Abstract
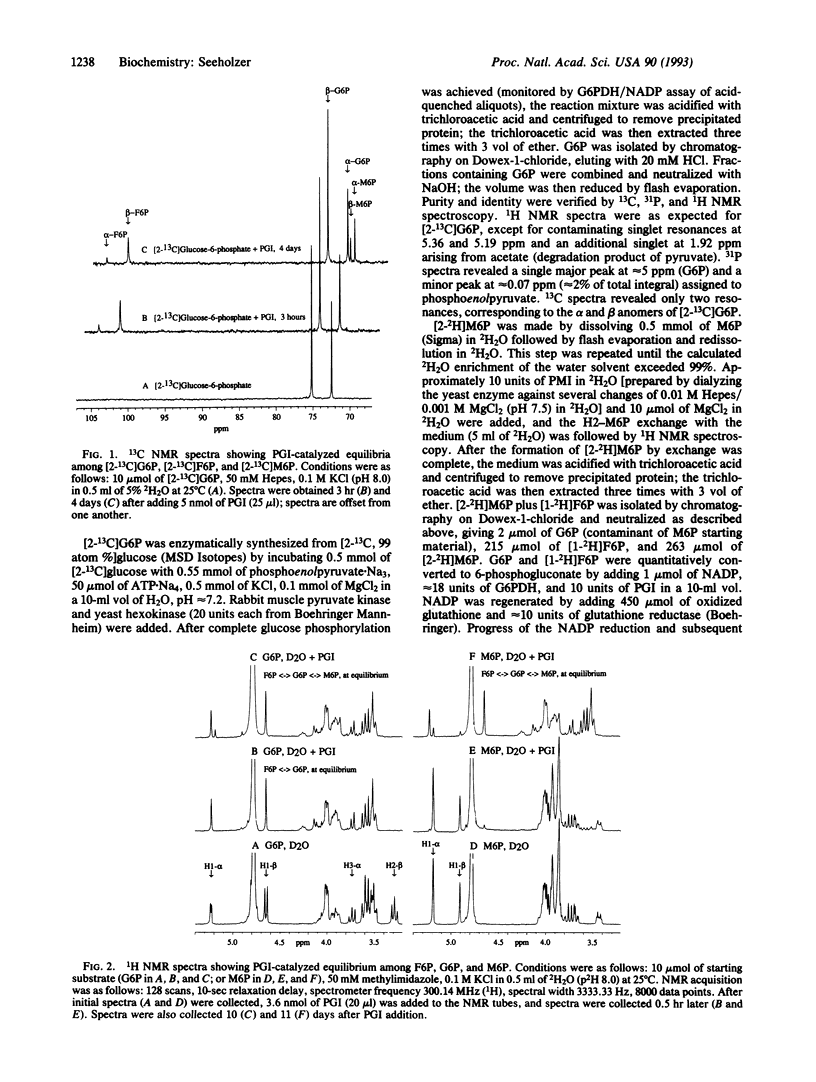
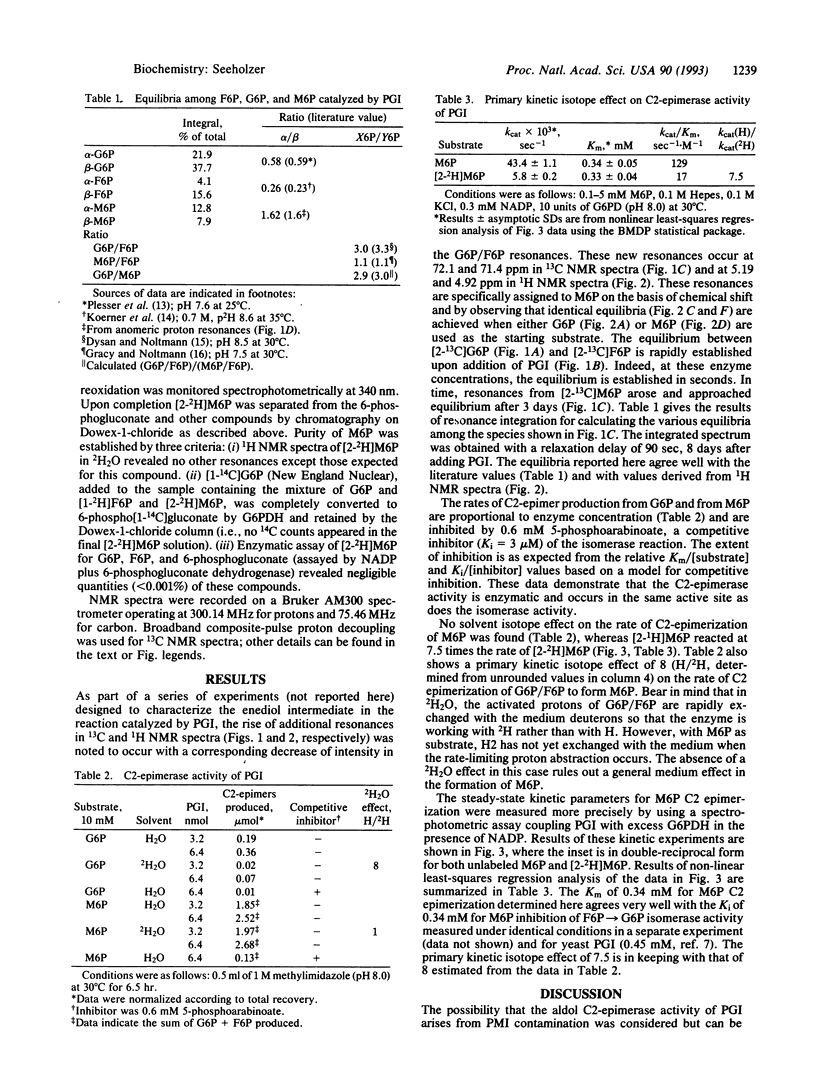
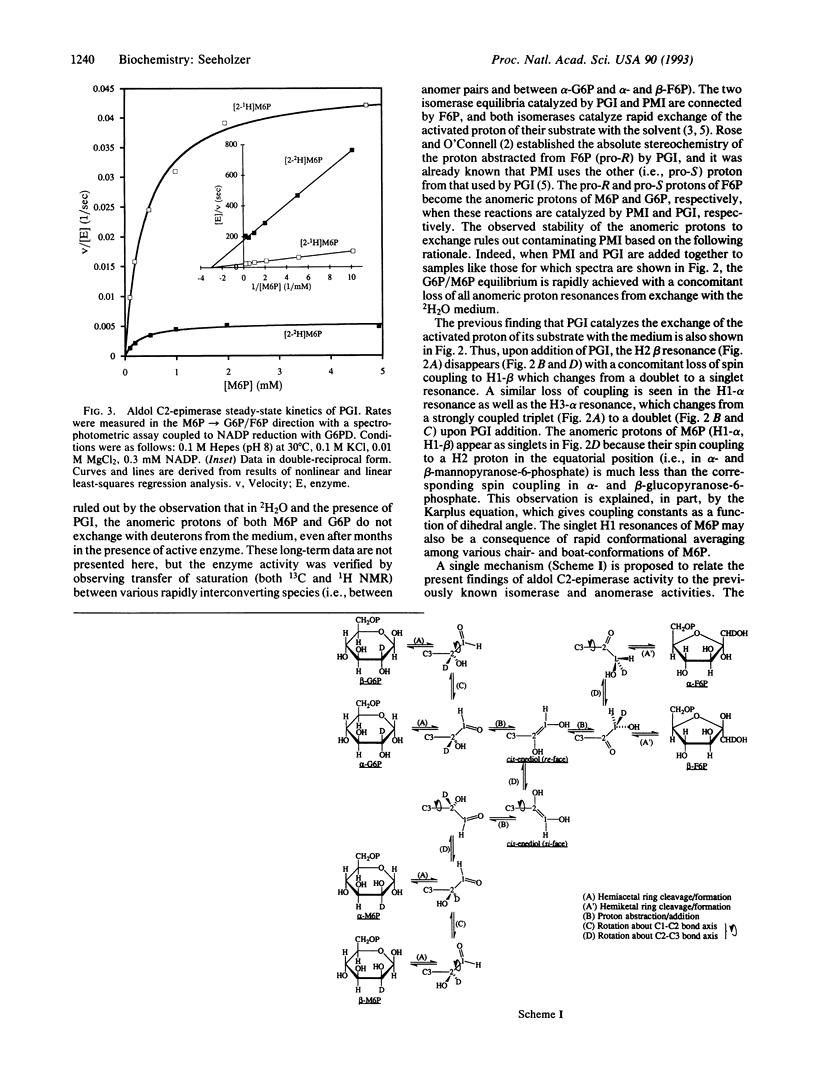
With 13C NMR, phosphoglucose isomerase (PGI; D-glucose-6-phosphate ketol-isomerase, EC 5.3.1.9) is shown to produce mannose 6-phosphate (M6P) slowly from a much more rapidly catalyzed equilibrium between glucose 6-phosphate (G6P) and fructose 6-phosphate (F6P). The identity of M6P and its formation from G6P plus F6P are confirmed by 1H NMR and by the ability of PGI to convert M6P to F6P plus G6P. The possibility of contaminating phosphomannose isomerase (PMI, D-mannose-6-phosphate ketol-isomerase, EC 5.3.1.8) is ruled out by finding no exchange of the C1 proton of G6P or of M6P, whereas exchange occurs with a mixture of PMI and PGI in 2H2O. The pro-R and pro-S protons of F6P become the anomeric protons of M6P and G6P through the actions of PMI and PGI, respectively. Both isomerases exchange the C2 proton of their substrate with the medium; hence, when PGI and PMI are added together to hexose phosphate solutions in 2H2O, both the substrate and anomeric protons are exchange rapidly with deuterons from the medium. The rates of C2-epimerization of G6P and M6P by PGI are shown to be proportional to enzyme concentration and inhibited by 5-phosphoarabinoate, a competitive inhibitor of the previously demonstrated isomerase and anomerase activities of PGI. These data show that the epimerization is enzymatically catalyzed and suggest the involvement of the same active site for all three activities. A primary kinetic isotope effect of 7.5 (H/2H) on the rate constant kcat of the M6P C2-epimerase activity was determined by using a coupled enzymatic assay. A model of the mechanism of PGI is offered, which relates C2-epimerase activity to the isomerase and anomerase activities by allowing the cis-enediol intermediate to rotate about the C2-C3 bond axis followed by protonation at C2 but not at C1 from the si face.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achari A., Marshall S. E., Muirhead H., Palmieri R. H., Noltmann E. A. Glucose-6-phosphate isomerase. Philos Trans R Soc Lond B Biol Sci. 1981 Jun 26;293(1063):145–157. doi: 10.1098/rstb.1981.0068. [DOI] [PubMed] [Google Scholar]
- Balaban R. S., Ferretti J. A. Rates of enzyme-catalyzed exchange determined by two-dimensional NMR: a study of glucose 6-phosphate anomerization and isomerization. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1241–1245. doi: 10.1073/pnas.80.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson J. E., Noltmann E. A. The effect of pH and temperature on the kinetic parameters of phosphoglucose isomerase. Participation of histidine and lysine in a proposed dual function mechanism. J Biol Chem. 1968 Apr 10;243(7):1401–1414. [PubMed] [Google Scholar]
- Gracy R. W., Noltmann E. A. Studies on phosphomannose isomerase. I. Isolation, homogeneity measurements, and determination of some physical properties. J Biol Chem. 1968 Jun 10;243(11):3161–3168. [PubMed] [Google Scholar]
- Koerner T. A., Jr, Cary L. W., Bhacca N. S., Younathan E. S. Tautomeric composition of D-fructose phosphates in solution by Fourier transform carbon-13 nuclear magnetic resonance. Biochem Biophys Res Commun. 1973 Apr 2;51(3):543–550. doi: 10.1016/0006-291x(73)91348-x. [DOI] [PubMed] [Google Scholar]
- Plesser T., Wurster B., Hess B. Determination of the kinetic constants of glucosephosphate isomerase by non-linear optimization. Isomerization and anomerization function. Eur J Biochem. 1979 Jul;98(1):93–98. doi: 10.1111/j.1432-1033.1979.tb13165.x. [DOI] [PubMed] [Google Scholar]
- Pon N. G., Schnackerz K. D., Blackburn M. N., Chatterjee G. C., Noltmann E. A. Molecular weight and amino acid composition of five-times-crystallized phosphoglucose isomerase from rabbit skeletal muscle. Biochemistry. 1970 Mar 31;9(7):1506–1514. doi: 10.1021/bi00809a005. [DOI] [PubMed] [Google Scholar]
- ROSE I. A. Mechanism of C-H bond cleavage in aldolase and isomerase reactions. Brookhaven Symp Biol. 1962 Dec;15:293–309. [PubMed] [Google Scholar]
- ROSE I. A., O'CONNELL E. L. Intramolecular hydrogen transfer in the phosphoglucose isomerase reaction. J Biol Chem. 1961 Dec;236:3086–3092. [PubMed] [Google Scholar]
- ROSE I. A., O'CONNELL E. L. Stereospecificity of the sugarphosphate isomerase reactions; a uniformity. Biochim Biophys Acta. 1960 Jul 29;42:159–160. doi: 10.1016/0006-3002(60)90765-4. [DOI] [PubMed] [Google Scholar]
- Richard J. P. Reaction of triosephosphate isomerase with L-glyceraldehyde 3-phosphate and triose 1,2-enediol 3-phosphate. Biochemistry. 1985 Feb 12;24(4):949–953. doi: 10.1021/bi00325a021. [DOI] [PubMed] [Google Scholar]
- Rose I. A., O'Connell E. L., Schray K. J. Mannose 6-phosphate: anomeric form used by phosphomannose isomerase and its 1-epimerization by phosphoglucose isomerase. J Biol Chem. 1973 Mar 25;248(6):2232–2234. [PubMed] [Google Scholar]
- SALAS M., VINUELA E., SOLS A. SPONTANEOUS AND ENZYMATICALLY CATALYZED ANOMERIZATION OF GLUCOSE 6-PHOSPHATE AND ANOMERIC SPECIFICITY OF RELATED ENZYMES. J Biol Chem. 1965 Feb;240:561–568. [PubMed] [Google Scholar]
- Schray K. J., Benkovic S. J., Benkovic P. A., Rose I. A. Catalytic reactions of phosphoglucose isomerase with cyclic forms of glucose 6-phosphate and fructose 6-phosphate. J Biol Chem. 1973 Mar 25;248(6):2219–2224. [PubMed] [Google Scholar]
- Shaw P. J., Muirhead H. The active site of glucose phosphate isomerase. FEBS Lett. 1976 May 15;65(1):50–55. doi: 10.1016/0014-5793(76)80619-9. [DOI] [PubMed] [Google Scholar]
- TOPPER Y. J. On the mechanism of action of phosphoglucose isomerase and phosphomannose isomerase. J Biol Chem. 1957 Mar;225(1):419–425. [PubMed] [Google Scholar]
- Willem R., Biesemans M., Hallenga K., Lippens G., Malaisse-Lagae F., Malaisse W. J. Dual anomeric specificity and dual anomerase activity of phosphoglucoisomerase quantified by two-dimensional phase-sensitive 13C EXSY NMR. J Biol Chem. 1992 Jan 5;267(1):210–217. [PubMed] [Google Scholar]



