatial” cue. Thus, olfactory cues may be very useful in some cases and redundant in others. The question asked here is whether feeding on items in some dietary categories is associated with greater reliance on the sense of smell than on others.
Although only a few studies have addressed this question, those that are available indicate that olfaction is particularly useful when feeding on fruits. Here, too, evidence comes from studies that quantified the frequency of sniffing behavior, or the tendency to sample the odor of feeding items, and from more direct interspecific comparisons of success rates in foraging tasks. In controlled experiments conducted in captivity, spider monkeys were found to increase their sniffing behavior more than squirrel monkeys did when addressing novel feeding items.67 This was explained by spider monkeys’ higher degree of frugivory as compared to the more insectivorous squirrel monkeys, thus indicating that more frugivorous species tend to rely more on their sense of smell. Another study compared the tendency to rely on olfactory cues (sniffing) as opposed to visual cues in discrimination tasks in three captive Malagasy primates of different dietary categories. Frugivorous ruffed lemurs showed the highest tendency to sniff items and could solve the task only when olfactory cues were present. Folivorous Coquerel's sifakas showed a lower level of sniffing and could not solve the task based solely on olfactory cues. Ring-tailed lemurs, as generalists, were intermediate. They tended to use more visual than olfactory investigation, but could solve the task using either of them alone.68 Taken together, these studies indicate that more frugivorous species have a higher tendency to sniff feeding items, while species that show a lesser degree of frugivory are less likely to employ olfactory investigation and rely more on visual cues.
In another set of studies, it has been shown that mouse lemurs are able, based solely on olfactory cues, to detect both fruits and invertebrate prey hidden under opaque lids. However, success rates were much higher for fruits, approximating 100%.57,77,78 Capuchin monkeys, which were reported to use their sense of smell when choosing fruits64, failed to locate invertebrates from close proximity when only olfactory cues were available in captive conditions.79 Capuchins were also shown to have higher olfactory discrimination capacity for fruity (as opposed to fishy) odors, thus indicating that their sense of smell is particularly tuned to fruity stimuli.80 Thus, species that consume both fruits and invertebrates achieve higher success rates in foraging tasks when using olfaction for fruit foraging. Finally, physiological studies in several primate species reported high sensitivity to and discrimination of odorants commonly present in fruits.81–83 Whether or not the primate sense of smell is similarly tuned to odorants emitted by invertebrates or leaves is unknown.
Thus, although comparative behavioral data are still based on a small number of species, they indicate that olfaction is more important in fruit selection than it is in foraging for invertebrates or leaves. This may derive from differences in the difficulty of using olfaction in these latter tasks. In fruit foraging, as noted earlier, the main function of olfaction is not to locate a fruit but to determine whether or not it is ripe. Fruit odor can be endlessly resampled (sniffed) from a very short distance; it may have even evolved to be olfactorily conspicuous – that is, to provide a reliable odor signal for ripeness.70,71 Thus, when choosing fruits, primates can extract more information via the olfactory trajectory. Species for which fruits constitute a large share of the diet may have evolved superior olfactory capabilities. This, in turn, makes frugivorous species more likely to resort to reliance on olfactory cues and show elevated levels of sniffing behavior. Invertebrates, on the other hand, are expected to be under selection pressures to reduce their olfactory signature. Even though there are descriptions of reliance on olfaction when finding invertebrates over short distances, visual and auditory cues probably play a bigger role in their location. Finally, there are not even anecdotal reports of sniffing behavior in leaf foraging, thus suggesting that folivores may rely even less on olfactory cues when selecting leaves. The most likely explanation is that other cues – specifically the location, texture, and possibly the color of leaves – provide sufficient sensory information.
although comparative behavioral data are still based on a small number of species, they indicate that olfaction is more important in fruit selection than it is in foraging for invertebrates or leaves.
there are not even anecdotal reports of sniffing behavior in leaf foraging, thus suggesting that folivores may rely even less on olfactory cues when selecting leaves. The most likely explanation is that other cues – specifically the location, texture, and possibly the color of leaves – provide sufficient sensory information.
Availability of Visual Cues
The importance of olfaction for food acquisition depends, then, on the information given by the target's volatile signature and also by the potential ability of primates to acquire similar information through other sensory trajectories, thus rendering olfaction redundant. Vision is often considered to be the main primate sensory modality84 and, indeed, it is likely that the availability of visual cues reduces the importance of olfaction. By extension, species having high visual capacities would tend to rely less on their sense of smell; that is, they will show reduced levels of sniffing and, over time, may evolve to possess less acute olfactory sensitivity or discriminatory capacity as a result of the relaxation of selection pressures.
The notion of a trade-off between olfaction and vision in primates goes back to the earliest stages of biological anthropology85 and has been in the heart of the hypothesis that primates evolved as “visual predators” in which enlargement of the orbits forced a reduction of the olfactory apparatus.86 This notion is further supported by the fact that primate olfactory and visual brain structures are negatively correlated.20 Here, we ask whether two factors that determine differences in the availability of visual cues, activity time and variation in color-vision capacities, predict differences in the level of reliance on olfaction.
Activity Time
Among anthropoids, the prediction that nocturnal owl monkeys tend to rely on olfaction more than do their diurnal counterparts87 has been confirmed in two independent studies.53,54 Both employed similar experimental approaches and demonstrated that owl monkeys perform better in olfactory-based food-detection tasks than do their diurnal counterparts. Yet these results are based on a single species. To show that this is indeed a pattern, the results need to be replicated in strepsirrhines, among which more nocturnal species are available. Another interesting parallel would be a comparison of day and night feeding in cathemeral species or diurnal species such as spider and woolly monkeys (Lagothrix spp.), which occasionally forage at night (Carlos Peres, personal communication).
Color Vision
Primates are the only eutherian mammals known to have acquired full trichromatic vision.88 However, not all species are fully trichromatic. Old World monkeys, apes, and New World howler monkeys (Alouatta spp.) are habitually trichromatic. All other species are di- or monochromatic or present population-level polymorphism in which all males and some females are dichromats and the rest of the females are trichromats.88,89
The prediction that full trichromatic vision is associated with a reduction in olfaction has been mainly addressed in genetic works (Table 1). Two behavioral studies measured the tendency to sniff fruits before ingestion in wild groups of two New World monkey species. They genotyped the females to determine whether they were di- or trichromatic and tested the hypothesis that dichromats sniff fruits more often to compensate for the lack of visual information available to trichromatic group members. The results were contradictory: An effect was found in capuchins,64 but not in spider monkeys.65
Evidence from comparative physiological studies provides a clearer picture. In a series of studies, Laska and colleagues compared olfactory sensitivity and discrimination capacity in three New and Old World monkey species: pigtailed macaques, squirrel monkeys, and spider monkeys.10,11,82,90–92 Although not addressing the question directly, these experiments included male New World monkeys, which necessarily are dichromats; female New World monkeys, which may have been trichromats; and Old World monkeys, which are trichromats. The results, encompassing a wide range of odorants, did not show any clear difference between New and Old World species and therefore imply that differences in color vision are not necessarily associated with interspecific differences in olfactory sensitivity or discrimination capacity.
Thus, the prediction of lesser reliance on olfaction by trichromats is not, to this point, unequivocally supported by the available data. Although based on only few studies, if the lack of a trade-off between full color vision and olfaction is indeed genuine, two nonmutually exclusive explanations come to mind. The first is that acquisition of trichromatic vision does not impose strong constraints on olfaction. Trichromacy was achieved through a duplication of the M-L opsin gene.88 The expression of three rather than two opsin genes in the eye should not impose any constraints on olfaction like those attributed to the enlargement of the orbits, which supposedly forced a reduction in olfaction.86 The second potential explanation, a functional one, is that there is little overlap in the information that color vision and the sense of smell provide. It has been speculated that trichromatic vision specifically helps in long-distance detection of fruits.93 Thus, if the sense of smell is indeed less important at anything beyond a short distance, color vision and olfaction may be complementary: Trichromacy helps identify fruit-bearing trees, while olfaction is used in the short distance, along with other senses, to determine whether an individual fruit is ripe.
the prediction of lesser reliance on olfaction by trichromats is not, to this point, unequivocally supported by the available data.
WHAT NEXT?
The proliferation of behavioral and physiological work in recent years has begun to establish trends that allow us to depart from the vague notion that olfaction may be important to primate feeding ecology and to begin to understand how it helps primates to acquire food and in which contexts it becomes useful. Currently available findings indicate that olfaction is used primarily in short distances and usually for food selection. These results also support arguments for some of the factors that have been suggested to affect the level of reliance on olfaction (diet, activity period), but provide less support for others (color vision). However, these data are still too scarce and unstandardized to reach clear conclusions. We are therefore hopeful that emphasizing these gaps in the available data will encourage future studies.
More specifically, we see three main approaches that would significantly improve our understanding of the roles of olfaction in primate feeding ecology. One is the chemical ecology of primate feeding behavior, which involves connecting primate physiological capacities and ecologically relevant chemically resolved stimuli; another is a systematic comparative approach in behavioral studies to control for the many confounding factors; the third is identification of similar and contrasting trends in nonprimate taxa.
Chemical ecology of primate feeding behavior
Something almost fully missing from available studies is an understanding of the chemical world within which the primate olfactory system has to pursue foraging and food selection. There are practically no published analyses of the odors of the fruits that primates consume or the invertebrates they catch in the wild. So far, to our knowledge, only one study conducted chemical analyses of natural odors of fruits consumed by primates,73 but even in it the actual chemical profiles of primate-consumed fruits have not been published.
Modern techniques allow relatively easy and cheap sampling and analysis of volatile compounds in the field.95 Introducing those techniques to the study of primate feeding ecology could resolve many questions. If, indeed, olfaction is more relied on in fruit selection and in some contexts in insect foraging, the targets for chemical investigation should be the odor profiles of ripe, unripe, and overripe fruits, as well as occupied and unoccupied tree holes or other microhabitats in which prey can be found. This can establish, first, whether there is even the potential for reliance on olfactory cues: If an unripe fruit smells just like a ripe one, there is no need to look further. Once the “olfactory distance” between desired and undesired items is understood, many questions can be asked. Can primates discriminate between the odors? Are success rates in choosing the right fruit or tree hole greater when the odor is present? Is this ability learned or innate? Further investigations can focus on individual compounds or compound classes and see which contribute to primates’ discrimination capacity. Finally, if certain compounds or compound classes turn out to be more important than others, functional genomics may be able to identify the olfactory receptors that allow their detection. Several studies have identified primate olfactory receptor genes that have gone through positive selection.42,46 Thus, by connecting the two, future work may be able to reveal in great detail which chemical stimuli are relevant enough to have exerted significant selection pressures on the primate olfactory receptor gene repertoire. This is a trail that can lead to very high-resolution understanding of lineage-specific olfactory adaptations.
A more comprehensive knowledge of the olfactory challenges faced by primates can then be taken to the lab and applied in controlled captive experiments that would connect physiological capacities and the ability to deal with ecologically relevant challenges. So far, there has been an almost complete separation: Studies have been about either physiology but less ecologically relevant information or vice versa. For example, available data suggest that in fruit acquisition the main function of olfaction is fruit selection, but it is unclear which aspects of the primate olfactory system have been under stronger positive selection as a consequence of this requirement. By measuring the olfactory sensitivity and discrimination capacity of ecologically relevant and chemically known stimuli, comparative analyses can test whether more frugivorous species show higher sensitivity to the odorants emitted by fruits or higher discrimination capacity between these odorants and the odors of unripe fruits. When choosing fruits, the most important information is not whether the fruit is there, but whether it is ripe, unripe, overripe, or potentially infested. Considering that fruits are not expected to be under selection pressures to lower their olfactory signature, olfactory discrimination capacity is expected to be more important than sensitivity. Thus it can be predicted that the degree of frugivory in a species is positively correlated with its ability to discriminate between relevant odorants.
A similar approach can be applied to the location of slow or sedentary invertebrates from short distances. Here, the information sought is mostly whether the prey is present or not. Furthermore, if primates and other predators use olfactory cues to locate these slow sedentary invertebrates, they are expected to be under selection pressure to lower their olfactory signature. Thus, it may turn out that olfactory sensitivity, not discrimination, is the olfactory capacity most important for insectivores.
Comparison of olfactory capacities when dealing with ecologically relevant stimuli could help us depart from a rather simplistic discussion regarding “olfactory elaboration.” Olfaction can be useful in more than one way and different ecological challenges are expected to exert different selection pressures on the various aspects of the main olfactory system. Moreover, establishing the connection between an ecological challenge and olfactory capacity could be used to reassess the quality of measurements such as the size of the main olfactory bulb and the olfactory receptor gene repertoire as proxies for actual olfactory performance in species-specific ecological challenges.
Comparative Behavioral Tests
The main issue in the search for olfactory adaptation is the presence of many confounding factors. For example, howler monkeys, the only New World primate with routine trichromacy,88 can be an interesting model taxon for examining whether elaboration of color vision is associated with a reduction in olfaction.40 However, their rather folivorous diet,96 which may also predict a reduction in the reliance on olfaction, requires controlling for diet before any conclusions can be drawn regarding the interplay between color vision and olfaction.
Other confounding factors may be related to nonfeeding functions of olfaction. Although some social signals are processed by the independent accessory olfactory system in mammals, the main and accessory olfactory systems show some functional overlap.6 Thus, the main olfactory system is expected to respond to selection pressures from various nonfeeding behaviors. Crucially, diet and activity time are somewhat correlated with social structure in primates; many insectivores are also nocturnal and solitary. Hence, it is likely that high olfactory capabilities are promoted by one factor and secondarily employed in other contexts, making it difficult to determine the relative contribution of each factor.
The problem of numerous confounding factors can only be addressed if future studies take a more comparative approach and are designed to control for all relevant variables. For instance, contrasting howler monkeys with muriquis (Brachyteles sp.), the other rather folivorous New World primate that does not share howlers’ habitual trichromacy, could help resolve the question of whether trichromatic vision is associated with less reliance on olfaction. Moreover, perhaps the most efficient approach to control for the relevant factors would be to focus on intraspecific variation, comparing conspecific dichromatic and trichromatic New World monkeys or lemurs.
Comparison to Other Taxa
Finally, the processes examined here are not confined to the primate order. Other taxonomic groups, such as bats or birds, are at least as ecologically diverse and also possess other strong sensory modalities that may have had an effect on the evolution of olfaction. Comparative anatomical and genetic studies that looked at carnivores,97 birds,98,99 bats,24 and other mammals100 are now available. Behavioral studies showed both similar and different trends: Long-distance olfactory-based detection is common in procellariiform birds,101 whereas bats use olfaction in both detection and selection.102,103 Considering the similarities and differences between primates and other taxa would shed additional light on ecological adaptations, proximate mechanisms and constraints, and help unravel universal trends in olfactory ecology and evolution.
Acknowledgments
We thank John Fleagle, Luca Pozzi and several anonymous reviewers for many helpful comments on previous drafts of this manuscript. We also thank all members of the Behavioral Ecology & Sociobiology Unit, German Primate Center, for their ideas and remarks in many discussions.
REFERENCES
- 1.Heymann EW. The neglected sense – olfaction in primate behavior, ecology, and evolution. Am J Primatol. 2006;68:519–524. doi: 10.1002/ajp.20249. [DOI] [PubMed] [Google Scholar]
- 2.Michael RP, Bonsall RW, Zumpe D. Evidence for chemical communication in primates. Vitam Horm. 1976;34:137–186. doi: 10.1016/s0083-6729(08)60075-8. [DOI] [PubMed] [Google Scholar]
- 3.Laska M, Seibt A, Weber A. “Microsmatic” primates revisited: olfactory sensitivity in the squirrel monkey. Chem Senses. 2000;25:47–53. doi: 10.1093/chemse/25.1.47. [DOI] [PubMed] [Google Scholar]
- 4.Laska M, Freyer D. Olfactory discrimination ability for aliphatic esters in squirrel monkeys and humans. Chem Senses. 1997;22:457–465. doi: 10.1093/chemse/22.4.457. [DOI] [PubMed] [Google Scholar]
- 5.Smith TD, Bhatnagar KP. Microsmatic primates: reconsidering how and when size matters. Anat Rec B New Anat. 2004;279:24–31. doi: 10.1002/ar.b.20026. [DOI] [PubMed] [Google Scholar]
- 6.Baxi KN, Dorries KM, Eisthen HL. Is the vomeronasal system really specialized for detecting pheromones? Trends Neurosci. 2006;29:1–7. doi: 10.1016/j.tins.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Lledo P, Gheusi G, Vincent J. Information processing in the mammalian olfactory system. Physiol Rev. 2005;85:281–317. doi: 10.1152/physrev.00008.2004. [DOI] [PubMed] [Google Scholar]
- 8.Mori K, Nagao H, Yoshihara Y. The olfactory bulb: coding and processing of odor molecule information. Science. 1999;286:711–715. doi: 10.1126/science.286.5440.711. [DOI] [PubMed] [Google Scholar]
- 9.Buck LB. Olfactory receptors and odor coding in mammals. Nutr Rev. 2004;62:S184–S188. doi: 10.1111/j.1753-4887.2004.tb00097.x. [DOI] [PubMed] [Google Scholar]
- 10.Laska M, Seibt A. Olfactory sensitivity for aliphatic esters in squirrel monkeys and pigtail macaques. Behav Brain Res. 2002;134:165–174. doi: 10.1016/s0166-4328(01)00464-8. [DOI] [PubMed] [Google Scholar]
- 11.Laska M, Genzel D, Wieser A. The number of functional olfactory receptor genes and the relative size of olfactory brain structures are poor predictors of olfactory discrimination performance with enantiomers. Chem Senses. 2005;30:171–175. doi: 10.1093/chemse/bji013. [DOI] [PubMed] [Google Scholar]
- 12.Palagi E, Dapporto L. Beyond odor discrimination: demonstrating individual recognition by scent in Lemur catta. Chem Senses. 2006;31:437–443. doi: 10.1093/chemse/bjj048. [DOI] [PubMed] [Google Scholar]
- 13.Sündermann D, Scheumann M, Zimmermann E. Olfactory predator recognition in predator-naïve gray mouse lemurs (Microcebus murinus. J Comp Psychol. 2008;122:146–155. doi: 10.1037/0735-7036.122.2.146. [DOI] [PubMed] [Google Scholar]
- 14.Dominy NJ, Lucas PW, Osorio D, et al. The sensory ecology of primate food perception. Evol Anthropol. 2001;10:171–186. doi: 10.1002/evan.21967. [DOI] [PubMed] [Google Scholar]
- 15.Dominy NJ, Lucas PW, Supardi Noor N. Primate sensory systems and foraging behavior. In: Hohmann G, Robbins MM, Boesch C, editors. Feeding ecololgy in apes and pther primates. Cambridge: Cambridge University Press; 2006. pp. 489–509. [Google Scholar]
- 16.Smith TD, Bhatnagar KP, Tuladhar P, et al. Distribution of olfactory epithelium in the primate nasal cavity: are microsmia and macrosmia valid morphological concepts? Anat Rec Part A. 2004;281:1173–1181. doi: 10.1002/ar.a.20122. [DOI] [PubMed] [Google Scholar]
- 17.Stephan H, Frahm HD, Baron G. New and revised data on volumes of brain structures in insectivores and primates. Folia Primatol. 1981;35:1–29. doi: 10.1159/000155963. [DOI] [PubMed] [Google Scholar]
- 18.Rouquier S, Blancher A, Giorgi D. The olfactory receptor gene repertoire in primates and mouse: evidence for reduction of the functional fraction in primates. Proc Natl Acad Sci USA. 2000;97:2870–2874. doi: 10.1073/pnas.040580197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baron G, Frahm HD, Bhatnagar KP, et al. Comparison of brain structure volumes in insectivora and primates. III. Main olfactory bulb (MOB) J Hirnforsch. 1983;24:551–568. [PubMed] [Google Scholar]
- 20.Barton R, Purvis A, Harvey PH. Evolutionary radiation of visual and olfactory brain systems in primates, bats and insectivores. Philos Trans R Soc B. 1995;348:381–392. doi: 10.1098/rstb.1995.0076. [DOI] [PubMed] [Google Scholar]
- 21.Barton R. Olfactory evolution and behavioral ecology in primates. Am J Primatol. 2006;68:545–558. doi: 10.1002/ajp.20251. [DOI] [PubMed] [Google Scholar]
- 22.Jerison HJ. Brain to body ratios and the evolution of intelligence. Science. 1955;121:447–449. doi: 10.1126/science.121.3144.447. [DOI] [PubMed] [Google Scholar]
- 23.Finlay BL, Darlington RB. Linked regularities in the development and evolution of mammalian brains. Science. 1995;268:1578–1584. doi: 10.1126/science.7777856. [DOI] [PubMed] [Google Scholar]
- 24.Hutcheon JM, Kirsch JAW, Garland T., Jr A comparative analysis of brain size in relation to foraging ecology and phylogeny in the chiroptera. Brain Behav Evol. 2002;60:165–180. doi: 10.1159/000065938. [DOI] [PubMed] [Google Scholar]
- 25.Barton R, Harvey PH. Mosaic evolution of brain structure in mammals. Nature. 2000;405:1055–1058. doi: 10.1038/35016580. [DOI] [PubMed] [Google Scholar]
- 26.Finlay BL, Darlington RB, Nicastro N. Developmental structure in brain evolution. Behav Brain Sci. 2001;24:263–308. [PubMed] [Google Scholar]
- 27.Ribeiro PFM, Manger PR, Catania KC, et al. Greater addition of neurons to the olfactory bulb than to the cerebral cortex of eulipotyphlans but not rodents, afrotherians or primates. Front Neuroanat. 2014;8:23. doi: 10.3389/fnana.2014.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Sousa AA, Proulx MJ. What can volumes reveal about human brain evolution? A framework for bridging behavioral, histometric and volumetric perspectives. Front Neuroanat. 2014;8:51. doi: 10.3389/fnana.2014.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heritage S. Modeling olfactory bulb evolution through primate phylogeny. PLoS One. 2014;9:e113904. doi: 10.1371/journal.pone.0113904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deaner RO, Nunn CL, van Schaik CP. Comparative tests of primate cognition: different scaling methods produce different results. Brain Behav Evol. 2000;55:44–52. doi: 10.1159/000006641. [DOI] [PubMed] [Google Scholar]
- 31.Kappeler PM, Heymann EW. Nonconvergence in the evolution of primate life history and socio-ecology. Biol J Linn Soc. 1996;59:297–326. [Google Scholar]
- 32.Felsenstein J. Phylogenies and the compartative method. Am Nat. 1985;125:1–15. [Google Scholar]
- 33.R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2014. URL http://www.R-project.org/ [Google Scholar]
- 34.Orme D, Freckleton RP, Thomas G, et al. 2012. Caper: comparative analyses of phylogenetics and evolution in R. R package version 0.5. http://CRAN.R-project.org/package=caper.
- 35.Springer MS, Meredith RW, Gatesy J, et al. Macroevolutionary dynamics and historical biogeography of primate diversification inferred from a species supermatrix. PLoS One. 2012;7:e49521. doi: 10.1371/journal.pone.0049521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Terborgh JW. Diversity and the tropical rain forest. New York: Scientific American Library; 1992. [Google Scholar]
- 37.Buck LB, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- 38.Young JM, Trask BJ. The sense of smell: genomics of vertebrate odorant receptors. Hum Mol Genet. 2002;11:1153–1160. doi: 10.1093/hmg/11.10.1153. [DOI] [PubMed] [Google Scholar]
- 39.Nei M, Niimura Y, Nozawa M. The evolution of animal chemosensory receptor gene repertoires: roles of chance and necessity. Nat Rev Genet. 2008;9:951–963. doi: 10.1038/nrg2480. [DOI] [PubMed] [Google Scholar]
- 40.Gilad Y, Wiebe V, Przeworski M, et al. Loss of olfactory receptor genes coincides with the acquisition of full trichromatic vision in primates. PLoS Biol. 2004;2:0120–0125. doi: 10.1371/journal.pbio.0020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang X, De la Cruz O, Pinto JM, et al. Characterizing the expression of the human olfactory receptor gene family using a novel DNA microarray. Genome Biol. 2007;8:R86. doi: 10.1186/gb-2007-8-5-r86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dong D, He G, Zhang S, et al. Evolution of olfactory receptor genes in primates dominated by birth-and-death process. Genome Biol Evol. 2009;1:258–264. doi: 10.1093/gbe/evp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsui A, Go Y, Niimura Y. Degeneration of olfactory receptor gene repertories in primates: no direct link to full trichromatic vision. Mol Biol Evol. 2010;27:1192–1200. doi: 10.1093/molbev/msq003. [DOI] [PubMed] [Google Scholar]
- 44.Rizvanovic A, Amundin M, Laska M. Olfactory discrimination ability of Asian elephants (Elephas maximus) for structurally related odorants. Chem Senses. 2013;38:107–118. doi: 10.1093/chemse/bjs097. [DOI] [PubMed] [Google Scholar]
- 45.Niimura Y. Evolution of chemosensory receptor genes in primates and other mammals. In: Hirai H, Go Y, Imai H, editors. Post-genome biology of primates. Tokyo: Springer; 2012. pp. 43–62. [Google Scholar]
- 46.Gilad Y, Man O, Glusman G. A comparison of the human and chimpanzee olfactory receptor gene repertoires. Genome Res. 2005;15:224–230. doi: 10.1101/gr.2846405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mori K, Manabe H. Unique characteristics of the olfactory system. In: Mori K, editor. The olfactory system: from molecules to motivational behaviors. Tokyo: Springer; 2014. pp. 1–18. [Google Scholar]
- 48.Bushdid C, Magnasco MO, Vosshall LB, et al. Humans can discriminate more than 1 trillion olfactory stimuli. Science. 2014;343:1370–1372. doi: 10.1126/science.1249168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weiss KM. I smell a rat! (and 999,999,999,999 other things, too) Evol Anthropol. 2014;23:166–171. doi: 10.1002/evan.21424. [DOI] [PubMed] [Google Scholar]
- 50.Touhara K. Odor and pheromone molecules, receptors and behavioral responses. In: Mori K, editor. The olfactory system: from molecules to motivational behaviors. Tokyo: Springer Japan; 2014. pp. 19–38. [Google Scholar]
- 51.Vickers NJ. Mechanisms of animal navigation in odor plumes. Biol Bull. 2000;198:203–212. doi: 10.2307/1542524. [DOI] [PubMed] [Google Scholar]
- 52.Porter J, Craven B, Khan RM, et al. Mechanisms of scent-tracking in humans. Nat Neurosci. 2007;10:27–29. doi: 10.1038/nn1819. [DOI] [PubMed] [Google Scholar]
- 53.Bolen RH, Green SM. Use of olfactory cues in foraging by owl monkeys (Aotus nancymai) and capuchin monkeys (Cebus apella. J Comp Psychol. 1997;111:152–158. doi: 10.1037/0735-7036.111.2.152. [DOI] [PubMed] [Google Scholar]
- 54.Bicca-Marques JC, Garber PA. Use of spatial, visual, and olfactory information during foraging in wild nocturnal and diurnal anthropoids: a field experiment comparing AotusCallicebus, and Saguinus. Am J Primatol. 2004;62:171–187. doi: 10.1002/ajp.20014. [DOI] [PubMed] [Google Scholar]
- 55.Da Costa RS, Bicca-Marques JC. Owl monkeys (Aotus nigriceps and A. infulatus) follow routes instead of food-related cues during foraging in captivity. PLoS One. 2014;9:e115188. doi: 10.1371/journal.pone.0115188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nekaris KA. Foraging behaviour of the slender loris (Loris lydekkerianus lydekkerianus): implications for theories of primate origins. J Hum Evol. 2005;49:289–300. doi: 10.1016/j.jhevol.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 57.Piep M, Radespiel U, Zimmermann E, et al. The sensory basis of prey detection in captive-born grey mouse lemurs, Microcebus murinus. Anim Behav. 2008;75:871–878. [Google Scholar]
- 58.Irwin MT, Raharison FJ, Rakotoarimanana H, et al. Diademed sifakas (Propithecus diadema) use olfaction to forage for the inflorescences of subterranean parasitic plants (Balanophoraceae: Langsdorffia sp, and Cytinaceae: Cytinus sp.) Int J Primatol. 2007;69:471–476. doi: 10.1002/ajp.20353. [DOI] [PubMed] [Google Scholar]
- 59.Mainland J, Sobel N. The sniff is part of the olfactory percept. Chem Senses. 2006;31:181–196. doi: 10.1093/chemse/bjj012. [DOI] [PubMed] [Google Scholar]
- 60.Verhagen JV, Wesson DW, Netoff TI, et al. Sniffing controls an adaptive filter of sensory input to the olfactory bulb. Nat Neurosci. 2007;10:631–639. doi: 10.1038/nn1892. [DOI] [PubMed] [Google Scholar]
- 61.Zschoke A, Thomsen R. Sniffing behaviours in guenons. Folia Primatol. 2014;85:244–251. doi: 10.1159/000363409. [DOI] [PubMed] [Google Scholar]
- 62.Matsumoto-Oda A, Kutsukake N, Hosaka K, et al. Sniffing behaviors in Mahale chimpanzees. Primates. 2007;48:81–85. doi: 10.1007/s10329-006-0006-1. [DOI] [PubMed] [Google Scholar]
- 63.Van Roosmalen MGM. Habitat preference, diet, feeding strategy and social organization of the black spider monkey (Ateles paniscus paniscus Linnaeus 1758) in Surinam. Acta Amaz. 1985;15:1–238. [Google Scholar]
- 64.Melin AD, Fedigan LM, Hiramatsu C, et al. Fig foraging by dichromatic and trichromatic Cebus capucinus in a tropical dry forest. Int J Primatol. 2009;30:753–775. [Google Scholar]
- 65.Hiramatsu C, Melin AD, Aureli F, et al. Interplay of olfaction and vision in fruit foraging of spider monkeys. Anim Behav. 2009;77:1421–1426. [Google Scholar]
- 66.Visalberghi E, Addessi E. Response to changes in food palatability in tufted capuchin monkeys, Cebus apella. Anim Behav. 2000;59:231–238. doi: 10.1006/anbe.1999.1297. [DOI] [PubMed] [Google Scholar]
- 67.Laska M, Freist P, Krause S. Which senses play a role in nonhuman primate food selection? A comparison between squirrel monkeys and spider monkeys. Am J Primatol. 2007;69:282–294. doi: 10.1002/ajp.20345. [DOI] [PubMed] [Google Scholar]
- 68.Rushmore J, Leonhardt SD, Drea CM. Sight or scent: lemur sensory reliance in detecting food quality varies with feeding ecology. PLoS One. 2012;7:e41558. doi: 10.1371/journal.pone.0041558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hübener F, Laska M. Assessing olfactory performance in an Old World primate, Macaca nemestrina. Physiol Behav. 1998;64:521–527. doi: 10.1016/s0031-9384(98)00099-7. [DOI] [PubMed] [Google Scholar]
- 70.Hodgkison R, Ayasse M, Kalko EKV, et al. Chemical ecology of fruit bat foraging behavior in relation to the fruit odors of two species of Paleotropical bat-dispersed figs (Ficus hispida and Ficus scortechinii. J Chem Ecol. 2007;33:2097–2110. doi: 10.1007/s10886-007-9367-1. [DOI] [PubMed] [Google Scholar]
- 71.Borges RM, Bessière JM, Hossaert-McKey M. The chemical ecology of seed dispersal in monoecious and dioecious figs. Funct Ecol. 2008;22:484–493. [Google Scholar]
- 72.Goff SA, Klee HJ. Plant volatile compounds: sensory cues for health and nutritional value? Science. 2006;311:815–819. doi: 10.1126/science.1112614. [DOI] [PubMed] [Google Scholar]
- 73.Valenta K, Burke RJ, Styler SA, et al. Colour and odour drive fruit selection and seed dispersal by mouse lemurs. Sci Rep. 2013;3:2424. doi: 10.1038/srep02424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kant MR, Bleeker PM, van Wijk M, et al. Plant volatiles in defence. Adv Bot Res. 2009;51:613–666. [Google Scholar]
- 75.Hilker M, McNeil J. Chemical and behavioral ecology in insect parasitoids: how to behave optimally in a complex odorous environment. In: Wajnberg E, Bernstein C, van Alphen J, editors. Behavioral ecology of insect parasitoids. Malden, MA: Blackwell Scientific; 2008. pp. 92–112. [Google Scholar]
- 76.Fleming AJ. Formation of primordia and phyllotaxy. Curr Opin Plant Biol. 2005;8:53–58. doi: 10.1016/j.pbi.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 77.Siemers BM, Goerlitz HR, Robsomanitrandrasana E, et al. Sensory basis of food detection in wild Microcebus murinus. Int J Primatol. 2007;28:291–304. [Google Scholar]
- 78.Siemers BM. The sensory ecology of foraging for animal prey. In: Masters J, Gamba M, Génin F, editors. Leaping ahead: advances in prosimian biology. New-York: Springer; 2013. pp. 257–263. [Google Scholar]
- 79.Phillips KA, Goodchild LMS, Haas ME, et al. Use of visual, acoustic, and olfactory information during embedded invertebrate foraging in brown capuchins (Cebus apella. J Comp Psychol. 2004;118:200–205. doi: 10.1037/0735-7036.118.2.200. [DOI] [PubMed] [Google Scholar]
- 80.Ueno Y. Olfactory discrimination of eight food flavors in the capuchin monkey (Cebus apella): comparison between fruity fishy odors. Primates. 1994;35:301–310. [Google Scholar]
- 81.Laska M, Rivas Bautista RM, Hernandez Salazar LT. Olfactory sensitivity for aliphatic alcohols and aldehydes in spider monkeys (Ateles geoffroyi. Am J Phys Anthropol. 2006;129:112–120. doi: 10.1002/ajpa.20252. [DOI] [PubMed] [Google Scholar]
- 82.Laska M, Höfelmann D, Huber D, et al. The frequency of occurrence of acyclic monoterpene alcohols in the chemical environment does not determine olfactory sensitivity in nonhuman primates. J Chem Ecol. 2006;32:1317–1331. doi: 10.1007/s10886-006-9090-3. [DOI] [PubMed] [Google Scholar]
- 83.Hernandez Salazar LT, Laska M, Rodriguez Luna E. Olfactory sensitivity for aliphatic esters in spider monkeys (Ateles geoffroyi. Behav Neurosci. 2003;117:1142–1149. doi: 10.1037/0735-7044.117.6.1142. [DOI] [PubMed] [Google Scholar]
- 84.Fobes JL, King JE. Vision: the dominant primate modality. In: Fobes JL, King JE, editors. Primate behavior. New York: Academic Press; 1982. pp. 219–243. [Google Scholar]
- 85.Elliot Smith G. The evolution of man. London: Oxford University Press; 1927. [Google Scholar]
- 86.Cartmill M. Rethinking primate origins. Science. 1974;184:436–443. doi: 10.1126/science.184.4135.436. [DOI] [PubMed] [Google Scholar]
- 87.Wright PC. The nocturnal primate niche in the New World. J Hum Evol. 1989;18:635–658. [Google Scholar]
- 88.Jacobs GH. Evolution of colour vision in mammals. Philos Trans R Soc B. 2009;364:2957–2967. doi: 10.1098/rstb.2009.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Leonhardt SD, Tung J, Camden JB, et al. Seeing red: behavioral evidence of trichromatic color vision in strepsirrhine primates. Behav Ecol. 2008;20:1–12. [Google Scholar]
- 90.Laska M, Seibt A. Olfactory sensitivity for aliphatic alcohols in squirrel monkeys and pigtail macaques. J Exp Biol. 2002;205:1633–1643. doi: 10.1242/jeb.205.11.1633. [DOI] [PubMed] [Google Scholar]
- 91.Laska M, Wieser A, Hernandez Salazar LT. Olfactory responsiveness to two odorous steroids in three species of nonhuman primates. Chem Senses. 2005;30:505–511. doi: 10.1093/chemse/bji043. [DOI] [PubMed] [Google Scholar]
- 92.Laska M, Bautista RMR, Höfelmann D, et al. Olfactory sensitivity for putrefaction-associated thiols and indols in three species of non-human primate. J Exp Biol. 2007;210:4169–4178. doi: 10.1242/jeb.012237. [DOI] [PubMed] [Google Scholar]
- 93.Melin AD, Hiramatsu C, Parr NA, et al. The behavioral ecology of color vision: considering fruit conspicuity, detection distance and dietary importance. Int J Primatol. 2014;35:258–287. [Google Scholar]
- 94.Gilad Y, Wiebe V, Przeworski M, et al. Correction: loss of olfactory receptor genes coincides with the acquisition of full trichromatic vision in primates. PLoS Biol. 2007;5:1383. doi: 10.1371/journal.pbio.0020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kalko EKV, Ayasse M. Study and analysis of odor involved in the behavioral ecology of bats. In: Kunz TH, Parsons S, editors. Ecological and behavioral methods for the study of bats. Baltimore: The Johns Hopkins University Press; 2009. pp. 491–499. [Google Scholar]
- 96.Neville MK, Glander KE, Braza F, et al. The howling monkeys. In: Mittermeier RA, Rylands AB, Coimbra-Fliho AF, et al., editors. Ecology and behavior of neotropical primates. Washington, D.C: World Wildlife Fund; 1988. pp. 349–354. [Google Scholar]
- 97.Gittleman JL. Carnivore olfactory bulb size: allometry, phylogeny and ecology. J Zool. 1991;225:253–272. [Google Scholar]
- 98.Healy SD, Guilford T. Olfactory-bulb size and nocturnality in birds. Evolution. 1990;44:339–346. doi: 10.1111/j.1558-5646.1990.tb05203.x. [DOI] [PubMed] [Google Scholar]
- 99.Steiger SS, Fidler AE, Valcu M, et al. Avian olfactory receptor gene repertoires: evidence for a well-developed sense of smell in birds? Proc R Soc Lond B Biol. 2008;275:2309–2317. doi: 10.1098/rspb.2008.0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hayden S, Bekaert M, Crider TA, et al. Ecological adaptation determines functional mammalian olfactory subgenomes. Genome Res. 2010;20:1–9. doi: 10.1101/gr.099416.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nevitt GA. Olfactory foraging by Antarctic procellariiform seabirds: life at high Reynolds numbers. Biol Bull. 2000;198:245–253. doi: 10.2307/1542527. [DOI] [PubMed] [Google Scholar]
- 102.Thies W, Kalko EKV, Schnitzler H-U. The roles of echolocation and olfaction in two neotropical fruit-eating bats, Carollia perspicillata and C. castanea, feeding on Piper. Behav Ecol Sociobiol. 1998;42:397–409. [Google Scholar]
- 103.Korine C, Kalko EKV. Fruit detection and discrimination by small fruit-eating bats (Phyllostomidae): echolocation call design and olfaction. Behav Ecol Sociobiol. 2005;59:12–23. [Google Scholar]


