The fact that we can only treat subsets of the population we are trying to make inferences on poses severe problems in any scientific discipline, primarily due to low replication. However, the sample's response may also be different from the population response due to what we call the ‘island effect’. Here, we systematically look at how global change experiments on vegetation may be subjected to this phenomenon, for example by treatment plots that are subjected to air whose characteristics (relative humidity, temperature) are influenced by the surrounding, non-treated vegetation. This may introduce a systematic artefact that experiments cannot account for.
Keywords: DGVM, elevated CO2, FACE, hydrology, land–atmosphere coupling, vegetation feedback effects, warming
Abstract
Most of the currently experienced global environmental changes (rising atmospheric CO2 concentrations, warming, altered amount and pattern of precipitation, and increased nutrient load) directly or indirectly affect ecosystem surface energy balance and plant transpiration. As a consequence, the relative humidity of the air surrounding the vegetation changes, thus creating a feedback loop whose net effect on transpiration and finally productivity is not trivial to quantify. Forcedly, in any global change experiment with the above drivers, we can only treat small plots, or ‘islands’, of vegetation. This means that the treated plots will likely experience the ambient humidity conditions influenced by the surrounding, non-treated vegetation. Experimental assessments of global change effects will thus systematically lack modifications originating from these potentially important feedback mechanisms, introducing a bias of unknown magnitude in all measurements of processes directly or indirectly depending on plant transpiration. We call this potential bias the ‘island effect’. Here, we discuss its implications in various global change experiments with plants. We also suggest ways to complement experiments using modelling approaches and observational studies. Ultimately, there is no obvious solution to deal with the island effect in field experiments and only models can provide an estimate of modification of responses by these feedbacks. However, we suggest that increasing the awareness of the island effect among both experimental researchers and modellers will greatly improve the interpretation of vegetation responses to global change.
Introduction
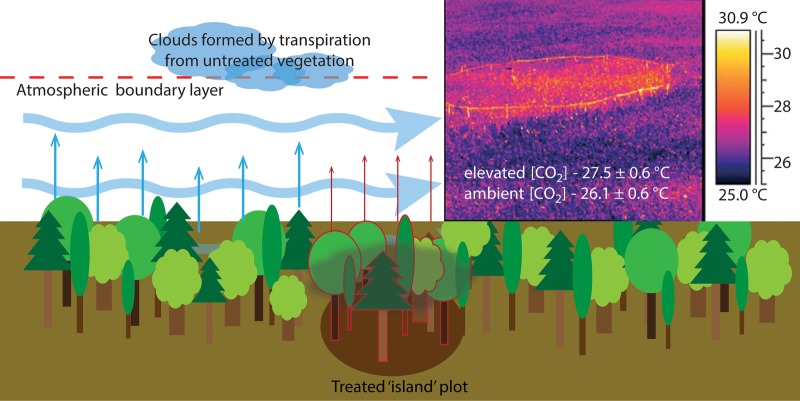
A manipulative field experiment can treat only a small subset of the area or population in question. This inevitably poses the question ‘what would the results look like if we were able to subject the whole area, or the whole population to our treatment?’ The answer to this question will generally be ‘we don't know’. We term this caveat of being able to treat only small ‘islands’ the ‘island effect’ in its broadest sense (sensu lato). Examples probably exist even outside biological disciplines, but within ecology, they are omnipresent: if we treat a patch of grass with fertilizer, food quality may increase and attract herbivores. However, if the whole landscape was exposed to the fertilizer treatment, no particular attraction of herbivores to our small experimental plots would occur. Generally speaking, by treating small subsets of a larger entity we wish to study, we are missing certain processes or mechanisms. Here, we focus on a problem where the island effect is likely very significant, i.e. manipulative experiments that are used to simulate environmental conditions that vegetation is expected to experience in the future. Most commonly, atmospheric CO2 concentration, temperature, precipitation amount and patterns, and nutrient input are altered according to certain global change scenarios, and plant responses (e.g. transpiration or biomass accumulation) are recorded. Although the physiological mechanisms of the responses to some of these drivers are generally well understood at the smallest scale (e.g. the leaf-level response to varying CO2 concentrations has been successfully modelled since the early 1980s, see Farquhar et al. 1980; Jarvis et al. 1999), it is difficult to scale leaf-level or single tree responses to stand or regional levels. Still to date, physiological processes in response to global change drivers are often implicitly scaled from the leaf to the stand, catchment or region, ignoring the multitude of potential atmospheric, soil and community feedback processes (Körner 2000; Leuzinger and Hättenschwiler 2013). Generally, feedback processes that occur at a smaller scale than the treated plot can be captured experimentally, but not those occurring at scales exceeding the plot size. Thus, the interpretation of experiments that treat small ‘islands’ of vegetation (e.g. Shaw et al. 2002; Morgan et al. 2004; Norby et al. 2005; Kongstad et al. 2012; Bader et al. 2013) are necessarily based on the assumption that feedback processes acting beyond the plot size are not important. Belowground, this assumption is generally met because soil conditions induced by treatments are likely less influenced by the surrounding, untreated conditions. One exception may be changes in the ground water table, which we would only observe if a larger region was treated. However, unlike air, soil from ambient plots is not transported to the treatment plots (Fig. 1). An example for this is a change in fine root biomass in free-air CO2 enrichment (FACE) experiments (Iversen et al. 2008; Bader et al. 2009; Ellsworth et al. 2012): for instance, changed soil conditions due to increased fine root turnover in an elevated CO2 plot will directly affect the experimental plot, as no soil from the surrounding ambient vegetation is transported to the treatment plot. In contrast, the constantly moving air over experimental plots will likely be influenced by vegetation growing in ambient conditions outside the experimental plot (Fig. 1). For example, a cloud that was formed due to transpiration over one region will shade and cause stomatal closure in leaves of distant plants (Fig. 1) (van der Ent et al. 2010; Gimeno et al. 2012). Thus, if a global change driver (e.g. temperature or elevated CO2) leads to changes in transpiration in a given experimental plot, the feedback effects arising from this change cannot be ‘sensed’ by the vegetation in the plot.
Figure 1.
A simple representation of the ‘island effect’. The small plot of treated vegetation with, for example, lowered transpiration is surrounded by untreated vegetation with unaltered and relatively higher transpiration. Clouds formed by the untreated plots influence the treated vegetation via altered VPD and lowered solar radiation. The darkened soil below the treated plot illustrates the weak coupling of neighbouring plots relative to the strong coupling of atmospheric conditions. The insert on the top right clearly shows a change in surface energy balance due to lowered stomatal conductance in response to elevated CO2 (from Leakey 2009 reproduced with permission from The Royal Society).
While there may be countless ‘island effects’ (broad sense, as defined above), in the following, we focus on atmospheric water dynamics and call the imperative failure of any manipulative experiment to account for atmospheric feedback ‘island effect’ (sensu stricto, as used henceforth), referring to small ‘islands’ of treated plots in the ‘sea’ of atmospheric conditions dominated by ambient vegetation (Fig. 1). In short, an island effect is a plant or ecosystem response that is due to a feedback loop triggered by an initial change in stomatal conductance (gs), which we are missing in our experiments. In the long term, the island effect can also be triggered by changes in the surface energy balance, for example through changes in leaf area index (LAI) or plant community composition. The phenomenon may thus occur in any field experiment in which we manipulate factors that can affect gs or the surface energy balance, such as experiments with elevated atmospheric CO2 (Ainsworth and Long 2005; Norby et al. 2010; Norby and Zak 2011), soil or air temperature changes (Melillo et al. 2002; Shaw et al. 2002), precipitation changes (Wu et al. 2011; Collins et al. 2012) and theoretically other factors such as nutrient availability (Krogman 1967) or ozone (Matyssek et al. 2010). The island effect may have regional and global implications for soil moisture, cloud formation, rainfall, run-off and finally plant productivity and vegetation structure, and any biogeochemical processes depending on these. Here, we first systematically characterize the island effect and then attempt to quantify its importance in various global change experiments. Finally, we provide an outlook on possible ways forward.
Identifying the Problem
Because the feedback loops associated with the island effect act across large spatial and temporal scales, the question of interest must be as follows: ‘what do we miss in an experiment due to the island effect?’ Admittedly, this question becomes almost infinitely complex and there is no definite answer, at least not from experiments. However, we can simplify the question by restricting ourselves to a given spatiotemporal scale (Fig. 2). All higher-order feedbacks missed in an experimentally treated vegetation island will have their origin in changes in transpiration caused by changes in gs. This is not quite true if long-term changes in LAI and community composition are considered, as those potentially entail changes in albedo, leaf energy balance and stand transpiration, irrespective of changes in gs. However, this long-term feedback can also arise from first-order changes in gs (see group 2.3), under which category they will be treated here.
Figure 2.
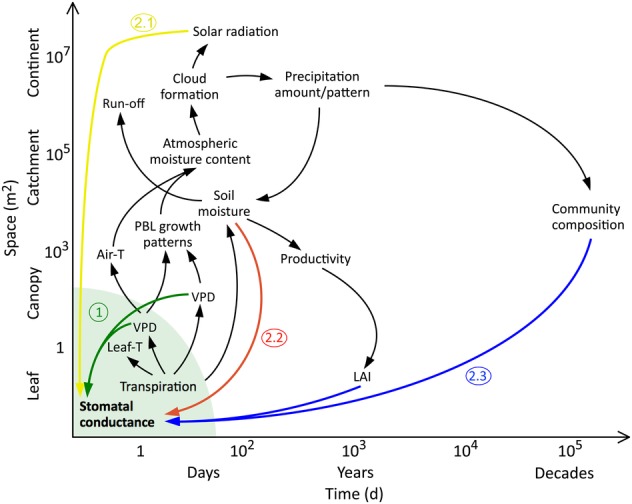
Potential feedback effects originating from an initial change in transpiration. In terrestrial global change experiments, these can turn into what we call ‘island effects’, depending on the considered spatial and temporal scale. At the lowest spatiotemporal scale (under the green arc), the island effect can be assumed to be zero, as the leaf boundary layer conditions are in fact influenced only by the respective leaf. The green arrows (1) represent first-order feedback effects, only leaf-level VPD is involved. Second-order feedback effects involve other factors such as cloud formation (2.1, yellow arrow), soil moisture (2.2, red arrow) or longer-term changes such as LAI or community composition (2.3, blue arrows).
At otherwise constant microclimatic conditions, transpiration of a leaf simply depends on gs and the vapour pressure deficit (VPD) in the boundary layer of that leaf (Jarvis and McNaughton 1986). If for now we ignore any transport of moisture away from the leaf, we can identify a positive feedback loop at the level of the leaf, initiated by a change in gs: for example, if water addition to the soil increases gs, transpiration will increase and the boundary layer VPD will decrease, which in turn increases stomatal conductance, decreases leaf temperature and so forth (loop under green area in Fig. 2). Thus, at the smallest spatiotemporal scale, there is no island effect, since all relevant feedbacks occur within the plot's size and the experiment's duration. Put simply, under these assumptions, the air ‘created’ or ‘influenced’ by the leaf is the air that the leaf ‘sees’ or is exposed to. However, as soon as we extend our spatial and/or temporal perspective, this is no longer true (area outside the green area in Fig. 2). For example, at landscape scale, the air that a leaf ‘sees’ may well have been affected (e.g. in terms of its moisture content or temperature) by plant transpiration hundreds of kilometres away. The plethora of potential feedbacks at these larger scales can roughly be grouped them into (1) first-order and (2) second-order or higher-order effects. Group (1) includes effects where gs affects transpiration and thus VPD, which then immediately feed back to gs—no further variables are involved (green arrows in Fig. 2). Group (2) includes effects that are more complex as they involve at least one more secondary effects before they feed back to gs: (2.1) those caused by changes in solar radiation, via alteration of atmospheric moisture and therefore cloud cover (de Arellano et al. 2012) (yellow arrow in Fig. 2); (2.2) those caused by changes in soil moisture, via first-order feedbacks through VPD and transpiration (red arrow in Fig. 2) and (2.3) effects that follow a few to many years after an initial change in gs, either via changes in LAI, adaptive responses of leaf and canopy conductance or eventually soil biogeochemistry and community composition (blue arrows in Fig. 2). Note again that all loops in Fig. 2 originate from, and feed back to, changes in stomatal conductance per unit leaf area as the ultimate hub of all island effects. Those feedback loops that operate outside the influence of gs may be important, but are ‘dead ends’, because they are not prone to further altering the island effect.
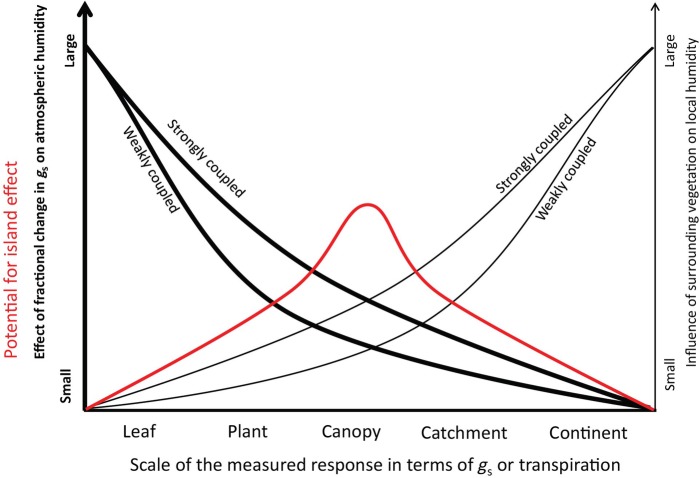
Can we identify at what spatiotemporal scale we are most likely to see an island effect in an experiment? The VPD conditions a leaf or plant ‘sees’ are a blend of its own transpiration as well as that of the neighbouring leaves, plants, stand, region or continent. On the one hand, the larger the spatial scale at which potential feedback effects act, the higher the risk that we miss them in a plot-size experiment. On the other hand, at increasing scales (from <1 m3 to many km3), the influence of a fractional change in gs on large-scale transpiration decreases (Fig. 3). This is because the bulk of Earth's evapotranspiration originates from oceans, with plant transpiration accounting for only ∼9–10 % of total water vapour input to the atmosphere (Roderick et al. 2014; Schlesinger and Jasechko 2014; Wild et al. 2015). Additionally, the feedbacks (and thus the risk of an island effect) are more pronounced in vegetation that is strongly coupled to the atmosphere, corresponding to a small aerodynamic resistance (Fig. 3) (Jarvis and McNaughton 1986).
Figure 3.
The potential for the island effect is maximal at intermediate spatial scales. This is because the two effects shown (effect of gs on atmospheric humidity, influence of surrounding vegetation on local humidity) are compensatory in terms of promoting the island effect. Generally, atmospherically well-coupled plants/stands such as tree canopies are more prone to the island effect than less coupled ones (e.g. grassland, see McNaughton and Jarvis 1991).
In summary, the two needed ‘ingredients’ or prerequisites for the island effect to occur are (i) a first-order change in gs that causes a change in transpiration following a global change treatment and (ii) that the air surrounding the leaf/plant/stand of interest is influenced to some degree by surrounding, non-treated leaves/plants/stands. In the following, we work through the most important experiments with global change drivers, characterizing where and when island effects may occur. For the rest of the text, we refer to the above-defined categories, which are also used in Fig. 2 (labelled 1, 2.1, 2.2 and 2.3).
Which Experiments Are Affected?
As long as the two ingredients for the island effect are present, it does not matter what triggers the first-order change in transpiration. The most prominent drivers, however, are elevated CO2, warming, precipitation and nutrient addition/depletion experiments. Once an initial change in gs has occurred, we need not worry much about what lies at the origin of this change, as the feedback effects (or the island effect as seen from the experimenter's perspective) will be identical. We will now briefly review what initial changes in gs have been found following experiments with the most common global change treatments.
Free-air CO2 enrichment experiments have been employed for >20 years testing responses of ecosystems to elevated atmospheric CO2 (Ainsworth and Long 2005). Generally, reduced stomatal opening is observed when plants are subjected to elevated atmospheric CO2, leading to lower levels of transpiration, i.e. decreased latent heat flux. Most meta-analyses report responses of stomatal conductance to elevated CO2 between −30 % and no response, with a mean around −15 % (at approximately doubled pre-industrial atmospheric CO2 concentration), strongly depending on the species and testing conditions (Field et al. 1995; Curtis and Wang 1998; Medlyn et al. 2001; Ainsworth et al. 2003; Ainsworth and Rogers 2007; Leuzinger and Körner 2007; Keel et al. 2007; Warren et al. 2011; Tor-ngern et al. 2015). In a nutrient-poor calcareous grassland, Niklaus and Körner (2004) found a ∼50 % reduction in leaf conductance in the dominant grass species, and no response in co-occurring sedges sharing the same root sphere. These strong responses in gs are, therefore, likely to trigger experimental island effects.
Warming experiments use several methods to heat the soil, usually with buried heating cables (e.g. Rustad et al. 2001; Melillo et al. 2002), or the atmosphere, usually with infrared lamps (e.g. Nijs et al. 1996; Hovenden et al. 2006; De Boeck et al. 2010). Plants respond to soil warming differently, depending on the biome with some reporting elevated gs (Rogiers and Clarke 2013) and others a decrease in gs. The net effect of warming on surface energy balance seems to be important: Nijs et al. (1997) found, for example, that despite a reduction in gs, leaf transpiration was elevated because the warmed canopy increased leaf-to-air vapour pressure difference. Trees in boreal forests may have stimulated gs, particularly in the morning and early spring, whereas tropical trees may not be affected much by temperature in terms of gs (Doughty and Goulden 2008). In cooler areas like the temperate zone, elevated soil temperatures have been shown to increase gs (e.g. Rogiers and Clarke 2013). Under well-watered conditions, warming can increase night-time gs and/or sap flow because of increased VPD, which can contribute considerably to total transpiration (Caird et al. 2007; Fisher et al. 2007). Furthermore, atmospheric warming increases VPD, which, everything else being equal, increases transpiration. This may evoke a potential feedback effect that is not immediately linked to gs. However, to avoid excessive water loss and/or cavitation, plants often lower gs in response to high transpiration or low leaf water potentials (e.g. Bunce 1997; Tardieu and Simonneau 1998; Brodribb and Holbrook 2003). Also, immediate canopy warming may be a feedback loop acting at small scales (under the arc in Fig. 2) and thus not evoke an island effect. Warming can also affect phenology (Keenan et al. 2014), with consequences for seasonal transpiration rates. For example, Zavaleta et al. (2003) found that under warming, soil water content in annual grassland increased not due to reduced transpiration at the leaf level, but a reduction in seasonal transpiration via an early onset of senescence. In this case, a potential island effect would act via transpiration, as phenology changes alone cannot cause an island effect.
Precipitation and drought experiments eventually alter soil water availability to plants. The influence of water availability on gs is well established (Hsiao 1973; Schulze et al. 2002; Dodd 2013); declining soil moisture reduces gs, whereas increasing soil moisture may elevate gs when plants are water limited. In addition, stomatal responses vary strongly between species so that elevated CO2 can trigger shifts in community composition via water use-related mechanisms (Volk et al. 2000; Morgan et al. 2011). Therefore, predicting indirect ecosystem responses to elevated CO2 requires a knowledge of the species’ characteristic stomatal regulation.
Finally, nutrient addition experiments have a long history in ecology, with growth and photosynthesis as typically measured responses. Increased productivity mostly translates to higher LAI, and possibly canopy conductance (at least at LAI <3, Krogman 1967; Schulze et al. 1994; Novick et al. 2009). Along with LAI come changes in albedo and surface energy balance. These changes likely occur over longer time periods, and the resulting feedbacks will be of group 2.3 in Fig. 2. Only if ultimately gs or transpiration are affected, which is likely the case, can an island effect (sensu strico) occur as a consequence of nutrient addition.
What Are We Missing?
It is clear from our above discussion that many global change drivers will, among other, cause changes in gs and/or transpiration, inevitably leading to changes in the atmospheric moisture conditions immediately surrounding the plant. If the scale of the feedback mechanism exceeds the plot scale, these new atmospheric conditions do not feed back on the plants in the treated plot, producing an island (Fig. 2). If, hypothetically, we could treat a large enough area so that the entire vegetation cover affected by the simulated global change driver transpired differently as in a realistic scenario, plants could in fact be subjected to a series of both positive and negative feedback mechanisms, with an unknown net outcome (Fig. 2).
A first, immediate and positive feedback loop could arise when, for example, drier air causes higher evaporative demand, leading to further stomatal closure and thus enhancing the first-order response (effect 1 in Fig. 2). This mechanism was indeed found early in a modelling attempt by Jacobs and de Bruin (1997), who used a planetary boundary layer (PBL)–vegetation model to simulate the vegetation–atmosphere interaction under elevated CO2. The problem is that an opposite effect is just as plausible, as higher VPD typically causes more transpiration despite stomatal closure. More generally, even if we are able to isolate one possible feedback as these authors did, other, less immediate effects acting at different spatiotemporal scales may overlay and thus cancel, mitigate or enhance the initial one. For example, on a spatial scale of a few to tens of kilometres, larger sensible heat flux caused by reduced transpiration could cause a decrease or even increase in cloud formation (D'Almeida et al. 2007; Knox et al. 2011), leading to modified radiation and thus altered surface energy balance, altered evapotranspiration and also altered stomatal conductance (effect 2.1 in Fig. 2). Other negative feedback loops could arise via reduced rain (following decreased cloud formation) or higher evaporative demand, both leading to lower soil moisture and eventually lower gs (effect 2.2 in Fig. 2). It is important to note that the latter two phenomena (effects on soil moisture via atmospheric feedback loops) are somewhat different from the direct influence of reduced gs on soil moisture, an effect that can actually be captured in an experiment (see below). The atmospheric island effect may, therefore, lead to an over- or underestimation, or even a sign reversal of the first-order response in terms of gs reported from an experiment.
While the island effect is inevitable aboveground, we can look at what happens belowground, where there is no island effect (sensu stricto). Soil, unlike air, is not transported to roots from far away, untreated vegetation in global change experiments. Apart from edge effects, the soil water conditions (e.g. soil moisture) that the roots ‘create’ are directly acting on them. If a tree transpires less due to elevated CO2 for example, it will cause the soil to be moist for longer. The magnitude of this measurable feedback effect in the soil, however, can be very large, to the point that increased soil moisture following a reduction in gs in FACE experiments often causes larger growth stimulation than the direct effects of elevated CO2 (Housman et al. 2006; Holtum and Winter 2010; Hartmann 2011). This is particularly true in grassland. In semi-natural grassland, lower stomatal conductance leads to significant soil moisture savings (Niklaus et al. 1998), which explained almost the entire variation in peak biomass CO2 response (Niklaus and Körner 2004). Similar effects were also found in other studies (Owensby et al. 1997; Morgan et al. 2004; Hovenden et al. 2014). In other words, if an island effect existed belowground, i.e. if the soil moisture that the roots of our CO2-treated plants experience came from ambient vegetation, we would be far off with our conclusions of ecosystem responses to elevated CO2! Are we missing something similar aboveground where we actually have to deal with the island effect? Probably not, but even if the island effect is much smaller than the elevated CO2–soil feedback effect we can measure, we would be likely to observe different net ecosystem responses.
Ultimately, the issue of the island effect will involve large temporal scales, as is often the case in ecology (Leuzinger et al. 2011). Effects acting over long time spans have the power to change the community structure or soil biota and therefore the nutrient cycles. These are very difficult to model or even to speculate about.
Ways Forward
We argue that the island effect is primarily a phenomenon that we need to be aware of when interpreting both experimental and modelling results. To date, few researchers are. No individual experiment or model will provide a conclusive answer, but by intelligently combining experimental and modelling approaches, we can better understand their sensitivities to the island effect. At the global scale, a fully coupled model technically takes care of the island effect, as vegetation is sensitive to changes in temperature, CO2, soil moisture, radiation, etc. and dynamically feeds back to the atmosphere (Cao et al. 2010). However, the coarse resolution and representation of vegetation diversity of such global models means that most of the above feedbacks will be only partially represented, and we will have to rely on more detailed vegetation modelling (e.g. Pappas et al. 2015a, b).
Because the island effect almost always means missing feedback on gs and therefore transpiration and surface energy balance, one crucial point of attack will be to better characterize VPD sensitivities. We know water fluxes may be ‘wrong’ (which cannot be fixed due to limitations in plot size), but we need to know whether the parameter investigated is sensitive to the changes in water fluxes that occur. This could be achieved by implementing factorial treatments that modify water availability. For example, a factorial CO2 × irrigation study could indicate that CO2 effects are dependent on water availability. If, for example, CO2 effects disappear when the soil moisture saving that occurs in experimental plots is offset (e.g. by equivalent water addition to control plots), then we know that we potentially have a problem. If the CO2 effects on a given variable remain largely constant, then the inferences are probably safe and not affected by the island effect. Another way to understand VPD responses are experiments where relative humidity (RH) is manipulated. Misting has been used in agriculture and horticulture (e.g. Katsoulas et al. 2001) to reduce leaf temperatures and transpiration, but RH experiments are rare in global change research and we know of just one in Tartu, Estonia (Kupper et al. 2011; Godbold et al. 2014). Kupper et al. (2011) found that higher atmospheric humidity increased both sap flow and canopy conductance, while Godbold et al. (2014) found that leaf longevity increased in one of two species measured in elevated RH. Taken together, these results suggest that annual transpiration can be higher in elevated RH conditions in this experiment, despite lower VPD. Another experimental approach to at least estimate the magnitude of a possible island effect could be to water different size (grassland) plots, e.g. from 1 m2 to several km2. Although watering even very large areas will not affect the diurnal PBL growth, if the plot size is the only variable that changes between plots of different sizes, we should see differences in ecosystem responses that are simply due to the island effect.
To constrain the potential bias that the island effect introduces in experiments, explicit simulations of changes in VPD using coupled vegetation–atmosphere models could help. As an example, we used a mechanistic ecohydrological model, T&C (Fatichi et al. 2012, 2015; Fatichi and Ivanov 2014), to carry out a sensitivity analysis of the potential errors one can commit assuming that RH does not change in a manipulation experiment, where, instead, a reduction in gs is expected (e.g. elevated CO2). We applied two CO2 levels (400 and 700 ppm), and different reduction factors (fRH) to the observed time series of RH, from fRH = 1.0 (no reduction) to fRH = 0.85 corresponding to a strong (−15 %) reduction of RH. We used four locations characterized by different climates and plant functional types to show the potential variability of the effect (Fig. 4A–D). Simulated errors in the long-term (5–10 years) transpiration may reach −5 to −10 %, but they are generally constrained to less than −2 % for realistic changes in RH (Fig. 4). The negative sign is expected since the island effect leads to an underestimation of transpiration because RH is not reduced. Simulated errors in net primary productivity (NPP) are constrained within ±2 %, except for one case study (UMBS in Fig. 4C), where they can reach up to +10 %. For this location, the changes in transpiration due to a lower RH are sufficient to increase the water stress in the forest stand with comparison to unchanged RH. Changes in soil moisture considerably affect NPP, which would be overestimated in the treated ‘island’. While the long-term expected errors may be small, there are specific seasons or periods where the island effect can be potentially very significant (>100 %), for example the dry period in the middle of the growing season (days 40 to 50, Fig. 4E and F). In this scenario, the difference in transpiration and NPP induced by a 5 % change in RH can affect the system response of a magnitude similar to that of an elevated CO2 treatment. Since many observations in manipulation experiments are typically carried out for limited periods during the growing season, the potential artefacts of the island effect may be significant. The presented results are a sensitivity analysis and likely dependent on model parameters, but they suggest that there may be situations or locations, where a small change in RH can lead to considerably different plant water stress, with potentially large implications in terms of ecosystem stability and composition.
Figure 4.
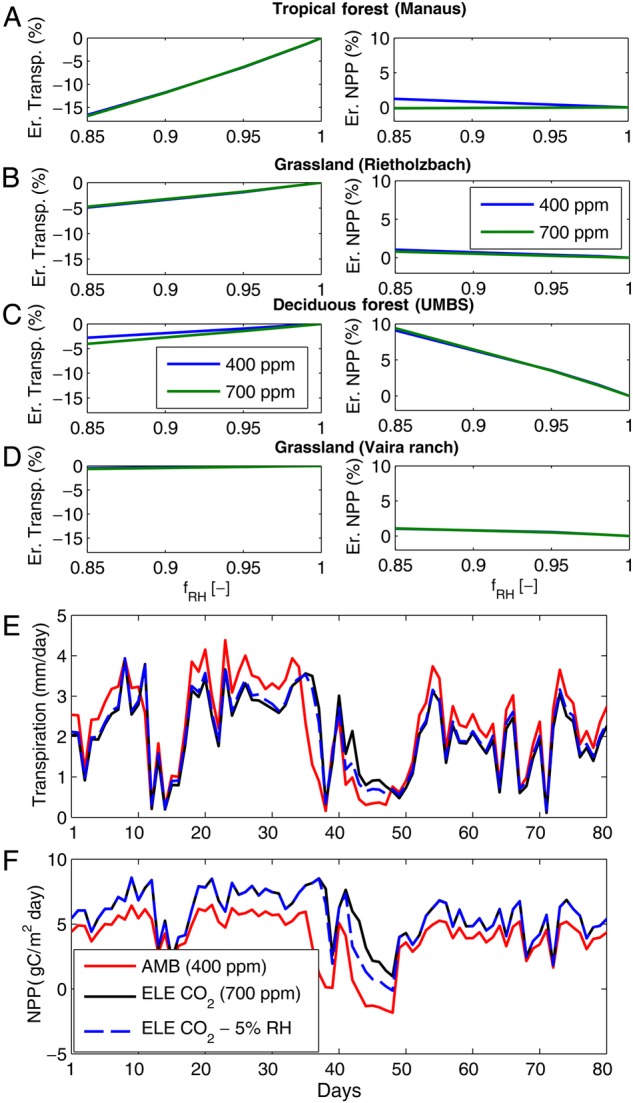
Sensitivity of the island effect in an elevated CO2 experiment. The potential error (Er.) committed in estimating transpiration and NPP with the current RH is shown. RH is reduced by a given factor (fRH), due to hypothetical local or regional feedbacks. The numerical experiment is carried out for two CO2 levels: 400 ppm (ambient, AMB) and 700 ppm (elevated, ELE CO2) and four locations: a tropical forest in Manaus (Brazil), a grassland in Rietholzbach (Switzerland), a deciduous forest near the University of Michigan Biological Station (UMBS) Michigan, USA, and a grassland in California, USA (Vaira ranch). The simulated time series of transpiration and NPP during a characteristic growing season at the UMBS are also shown (subplots E and F); note the dry period between days 2040 and 2050.
Conclusions
In conclusion, we advocate an increasing awareness for the systematic biases that may emerge from plot-size dependence of responses to simulated global change. Generally, it appears imperative that future global change field experiments are designed in a way that enables effective up-scaling strategies or that at least allow to constrain the magnitude of potential island artefacts. Ideally, these bias estimates are then published together with the study results. Modelling efforts to explicitly simulate the magnitude of the island effect dependent on the affected plot size could help to successfully tackle this challenging endeavour. Water-vapour-related scaling issues have now been discussed for almost three decades (Jarvis and McNaughton 1986; Amthor 1999), but in our opinion, the problem has not been effectively dealt with to date. We believe that this situation could be overcome by adopting approaches along the lines we discussed here and by explicitly dealing with the island effect when designing experimental studies or when using field experimental data to parameterize models used for regional or global simulations. While models are often based on physical laws (e.g. energy conservation) and physiological principles (e.g. the photosynthesis scheme based on the Farquhar model), special attention needs to be given to the degree to which they might have been implicitly or explicitly ‘tuned’ to match experimental data (Leuzinger and Thomas 2011)—experimental data which, as we stress here, might suffer from bias due to the island effect. Such a ‘trickling’ of bias into models is likely since models are abstractions that necessarily capture only part of the real-world complexity, and are gauged against experimental data. Most ecosystem models are based on a ‘carbon-centred’ approach and only implement a limited number of feedback mechanisms that may dampen this ‘C-driven’ (i.e. photosynthetic) response (Leuzinger et al. 2011; Fatichi et al. 2014). This general issue has been recognized (Hungate et al. 2003; Piao et al. 2013), but our understanding is that the island effect in experimental data against which models are gauged has been largely ignored so far.
Sources of Funding
J.C. acknowledges the supported provided by a Vice Chancellor's Doctoral Scholarship. S.L. and S.F. received a travel grant from the institute of Applied Ecology New Zealand, Auckland University of Technology. P.A.N. is supported by the University of Zurich Priority Programme on Global Change and Biodiversity.
Contributions by the Authors
S.L., P.A.N. and C.K. conceived the idea. S.F. performed the model analyses. J.C. developed the idea further and drafted the section ‘Which Experiments Are Affected’. All authors wrote the manuscript.
Conflict of Interest Statement
None declared.
Acknowledgement
We thank one anonymous reviewer for useful comments on an early draft of the manuscript.
Literature Cited
- Ainsworth EA, Long SP. 2005. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytologist 165:351–372. 10.1111/j.1469-8137.2004.01224.x [DOI] [PubMed] [Google Scholar]
- Ainsworth EA, Rogers A. 2007. The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant, Cell and Environment 30:258–270. 10.1111/j.1365-3040.2007.01641.x [DOI] [PubMed] [Google Scholar]
- Ainsworth EA, Davey PA, Hymus GJ, Osborne CP, Rogers A, Blum H, Nösberger J, Long SP. 2003. Is stimulation of leaf photosynthesis by elevated carbon dioxide concentration maintained in the long term? A test with Lolium perenne grown for 10 years at two nitrogen fertilization levels under Free Air CO2 Enrichment (FACE). Plant, Cell and Environment 26:705–714. 10.1046/j.1365-3040.2003.01007.x [DOI] [Google Scholar]
- Amthor JS. 1999. Increasing atmospheric CO2 concentration, water use, and water stress: scaling up from the plant to the landscape. In: Mooney HA, Luo Y, eds. Carbon dioxide and environmental stress. San Diego: Academic Press. [Google Scholar]
- Bader M, Hiltbrunner E, Körner C. 2009. Fine root responses of mature deciduous forest trees to free air carbon dioxide enrichment (FACE). Functional Ecology 23:913–921. 10.1111/j.1365-2435.2009.01574.x [DOI] [Google Scholar]
- Bader MK-F, Leuzinger S, Keel SG, Siegwolf RTW, Hagedorn F, Schleppi P, Körner C. 2013. Central European hardwood trees in a high-CO2 future: synthesis of an 8-year forest canopy CO2 enrichment project. Journal of Ecology 101:1509–1519. 10.1111/1365-2745.12149 [DOI] [Google Scholar]
- Brodribb TJ, Holbrook NM. 2003. Stomatal closure during leaf dehydration, correlation with other leaf physiological traits. Plant Physiology 132:2166–2173. 10.1104/pp.103.023879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunce JA. 1997. Does transpiration control stomatal responses to water vapour pressure deficit? Plant, Cell and Environment 20:131–135. 10.1046/j.1365-3040.1997.d01-3.x [DOI] [Google Scholar]
- Caird MA, Richards JH, Donovan LA. 2007. Nighttime stomatal conductance and transpiration in C3 and C4 plants. Plant Physiology 143:4–10. 10.1104/pp.106.092940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Bala G, Caldeira K, Nemani R, Ban-Weiss G. 2010. Importance of carbon dioxide physiological forcing to future climate change. Proceedings of the National Academy of Sciences of the USA 107:9513–9518. 10.1073/pnas.0913000107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SL, Koerner SE, Plaut JA, Okie JG, Brese D, Calabrese LB, Carvajal A, Evansen RJ, Nonaka E. 2012. Stability of tallgrass prairie during a 19-year increase in growing season precipitation. Functional Ecology 26:1450–1459. 10.1111/j.1365-2435.2012.01995.x [DOI] [Google Scholar]
- Curtis PS, Wang X. 1998. A meta-analysis of elevated CO2 effects on woody plant mass, form, and physiology. Oecologia 113:299–313. 10.1007/s004420050381 [DOI] [PubMed] [Google Scholar]
- D'Almeida C, Vörösmarty CJ, Hurtt GC, Marengo JA, Dingman SL, Keim BD. 2007. The effects of deforestation on the hydrological cycle in Amazonia: a review on scale and resolution. International Journal of Climatology 27:633–647. 10.1002/joc.1475 [DOI] [Google Scholar]
- de Arellano JVG, van Heerwaarden CC, Lelieveld J. 2012. Modelled suppression of boundary-layer clouds by plants in a CO2-rich atmosphere. Nature geoscience 5:701–704. [Google Scholar]
- De Boeck HJ, Dreesen FE, Janssens IA, Nijs I. 2010. Climatic characteristics of heat waves and their simulation in plant experiments. Global Change Biology 16:1992–2000. 10.1111/j.1365-2486.2009.02049.x [DOI] [Google Scholar]
- Dodd IC. 2013. Abscisic acid and stomatal closure: a hydraulic conductance conundrum? New Phytologist 197:6–8. 10.1111/nph.12052 [DOI] [PubMed] [Google Scholar]
- Doughty CE, Goulden ML. 2008. Are tropical forests near a high temperature threshold? Journal of Geophysical Research 113:G00B07. [Google Scholar]
- Ellsworth DS, Thomas R, Crous KY, Palmroth S, Ward E, Maier C, DeLucia E, Oren R. 2012. Elevated CO2 affects photosynthetic responses in canopy pine and subcanopy deciduous trees over 10 years: a synthesis from Duke FACE. Global Change Biology 18:223–242. 10.1111/j.1365-2486.2011.02505.x [DOI] [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA. 1980. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90. 10.1007/BF00386231 [DOI] [PubMed] [Google Scholar]
- Fatichi S, Ivanov VY. 2014. Interannual variability of evapotranspiration and vegetation productivity. Water Resources Research 50:3275–3294. 10.1002/2013WR015044 [DOI] [Google Scholar]
- Fatichi S, Ivanov VY, Caporali E. 2012. A mechanistic ecohydrological model to investigate complex interactions in cold and warm water-controlled environments. 1. Theoretical framework and plot-scale analysis. Journal of Advances in Modeling Earth Systems 4: 10.1029/2011MS000086. [DOI] [Google Scholar]
- Fatichi S, Leuzinger S, Körner C. 2014. Moving beyond photosynthesis: from carbon source to sink-driven vegetation modeling. New Phytologist 201:1086–1095. 10.1111/nph.12614 [DOI] [PubMed] [Google Scholar]
- Fatichi S, Katul GG, Ivanov VY, Pappas C, Paschalis A, Consolo A, Kim J, Burlando P. 2015. Abiotic and biotic controls of soil moisture spatio-temporal variability and the occurrence of hysteresis. Water Resources Research; 10.1002/2014WR016102. [DOI] [Google Scholar]
- Field CB, Jackson RB, Mooney HA. 1995. Stomatal responses to increased CO2: implications from the plant to the global scale. Plant, Cell and Environment 18:1214–1225. 10.1111/j.1365-3040.1995.tb00630.x [DOI] [Google Scholar]
- Fisher JB, Baldocchi DD, Misson L, Dawson TE, Goldstein AH. 2007. What the towers don't see at night: nocturnal sap flow in trees and shrubs at two AmeriFlux sites in California. Tree Physiology 27:597–610. 10.1093/treephys/27.4.597 [DOI] [PubMed] [Google Scholar]
- Gimeno L, Stohl A, Trigo RM, Dominguez F, Yoshimura K, Yu L, Drumond A, Durán-Quesada AM, Nieto R. 2012. Oceanic and terrestrial sources of continental precipitation. Reviews of Geophysics 50: 10.1029/2012RG000389. 10.1029/2012RG000389 [DOI] [Google Scholar]
- Godbold D, Tullus A, Kupper P, Sõber J, Ostonen I, Godbold JA, Lukac M, Ahmed IU, Smith AR. 2014. Elevated atmospheric CO2 and humidity delay leaf fall in Betula pendula, but not in Alnus glutinosa or Populus tremula× tremuloides. Annals of Forest Science 71:831–842. 10.1007/s13595-014-0382-4 [DOI] [Google Scholar]
- Hartmann H. 2011. Will a 385 million year-struggle for light become a struggle for water and for carbon?—How trees may cope with more frequent climate change-type drought events. Global Change Biology 17:642–655. 10.1111/j.1365-2486.2010.02248.x [DOI] [Google Scholar]
- Holtum JAM, Winter K. 2010. Elevated [CO2] and forest vegetation: more a water issue than a carbon issue? Functional Plant Biology 37:694–702. 10.1071/FP10001 [DOI] [Google Scholar]
- Housman DC, Naumburg E, Huxman TE, Charlet TN, Nowak RS, Smith SD. 2006. Increases in desert shrub productivity under elevated carbon dioxide vary with water availability. Ecosystems 9:374–385. 10.1007/s10021-005-0124-4 [DOI] [Google Scholar]
- Hovenden MJ, Miglietta F, Zaldei A, Vander Schoor JK, Wills KE, Newton PCD. 2006. The TasFACE climate-change impacts experiment: design and performance of combined elevated CO2 and temperature enhancement in a native Tasmanian grassland. Australian Journal of Botany 54:1–10. 10.1071/BT04194 [DOI] [Google Scholar]
- Hovenden MJ, Newton PCD, Willis KE. 2014. Seasonal not annual rainfall determines grassland biomass response to carbon dioxide. Nature 511:583–586. [DOI] [PubMed] [Google Scholar]
- Hsiao TC. 1973. Plant responses to water stress. Annual Review of Plant Physiology 24:519–570. 10.1146/annurev.pp.24.060173.002511 [DOI] [Google Scholar]
- Hungate BA, Dukes JS, Shaw MR, Luo Y, Field CB. 2003. Nitrogen and climate change. Science 302:1512–1513. 10.1126/science.1091390 [DOI] [PubMed] [Google Scholar]
- Iversen CM, Ledford J, Norby RJ. 2008. CO2 enrichment increases carbon and nitrogen input from fine roots in a deciduous forest. New Phytologist 179:837–847. 10.1111/j.1469-8137.2008.02516.x [DOI] [PubMed] [Google Scholar]
- Jacobs CMJ, de Bruin HAR. 1997. Predicting regional transpiration at elevated atmospheric CO2: influence of the PBL–vegetation interaction. Journal of Applied Meteorology 36:1663–1675. [DOI] [Google Scholar]
- Jarvis AJ, Mansfield TA, Davies WJ. 1999. Stomatal behaviour, photosynthesis and transpiration under rising CO2. Plant, Cell and Environment 22:639–648. 10.1046/j.1365-3040.1999.00407.x [DOI] [Google Scholar]
- Jarvis PG, McNaughton KG. 1986. Stomatal control of transpiration: scaling up from leaf to region. Advances in Ecological Research 15:49. [Google Scholar]
- Katsoulas N, Baille A, Kittas C. 2001. Effect of misting on transpiration and conductances of a greenhouse rose canopy. Agricultural and Forest Meteorology 106:233–247. 10.1016/S0168-1923(00)00211-2 [DOI] [Google Scholar]
- Keel SG, Pepin S, Leuzinger S, Körner C. 2007. Stomatal conductance in mature deciduous forest trees exposed to elevated CO2. Trees 21:151–159. [Google Scholar]
- Keenan TF, Gray J, Friedl MA, Toomey M, Bohrer G, Hollinger DY, Munger JW, O'Keefe J, Schmid HP, Wing IS, Yang B, Richardson AD. 2014. Net carbon uptake has increased through warming-induced changes in temperate forest phenology. Nature Climate Change 4:598–604. 10.1038/nclimate2253 [DOI] [Google Scholar]
- Knox R, Bisht G, Wang J, Bras R. 2011. Precipitation variability over the forest-to-nonforest transition in southwestern Amazonia. Journal of Climate 24:2368–2377. 10.1175/2010JCLI3815.1 [DOI] [Google Scholar]
- Kongstad J, Schmidt IK, Riis-Nielsen T, Arndal MF, Mikkelsen TN, Beier C. 2012. High resilience in heathland plants to changes in temperature, drought, and CO2 in combination: results from the CLIMAITE experiment. Ecosystems 15:269–283. 10.1007/s10021-011-9508-9 [DOI] [Google Scholar]
- Körner C. 2000. Biosphere responses to CO2 enrichment. Ecological Applications 10:1590–1619. [Google Scholar]
- Krogman KK. 1967. Evapotranspiration by irrigated grass as related to fertilizer. Canadian Journal of Plant Science 47:281–287. 10.4141/cjps67-050 [DOI] [Google Scholar]
- Kupper P, Sõber J, Sellin A, Lõhmus K, Tullus A, Räim O, Lubenets K, Tulva I, Uri V, Zobel M, Kull O, Sõber A. 2011. An experimental facility for free air humidity manipulation (FAHM) can alter water flux through deciduous tree canopy. Environmental and Experimental Botany 72:432–438. 10.1016/j.envexpbot.2010.09.003 [DOI] [Google Scholar]
- Leakey ADB. 2009. Rising atmospheric carbon dioxide concentration and the future of C4 crops for food and fuel. Proceedings of the Royal Society B: Biological Sciences 276:2333–2343. 10.1098/rspb.2008.1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuzinger S, Körner C. 2007. Water savings in mature deciduous forest trees under elevated CO2. Global Change Biology 13:2498–2508. [Google Scholar]
- Leuzinger S, Hättenschwiler S. 2013. Beyond global change: lessons from 25 years of CO2 research. Oecologia 171:639–651. 10.1007/s00442-012-2584-5 [DOI] [PubMed] [Google Scholar]
- Leuzinger S, Thomas RQ. 2011. How do we improve Earth system models? Integrating Earth system models, ecosystem models, experiments and long-term data. New Phytologist 191:15–18. 10.1111/j.1469-8137.2011.03778.x [DOI] [PubMed] [Google Scholar]
- Leuzinger S, Luo Y, Beier C, Dieleman W, Vicca S, Körner C. 2011. Do global change experiments overestimate impacts on terrestrial ecosystems? Trends in Ecology and Evolution 26:236–241. 10.1016/j.tree.2011.02.011 [DOI] [PubMed] [Google Scholar]
- Matyssek R, Wieser G, Ceulemans R, Rennenberg H, Pretzsch H, Haberer K, Löw M, Nunn AJ, Werner H, Wipfler P, Oßwald W, Nikolova P, Hanke DE, Kraigher H, Tausz M, Bahnweg G, Kitao M, Dieler J, Sandermann H, Herbinger K, Grebenc T, Blumenröther M, Deckmyn G, Grams TEE, Heerdt C, Leuchner M, Fabian P, Häberle KH. 2010. Enhanced ozone strongly reduces carbon sink strength of adult beech (Fagus sylvatica)—resume from the free-air fumigation study at Kranzberg Forest. Environmental Pollution 158:2527–2532. 10.1016/j.envpol.2010.05.009 [DOI] [PubMed] [Google Scholar]
- McNaughton KG, Jarvis PG. 1991. Effects of spatial scale on stomatal control of transpiration. Agricultural and Forest Meteorology 54:279–302. 10.1016/0168-1923(91)90010-N [DOI] [Google Scholar]
- Medlyn BE, Barton CVM, Broadmeadow MSJ, Ceulemans R, De Angelis P, Forstreuter M, Freeman M, Jackson SB, Kellomäki S, Laitat E, Rey A, Roberntz P, Sigurdsson BD, Strassemeyer J, Wang K, Curtis PS, Jarvis PG. 2001. Stomatal conductance of forest species after long-term exposure to elevated CO2 concentration: a synthesis. New Phytologist 149:247–264. 10.1046/j.1469-8137.2001.00028.x [DOI] [PubMed] [Google Scholar]
- Melillo JM, Steudler PA, Aber JD, Newkirk K, Lux H, Bowles FP, Catricala C, Magill A, Ahrens T, Morrisseau S. 2002. Soil warming and carbon-cycle feedbacks to the climate system. Science 298:2173–2176. 10.1126/science.1074153 [DOI] [PubMed] [Google Scholar]
- Morgan JA, Pataki DE, Körner C, Clark H, Del Grosso SJ, Grünzweig JM, Knapp AK, Mosier AR, Newton PCD, Niklaus PA, Nippert JB, Nowak RS, Parton WJ, Polley HW, Shaw MR. 2004. Water relations in grassland and desert ecosystems exposed to elevated atmospheric CO2. Oecologia 140:11–25. 10.1007/s00442-004-1550-2 [DOI] [PubMed] [Google Scholar]
- Morgan JA, Lecain DR, Pendall E, Blumenthal DM, Kimball BA, Carrillo Y, Williams DG, Heisler-White J, Dijkstra FA, West M. 2011. C4 grasses prosper as carbon dioxide eliminates desiccation in warmed semi-arid grassland. Nature 476:202–205. 10.1038/nature10274 [DOI] [PubMed] [Google Scholar]
- Nijs I, Kockelbergh F, Teughels H, Blum H, Hendrey G, Impens I. 1996. Free Air Temperature Increase (FATI): a new tool to study global warming effects on plants in the field. Plant, Cell and Environment 19:495–502. 10.1111/j.1365-3040.1996.tb00343.x [DOI] [Google Scholar]
- Nijs I, Ferris R, Blum H, Hendrey G, Impens I. 1997. Stomatal regulation in a changing climate: a field study using Free Air Temperature Increase (FATI) and Free Air CO2 Enrichment (FACE). Plant, Cell and Environment 20:1041–1050. 10.1111/j.1365-3040.1997.tb00680.x [DOI] [Google Scholar]
- Niklaus PA, Körner C. 2004. Synthesis of a six-year study of calcareous grassland responses to in situ CO2 enrichment. Ecological Monographs 74:491–511. 10.1890/03-4047 [DOI] [Google Scholar]
- Niklaus PA, Spinnler D, Körner C. 1998. Soil moisture dynamics of calcareous grassland under elevated CO2. Oecologia 117:201–208. 10.1007/s004420050649 [DOI] [PubMed] [Google Scholar]
- Norby RJ, Zak DR. 2011. Ecological lessons from free-air CO2 enrichment (FACE) experiments. Annual Review of Ecology, Evolution, and Systematics 42:181–203. 10.1146/annurev-ecolsys-102209-144647 [DOI] [Google Scholar]
- Norby RJ, DeLucia EH, Gielen B, Calfapietra C, Giardina CP, King JS, Ledford J, McCarthy HR, Moore DJP, Ceulemans R, De Angelis P, Finzi AC, Karnosky DF, Kubiske ME, Lukac M, Pregitzer KS, Scarascia-Mugnozza GE, Schlesinger WH, Oren R. 2005. Forest response to elevated CO2 is conserved across a broad range of productivity. Proceedings of the National Academy of Sciences of the USA 102:18052–18056. 10.1073/pnas.0509478102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norby RJ, Warren JM, Iversen CM, Medlyn BE, McMurtrie RE. 2010. CO2 enhancement of forest productivity constrained by limited nitrogen availability. Proceedings of the National Academy of Sciences of the USA 107:19368–19373. 10.1073/pnas.1006463107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick K, Oren R, Stoy P, Juang J-Y, Siqueira M, Katul G. 2009. The relationship between reference canopy conductance and simplified hydraulic architecture. Advances in Water Resources 32:809–819. 10.1016/j.advwatres.2009.02.004 [DOI] [Google Scholar]
- Owensby C, Ham J, Knapp A, Bremer D, Auen L. 1997. Water vapour fluxes and their impact under elevated CO2 in a C4-tallgrass prairie. Global Change Biology 3:189–195. 10.1046/j.1365-2486.1997.00084.x [DOI] [Google Scholar]
- Pappas C, Fatichi S, Rimkus S, Burlando P, Huber MO. 2015a. The role of local-scale heterogeneities in terrestrial ecosystem modeling. Journal of Geophysical Research: Biogeosciences 120:341–360. 10.1002/2014JG002735 [DOI] [Google Scholar]
- Pappas C, Fatichi S, Burlando P. 2015b. Modeling terrestrial carbon and water dynamics across climatic gradients: does plant diversity matter? New Phytologist. 10.1111/nph.13590. [DOI] [PubMed] [Google Scholar]
- Piao S, Sitch S, Ciais P, Friedlingstein P, Peylin P, Wang X, Ahlström A, Anav A, Canadell JG, Cong N, Huntingford C, Jung M, Levis S, Levy PE, Li J, Lin X, Lomas MR, Lu M, Luo Y, Ma Y, Myneni RB, Poulter B, Sun Z, Wang T, Viovy N, Zaehle S, Zeng N. 2013. Evaluation of terrestrial carbon cycle models for their response to climate variability and to CO2 trends. Global Change Biology 19:2117–2132. 10.1111/gcb.12187 [DOI] [PubMed] [Google Scholar]
- Roderick ML, Sun F, Lim WH, Farquhar GD. 2014. A general framework for understanding the response of the water cycle to global warming over land and ocean. Hydrology and Earth System Sciences 18:1575–1589. 10.5194/hess-18-1575-2014 [DOI] [Google Scholar]
- Rogiers SY, Clarke SJ. 2013. Nocturnal and daytime stomatal conductance respond to root-zone temperature in ‘Shiraz’ grapevines. Annals of Botany 111:433–444. 10.1093/aob/mcs298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustad L, Campbell J, Marion G, Norby R, Mitchell M, Hartley A, Cornelissen J, Gurevitch J, GCTE-NEWS. 2001. A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 126:543–562. 10.1007/s004420000544 [DOI] [PubMed] [Google Scholar]
- Schlesinger WH, Jasechko S. 2014. Transpiration in the global water cycle. Agricultural and Forest Meteorology 189–190:115–117. 10.1016/j.agrformet.2014.01.011 [DOI] [Google Scholar]
- Schulze E, Kelliher FM, Körner C, Lloyd J, Leuning R. 1994. Relationships among maximum stomatal conductance, ecosystem surface conductance, carbon assimilation rate, and plant nitrogen nutrition: a global ecology scaling exercise. Annual Review of Ecology and Systematics 25:629–660. 10.1146/annurev.es.25.110194.003213 [DOI] [Google Scholar]
- Schulze E-D, Beck E, Müller-Hohenstein K. 2002. Plant ecology. Heidelberg: Springer. [Google Scholar]
- Shaw MR, Zavaleta ES, Chiariello NR, Cleland EE, Mooney HA, Field CB. 2002. Grassland responses to global environmental changes suppressed by elevated CO2. Science 298:1987–1990. 10.1126/science.1075312 [DOI] [PubMed] [Google Scholar]
- Tardieu F, Simonneau T. 1998. Variability among species of stomatal control under fluctuating soil water status and evaporative demand: modelling isohydric and anisohydric behaviours. Journal of Experimental Botany 49:419–432. 10.1093/jxb/49.Special_Issue.419 [DOI] [Google Scholar]
- Tor-ngern P, Oren R, Ward EJ, Palmroth S, McCarthy HR, Domec J-C. 2015. Increases in atmospheric CO2 have little influence on transpiration of a temperate forest canopy. New Phytologist 205:518–525. 10.1111/nph.13148 [DOI] [PubMed] [Google Scholar]
- van der Ent RJ, Savenije HHG, Schaefli B, Steele-Dunne SC. 2010. Origin and fate of atmospheric moisture over continents. Water Resources Research 46: 10.1029/2010WR009127 10.1029/2010WR009127 [DOI] [Google Scholar]
- Volk M, Niklaus PA, Körner C. 2000. Soil moisture effects determine CO2 responses of grassland species. Oecologia 125:380–388. 10.1007/s004420000454 [DOI] [PubMed] [Google Scholar]
- Warren JM, Pötzelsberger E, Wullschleger SD, Thornton PE, Hasenauer H, Norby RJ. 2011. Ecohydrologic impact of reduced stomatal conductance in forests exposed to elevated CO2. Ecohydrology 4:196–210. 10.1002/eco.173 [DOI] [Google Scholar]
- Wild M, Folini D, Hakuba MZ, Schär C, Seneviratne SI, Kato S, Rutan D, Ammann C, Wood EF, König-Langlo G. 2015. The energy balance over land and oceans: an assessment based on direct observations and CMIP5 climate models. Climate Dynamics 44:3393–3429. 10.1007/s00382-014-2430-z [DOI] [Google Scholar]
- Wu Z, Dijkstra P, Koch GW, Peñuelas J, Hungate BA. 2011. Responses of terrestrial ecosystems to temperature and precipitation change: a meta-analysis of experimental manipulation. Global Change Biology 17:927–942. 10.1111/j.1365-2486.2010.02302.x [DOI] [Google Scholar]
- Zavaleta ES, Thomas BD, Chiariello NR, Asner GP, Shaw MR, Field CB. 2003. Plants reverse warming effect on ecosystem water balance. Proceedings of the National Academy of Sciences of the USA 100:9892–9893. 10.1073/pnas.1732012100 [DOI] [PMC free article] [PubMed] [Google Scholar]