Abstract
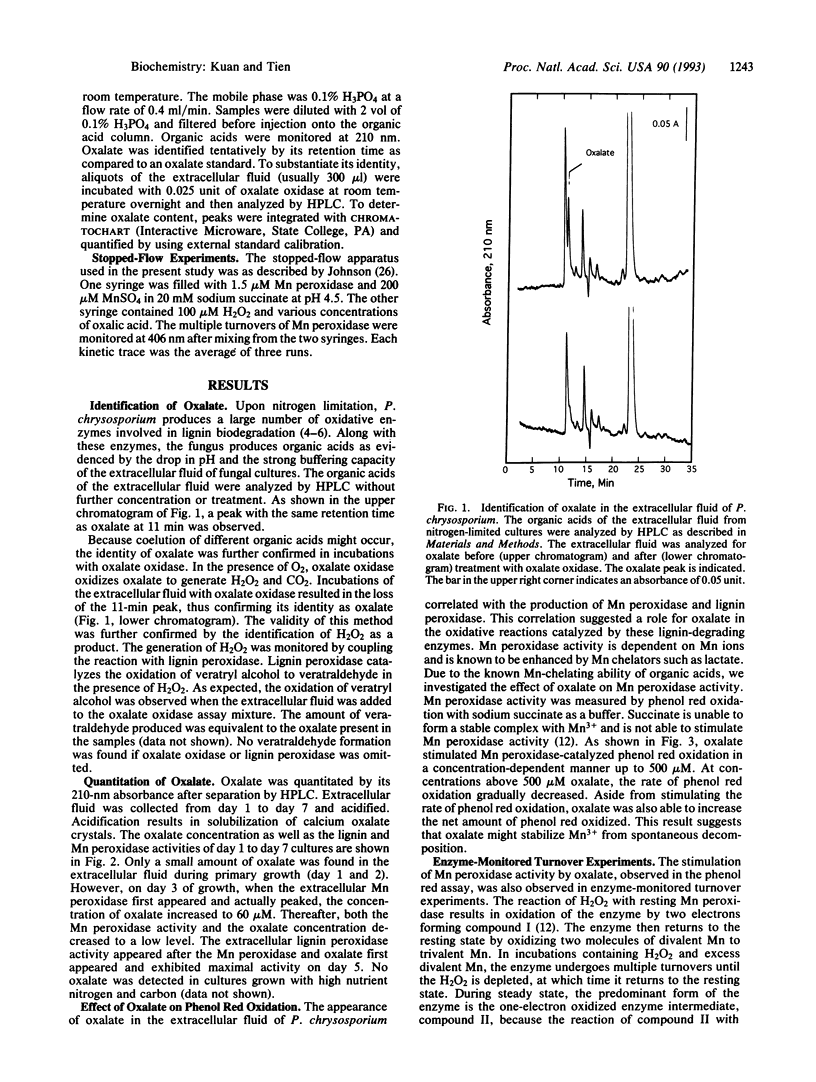
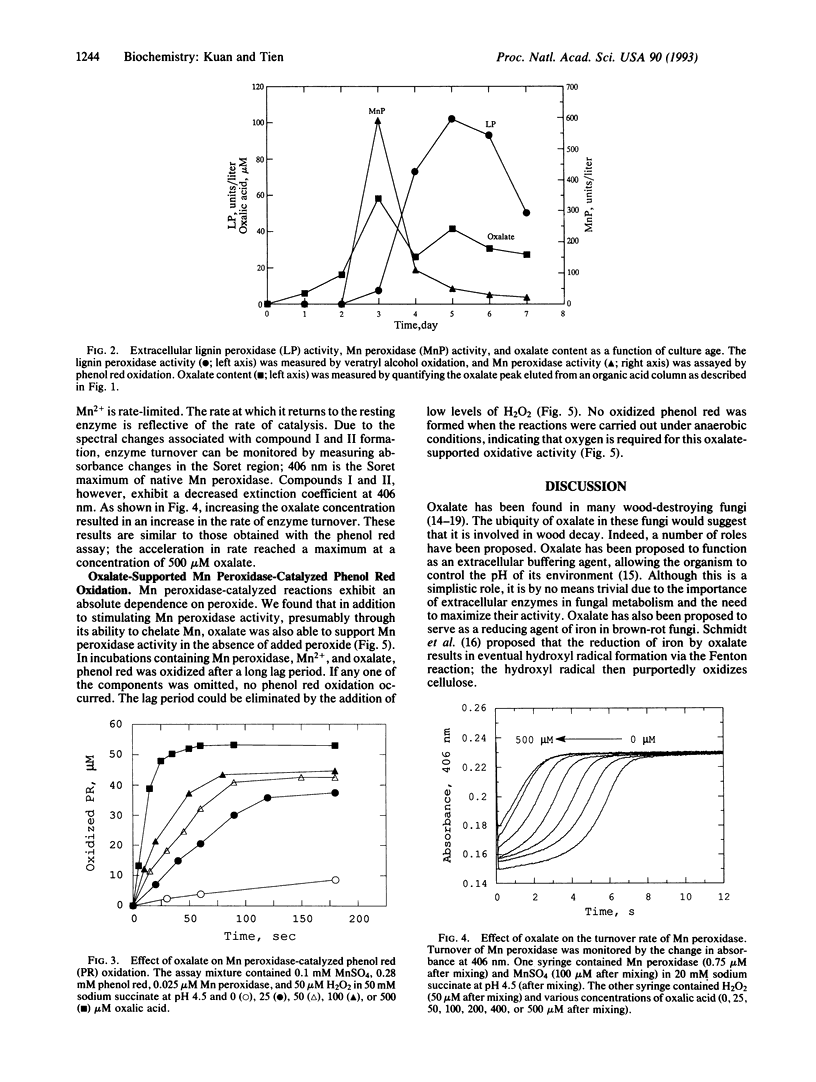
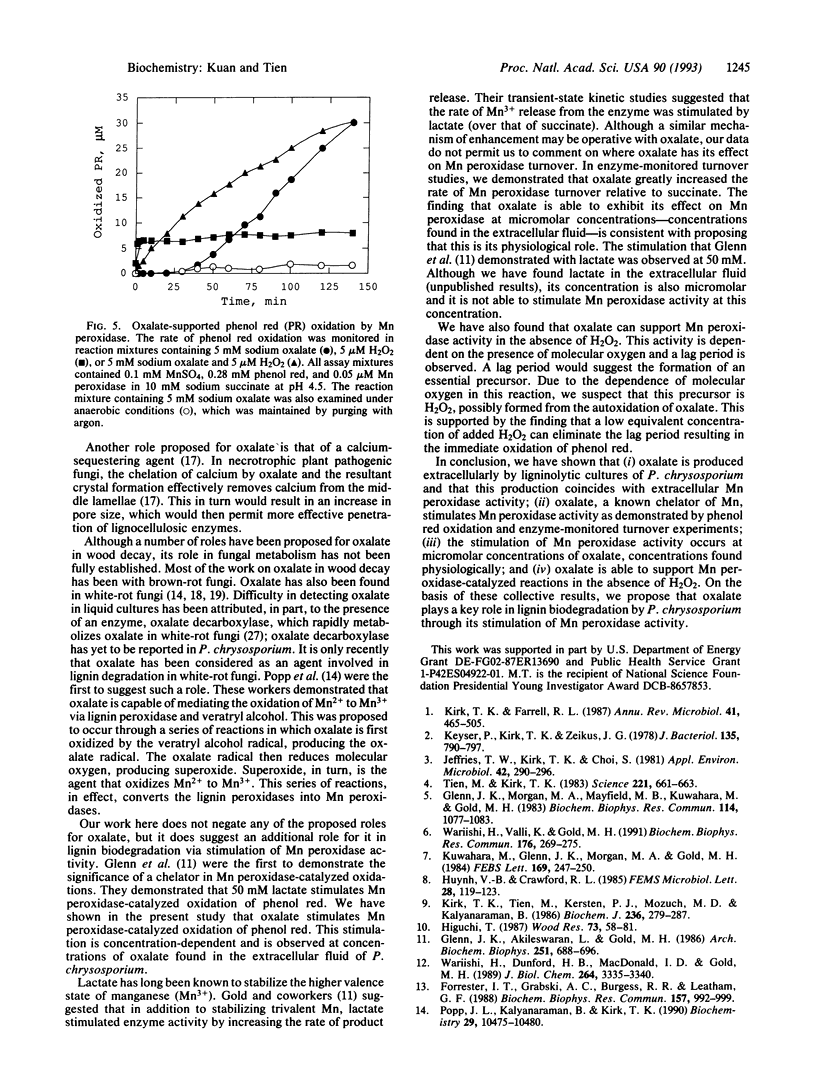
Oxalate is produced by numerous wood-degrading fungi. Our studies here show that the white-rot fungus Phanerochaete chrysosporium produces extracellular oxalate under conditions that induce synthesis of the ligninolytic system. Little or no oxalate was detected in cultures grown under high nutrient nitrogen or carbon. This extracellular oxalate was identified and quantitated by HPLC. Its identity was further substantiated by its decomposition by the enzyme oxalate oxidase. The oxalate content of the extracellular fluid (peaking at 60 microM) paralleled the extracellular activity of the lignin-degrading enzyme, Mn peroxidase. Significantly, we demonstrated that oxalate, at physiological concentrations, substantially stimulated Mn peroxidase-catalyzed phenol red oxidation, presumably by its ability to chelate Mn. Stopped flow studies also indicate that oxalate accelerates the turnover of Mn peroxidase. Furthermore, we discovered that oxalate can support Mn peroxidase-catalyzed oxidations in the absence of exogenous H2O2 and in the presence of dioxygen. These results allow us to propose an important role for oxalate, a ubiquitous compound produced by wood-destroying fungi, in lignin biodegradation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Forrester I. T., Grabski A. C., Burgess R. R., Leatham G. F. Manganese, Mn-dependent peroxidases, and the biodegradation of lignin. Biochem Biophys Res Commun. 1988 Dec 30;157(3):992–999. doi: 10.1016/s0006-291x(88)80972-0. [DOI] [PubMed] [Google Scholar]
- Glenn J. K., Akileswaran L., Gold M. H. Mn(II) oxidation is the principal function of the extracellular Mn-peroxidase from Phanerochaete chrysosporium. Arch Biochem Biophys. 1986 Dec;251(2):688–696. doi: 10.1016/0003-9861(86)90378-4. [DOI] [PubMed] [Google Scholar]
- Glenn J. K., Gold M. H. Purification and characterization of an extracellular Mn(II)-dependent peroxidase from the lignin-degrading basidiomycete, Phanerochaete chrysosporium. Arch Biochem Biophys. 1985 Nov 1;242(2):329–341. doi: 10.1016/0003-9861(85)90217-6. [DOI] [PubMed] [Google Scholar]
- Glenn J. K., Morgan M. A., Mayfield M. B., Kuwahara M., Gold M. H. An extracellular H2O2-requiring enzyme preparation involved in lignin biodegradation by the white rot basidiomycete Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1983 Aug 12;114(3):1077–1083. doi: 10.1016/0006-291x(83)90672-1. [DOI] [PubMed] [Google Scholar]
- Jeffries T. W., Choi S., Kirk T. K. Nutritional Regulation of Lignin Degradation by Phanerochaete chrysosporium. Appl Environ Microbiol. 1981 Aug;42(2):290–296. doi: 10.1128/aem.42.2.290-296.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. A. Rapid kinetic analysis of mechanochemical adenosinetriphosphatases. Methods Enzymol. 1986;134:677–705. doi: 10.1016/0076-6879(86)34129-6. [DOI] [PubMed] [Google Scholar]
- Keyser P., Kirk T. K., Zeikus J. G. Ligninolytic enzyme system of Phanaerochaete chrysosporium: synthesized in the absence of lignin in response to nitrogen starvation. J Bacteriol. 1978 Sep;135(3):790–797. doi: 10.1128/jb.135.3.790-797.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk T. K., Farrell R. L. Enzymatic "combustion": the microbial degradation of lignin. Annu Rev Microbiol. 1987;41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- Kirk T. K., Tien M., Kersten P. J., Mozuch M. D., Kalyanaraman B. Ligninase of Phanerochaete chrysosporium. Mechanism of its degradation of the non-phenolic arylglycerol beta-aryl ether substructure of lignin. Biochem J. 1986 May 15;236(1):279–287. doi: 10.1042/bj2360279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth A. B., Denny M., Tien M. Overproduction of lignin-degrading enzymes by an isolate of Phanerochaete chrysosporium. Appl Environ Microbiol. 1991 Sep;57(9):2591–2596. doi: 10.1128/aem.57.9.2591-2596.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pease E. A., Aust S. D., Tien M. Heterologous expression of active manganese peroxidase from Phanerochaete chrysosporium using the baculovirus expression system. Biochem Biophys Res Commun. 1991 Sep 16;179(2):897–903. doi: 10.1016/0006-291x(91)91903-p. [DOI] [PubMed] [Google Scholar]
- Popp J. L., Kalyanaraman B., Kirk T. K. Lignin peroxidase oxidation of Mn2+ in the presence of veratryl alcohol, malonic or oxalic acid, and oxygen. Biochemistry. 1990 Nov 20;29(46):10475–10480. doi: 10.1021/bi00498a008. [DOI] [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-Degrading Enzyme from the Hymenomycete Phanerochaete chrysosporium Burds. Science. 1983 Aug 12;221(4611):661–663. doi: 10.1126/science.221.4611.661. [DOI] [PubMed] [Google Scholar]
- Tien M., Myer S. B. Selection and characterization of mutants of Phanerochaete chrysosporium exhibiting ligninolytic activity under nutrient-rich conditions. Appl Environ Microbiol. 1990 Aug;56(8):2540–2544. doi: 10.1128/aem.56.8.2540-2544.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wariishi H., Dunford H. B., MacDonald I. D., Gold M. H. Manganese peroxidase from the lignin-degrading basidiomycete Phanerochaete chrysosporium. Transient state kinetics and reaction mechanism. J Biol Chem. 1989 Feb 25;264(6):3335–3340. [PubMed] [Google Scholar]
- Wariishi H., Valli K., Gold M. H. In vitro depolymerization of lignin by manganese peroxidase of Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1991 Apr 15;176(1):269–275. doi: 10.1016/0006-291x(91)90919-x. [DOI] [PubMed] [Google Scholar]



