Abstract
Hepatitis B virus (HBV) currently infects an estimated population of 2 billion individuals in the world, including 400 million people with chronic HBV infection. HBV virology, replication and the host’s immune response to HBV infection contribute to different infection outcomes. Acute hepatitis HBV infection is self-limiting but it leaves a residual infection that can become active in an individual during immunosuppression. In chronic HBV infection, the virus persistently replicates in hepatocytes leading to immune mediated hepatocellular damage. Despite the inability to remove the virus in more than 70 % of patients, current treatments for chronic HBV infection, interferon alpha and antiviral nucleotide/nucleoside analogues, aim to reduce viral replication to prevent or at least delay the progression to cirrhosis and hepatocellular carcinoma. In both self resolved acute and persistent HBV infection, the long term existence of chromatinised covalently closed circular DNA (cccDNA) in the nuclei of infected hepatocytes cannot be targeted by current treatments to eliminate these templates to eradicate the viral persistence. Identifying the mechanisms involve in the removal of infected hepatocytes will be useful as treatment options. In this context, DNA based novel therapeutic and immunization strategies might help to remove stable cccDNA and thus viral persistence.
Keywords: Hepatitis B virus, Natural history, Infection outcomes, Vaccination and treatment
Introduction
Hepatitis B virus (HBV) infection is a major public health problem in Southeast Asian, South Asian and African countries with increasingly high prevalence and mortality. HBV currently infects an estimated population 2 billion people globally, including 400 million individuals with chronic HBV infection [1, 2]. HBV transmission occurs via parenteral routes that include needle stick injury, blood transfusion, organ transplantation and sexual contact. Horizontal transmission from infected mothers to neonates is also an important route of transmission in areas where HBV is hyper-endemic. Patients such as hemophiliacs who undergo blood transfusion, individuals in occupations such as dentistry and surgery, health care staff in other disciplines, neonates of infected mothers and intravenous drug users are at high risk of HBV infection due to possible frequent exposures. HBV carriers can be a major source of infection for individuals who live in the same household and share razors or toothbrushes [1, 2].
The highest prevalence of HBV (>8 %) is seen sub-Saharan Africa, Southeast Asia, the South Pacific Islands and the Artic/sub-Artic region, including Western Alaska, the Baffin Archipelago of Canada and all of Greenland [1, 3]. Some regions have an intermediate HBV prevalence. These include much of the Mediterranean region, Southern Europe and North Africa, Eastern Europe, Russia, the Middle East, and the Indian subcontinent and parts of South America [1]. HBV transmission in endemic regions mainly occurs through chronically infected carrier mothers to neonates and by un-recognized accidental exposure to infected blood or body fluids, especially in children and adults who are in the same household as infected individuals. The prevalence of chronic HBV infection is inversely correlated with age at the time of infection, hence, >90 % perinatal infections progress to chronic HBV infection. Based on a long-term study conducted in Taiwan, >25 % of HBV infections in preschool children and <4 % of infections in adolescents progressed to chronicity [4, 5].
In regions where HBV is moderately (2–7 %) or less (<2 %) prevalent, infection of adults is very common. Adults can be infected via unprotected sexual intercourse or by intravenous drug use. It is also possible that HBV transmission is not restricted to those who practice risky behaviours. HBV is viable for a long time in the environment as shown in a study conducted by the Centre for Disease Control (CDC). A highly infective serum placed on a glass slide left in the environment for a week, was infective to a chimpanzee (McMahon 2005). This suggests that any kind of accidental exposure to articles contaminated by blood and body fluids of HBV carriers could also transmit the infection. In countries where HBV prevalence is low or moderate, immunization of health care workers is carried out as they are at increased risk. However, immunizing specific groups has not reduced the overall prevalence of HBV worldwide [1, 4]. Hence, in the last decade many countries have moved towards universal vaccination against HBV infection.
This review focuses on HBV infection with reference to infection outcomes, immune response to HBV, existing treatment options including prophylactic vaccination and new therapeutic approaches for chronic hepatitis B (CHB).
Natural history of HBV infections
Historical aspects
Although viral hepatitis dates back to the fifth century BC, it was only in 1963 that Blumberg and colleagues, who were searching for polymorphic serum proteins, discovered HBsAg in the blood of an Aboriginal Australian [6] and HBV was then distinguished from other serum hepatitis viruses [7, 8]. A few years later Dane and his colleagues showed the presence of virus-like particles (VLP) in the serum of HBV infected patients using immuno-electron microscopy [9]. Dane’s discovery was confirmed with the identification of an endogenous polymerase activity within HBV core particles [10]. Subsequently, the proteins and genome of the HBV were identified and HBV became the first recognized human hepatitis virus [10–12].
Basic properties of HBV
HBV belongs to the genus Orthohepadnavirus of the family Hepadnaviridae. The HBV virion is spherical with a diameter of 42 nm. The virion is composed of an envelope enclosing a capsid, which contains the viral DNA which is partially double stranded, and a polymerase protein [13]. The HBV virus has a genome of 3.2 kb which contains partially overlapping open reading frames (ORF): encoding for polymerase (P), core protein (C), envelope proteins (S) (which includes three forms of HBsAg called small (S), middle (M) and large (L)) and a transcriptional trans-activator protein (X), which is believed to be associated with oncogenesis in hepatocellular carcinoma (HCC) [13, 14].
HBV replication
Virus entry is believed to occur through receptor mediated endocytosis (RME), but the actual receptors involved have not been identified. A region within the pre-S1 of L-HBs has been identified as being involved in binding to hepatocytes [15]. More recent data suggest that pre-S1 of L governs the entry process. Only a small number of virions that have pre-S1 with the correct topology will bind and enter the host cell. Furthermore, monoclonal antibodies against amino acid (AA) 31–31 of the pre-S1-domain inhibit the attachment of HBV to the primary human hepatocytes (PHH) supporting the importance of pre-S1 in the entry process [16].
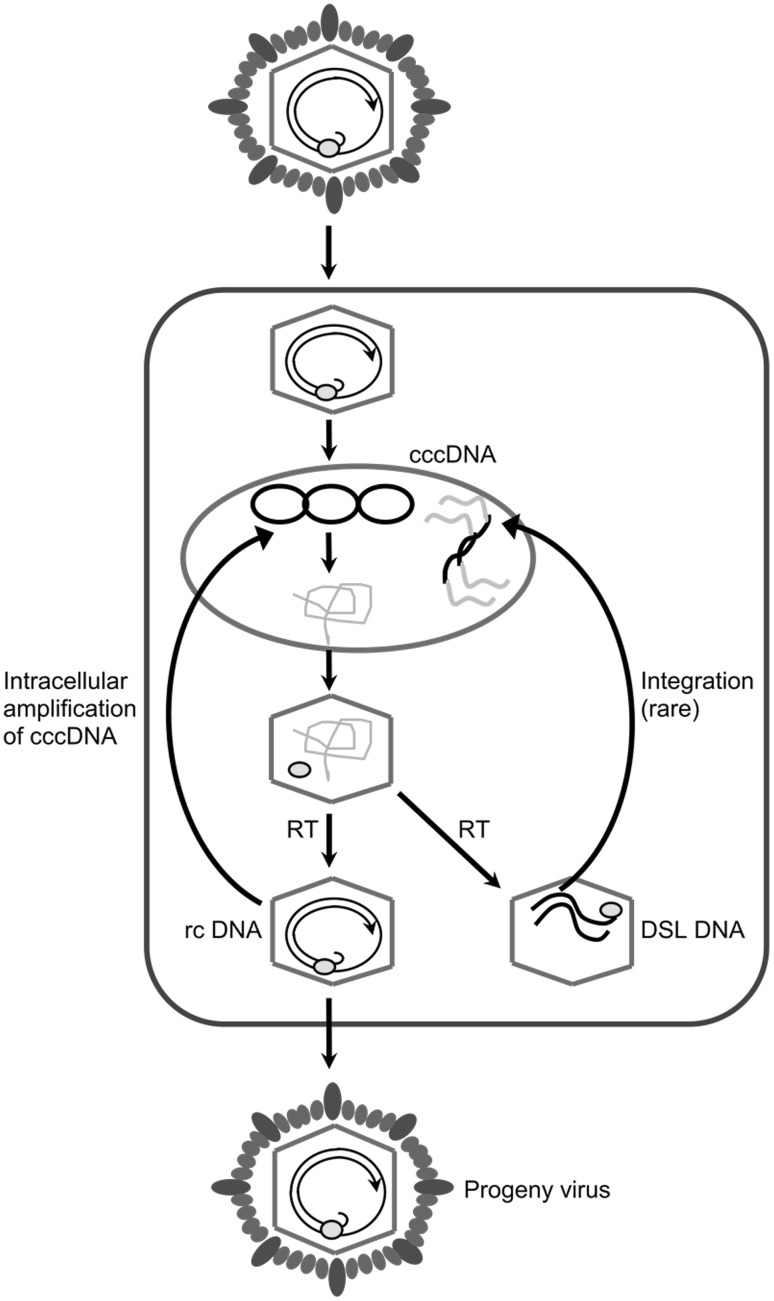
After entering the hepatocyte nucleus, the rcDNA genome is converted to covalently closed circular DNA (cccDNA) through a series of molecular changes such as completion of the positive DNA strand, removal of the covalently bound Pol, removal of the RNA primer, ligation of the gaps in the positive and negative strands, and supercoiling of the DNA [17, 18]. These cccDNA molecules are stable and act as a reservoir for HBV infection. Furthermore, cccDNA is highly resistant to anti-viral therapies and thus it is difficult to clear these molecules from the liver in the absence of hepatocyte division [19].
Immature nucleocapsids are formed by packaging of pgRNA within nucleocapsids in the cytoplasm of the cell [20]. After packaging of pgRNA, the viral polymerase facilitates the conversion of pgRNA to the rcDNA genome. Mature nucleocapsids containing rcDNA genomes are then recycled to the nucleus to form a pool of cccDNA (Fig. 1), ranging from 30 to 50 copies of cccDNA per cell [21], or are exported from the cell via the endoplasmic reticulum (ER), where nucleocapsids are enveloped in surface proteins embedded in host-derived lipid [22–24].
Fig. 1.
Schematic diagram showing the different stages of hepadnavirus life cycle during replication—adapted from Jilbert et al. [17]. cccDNA Covalently closed circular DNA, RT Reverse transcriptase, rcDNA Relaxed circular DNA, DSL DNA Double stranded linear DNA
However, there are more studies emerging on HBV virion formation and release. Surface protein synthesis occurs in the ER where the cytoplasmically preformed nucleocapsids are enveloped with these surface proteins and then released via the constitutional secretion pathway [25]. The envelopment results from highly co-ordinated biological interactions between the nucleocapsids and the cytoplasmically exposed pre-S region of the L protein. The S protein plays a major role in the secretion and the budding of virus particles. Budding of surface proteins without the nucleocapsids and virus genome into the ER lumen results in the production of sub-viral particles (SVPs) [24].
Infection outcomes
Hepadnavirus infection gives rise to two possible outcomes; acute or chronic infection [1]. In humans, 1–5 % of adults infected with HBV develop chronic infection, while the remainder clears the infection. Approximately 25–50 % of children infected with HBV at ages 1–5 develop chronic infection, as do 90 % of infants infected at birth, suggesting an age-dependent infection outcome [26].
Acute or transient HBV infection
An acute HBV infection occurs when HBsAg is eliminated from the serum and alanine aminotransferase (ALT) levels return to normal in less than 6 months. Although it was initially believed that HBV DNA is eliminated from the serum and liver, traces of DNA may be detectable by sensitive PCR assays [27]. Acute infection lasting for 6 months is quite unusual for a viral infection, as in most cases acute infections occur if the virus is rapidly eliminated, but given the long incubation period of HBV and the resultant slow development of the immune response, this is not surprising.
Strong cell-mediated immune (CMI) responses to surface, core and polymerase proteins are described during transient HBV infections, as well as humoral immune responses to the surface, core, e-antigen and polymerase proteins [28]. Both types of immune response can facilitate clearance of the virus. Anti-HBs antibodies produced by the humoral immune response are able to bind HBV surface proteins, which may serve two purposes. Firstly, they may block receptors necessary for attachment to hepatocytes, and secondly, they may facilitate the removal of virus from the blood by opsonising virus particles for engulfment by phagocytes [29]. T-cells generated by CMI response may facilitate clearance of the virus in two ways. Firstly, CD8+ T-cells may cause infected hepatocytes to undergo apoptosis and secondly, T-cells may secrete cytokines, which may lead to non-cytolytic clearance of the virus [30]. The relative contribution of the both types of immune response in the clearance of HBV infection in humans is not well understood.
In addition to adaptive immunity, innate immunity may also play a role in the clearance of hepadnavirus infections. An early innate immune response in many viral infections is the production of interferon alpha (IFN-α), interferon beta (IFN-β) and interleukin-2 (IL-2) [31]. In addition, various cells of the innate immune system may also have roles in clearing hepadnavirus infection. Natural killer (NK) cells have been shown to play a role in controlling hepadnavirus infections. They have both cytolytic and non-cytolytic antiviral functions, being able to induce apoptosis of cells by down-regulating expression of major histocompatibility complex (MHC) class I, and also producing antiviral cytokines such as IFN-α and tumour necrosis factor alpha (TNF-α) [32]. Macrophages, granulocytes and NK T cells may also play a role in clearance of hepadnavirus infections, although the role of these cells in viral clearance is poorly understood [33]. Other components of the innate immune response that may be important in clearance of viruses are the toll-like receptors (TLRs). These are a range of molecules able to recognise certain foreign structures which are also called pathogen-specific molecular patterns (PAMPs). They activate cell-signalling pathways leading to maturation of dendritic cells, expression of co-stimulatory molecules and secretion of type I interferons. The TLRs that have been shown to be activated during virus infection are TLR3, TLR9, TLR7, TLR8, TLR2 and TLR4 [34] and they may make some contribution to hepadnavirus clearance [35].
Chronic infection
Chronic HBV infection is defined as the failure to clear HBsAg from the serum of a HBV-infected individual for more than 6 months (McMahon 2005). There are three common phases in the natural history of chronic HBV infection (Fig. 2) [27].
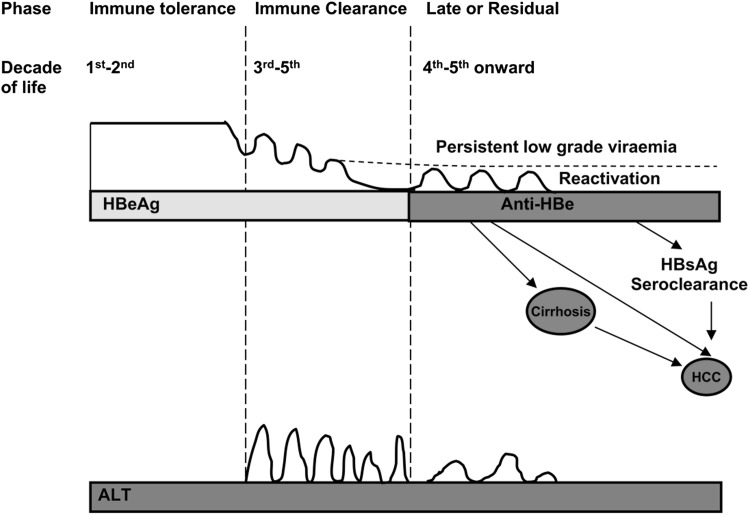
Fig. 2.
Natural history illustrating the different phases of chronic HBV infection—adapted from Yuen and Lai [39]. HBeAg HBV e antigen, HBsAg HBV surface antigen, HCC hepatocellular carcinoma, ALT alanine aminotransferase
The first phase is called the immune tolerance phase and this occurs when the infection is contracted during childhood and lasts for 1–2 decades. Individuals in this phase have high levels of serum HBV DNA (107–1011 copies/mL) with normal ALT levels as the liver necroinflammatory changes are minimal [36]. HBeAg, a truncated transcriptional product of HBV core gene, is present in the serum during immune tolerance phase. This scenario supports the postulates of HBeAg being a tolerising protein to induce T cell tolerance.
The second phase is called the immune clearance phase in which there is an increased level of HBcAg expression in the infected hepatocytes presented by the antigen presenting cells (APC) leading to immune mediated death of infected hepatocytes by the CTL response, resulting in increased ALT levels in the serum. However, the immune response is often not sufficient to eradicate the virus but it contributes to continued liver damage [37]. Based on the nature of the immune response during the immune clearance phase, chronically HBV infected (CHB) patients may develop fibrosis or cirrhosis of the liver [1, 27]. After repeated attempts of immune response, virus replication will be suppressed due to the reduced number of infected hepatocytes and then the CHB patients often seroconvert to anti-HBe antibodies [1, 38, 39].
Seroconversion to anti-HBe antibodies indicates entry into the third “residual” phase or “inactive” carrier state where patients have lower serum HBV DNA levels (Fig. 2) and persistently lower ALT levels [27]. This is a long phase where the patients have anti-HBe antibodies and relatively low levels of HBV DNA between 103 and 105 copies/mL. Although most patients have normal ALT levels, some may have mild to moderate elevations. During this phase 0.2–2.0 % of CHB patients may clear HBsAg from the serum and seroconvert to anti-HBs antibodies [1, 38, 39].
Approximately 30 % patients will undergo reactivation (sometimes called the fourth phase) with occasional reversion to HBeAg-positivity. Reactivation after the sero-conversion to anti-HBe antibodies is called HBeAg-negative chronic HBV infection. Some think that it is misleading to call reactivation the fourth phase because some patients may have continued liver damage and elevated ALT levels even after sero-converting anti-HBe antibodies [1, 38, 39]. Moreover, the majority of the patients who acquired the infection in childhood, after sero-converting to anti-HBe antibodies, will go on to the reactivation phase without symptoms of cirrhosis or hepatocellular carcinoma (HCC) [27, 40, 41].
Vaccines for prevention of HBV infection
In general two types of HBV vaccines are widely used for prophylaxis. The first is a plasma derived vaccine composed of HBsAg particles purified from the serum of chronic HBV carriers. The second is a genetically engineered recombinant vaccine where the HBV S gene is expressed in yeast via a plasmid vector to produce HBsAg VLP. Both of these HBV vaccines are highly immunogenic and result in the production of anti-HBs antibodies and restrict infections caused by all subtypes and genotypes of HBV. Three intramuscular doses of HBV vaccine confer protective immunity in 95–99 % of healthy infants, children, and young adults [4]. However, these vaccines fail to produce protective immunity in individuals who are more than 40 years of age and in individuals who are immuno-compromised [42]. Immunising neonates born to carrier mothers with immuno-globulins against HBV plus HBV vaccine can prevent the development of chronic infection in >90 % of neonates [4, 43]. Universal neonatal immunisation is practiced in some countries that experience hyper-endemic HBV infection. Adopting universal neonatal immunisation has been shown to reduce the number of HBV carriers and HBV associated primary liver cancer or HCC in Taiwan [4, 43].
The immune response to vaccination is assessed by measuring the anti-HBs level 6–8 weeks after the last dose of the vaccine. It is shown that about 5–10 % of immune-competent individuals do not develop a sufficient immune response to a standard HBV vaccination [44]. Non-responsiveness to HBV vaccination is defined as antibody titre of anti-HBs <10 mIU/mL. Anti-HBs levels between 10–100 mIU/mL is referred as hypo-responsiveness to the HBV vaccine and levels >100 mIU/mL is taken as high immune response. The anti-HBs levels of >10 mIU/mL is generally considered as protective against HBV infection [45]. In immuno-competent individuals booster of HBV vaccination is not recommended up to 20 years after completing the HBV vaccination. Most of the available data on immunity against HBV are done between 1 and 20 years after completing the HBV vaccination [46, 47]. However, in immuno-compromised or non-immune individuals booster of HBV vaccination is recommended [48].
Treatment for chronic HBV
In the majority of adults HBV infection is self-limiting and does not require any treatment similar to many other viral infections. In chronic HBV infection the virus persistently replicates in hepatocytes leading to immune mediated hepatocyte damage. Therapies have been instituted to inhibit viral replication to prevent or at least to delay the progression of hepatitis to cirrhosis and HCC. A number of prophylactic and treatment measures using HBV vaccines, immunomodulators such as interferon alpha (IFN-α) and pegylated IFN-α (peg IFN-α) and antiviral agents called nucleotide/nucleoside analogues (NAs) have been developed for the treatment of chronic HBV infection [49–51].
Currently approved therapies for chronic HBV infection
Currently approved therapies by the Food and Drug Administration (FDA) for chronic HBV infection are IFN-α and pegIFN-α and the NAs, 3TC, AFV and ETV. Permanent loss of HBV DNA including cccDNA and sero-conversion from HBeAg to anti-HBe antibodies and/or HBsAg to anti-HBsAg antibodies indicates a positive response to treatment. A positive response to treatment is usually associated with an improvement in necro-inflammation of the liver according to the protocols developed by the World Health Organisation (WHO) for therapy for chronic HBV infection [50–53]. However, more recent data suggest that loss of HBsAg gives a better SVR than the loss of HBV DNA among patients who respond to the therapy with pegIFN-α. Approximately 10 % pegIFN-α treated patients who lost HBsAg in the serum at the end of treatment endpoint eventually cleared HBV [54].
Antiviral resistance to NAs
In general, NA treatments are administered until the patients seroconvert to anti-HBs or anti-HBe antibodies [52]. In many clinical cases sero-conversion takes more than a year and in this time HBV becomes resistant to NA, creating a NA-resistant HBV mutant strain. Emergence of resistant strains is associated with a reduction in the viral fitness due to the inherent rate of mutations that occurs in HBV due to error-prone proof-reading capacity [51]. The selection of additional mutations that favour growth of the virus under a drug treatment modifies the resistance dynamics of the virus [55].
This has been shown by in vitro experiments as replication deficient phenotype of HBV or reduction plasma virus load in treated patients. However, under the selection pressure of a drug, the resistant clone of a HBV can replicate relatively faster due to the growth advantage [55].
Hence, drug resistance has been widely reported with NA monotherapy, which is the common treatment modality used against chronic hepatitis B virus infection [19, 52]. Conventional NA therapies also fail to reduce the levels of cccDNA in infected hepatocytes to a significantly low level at which the immune system can counteract to overcome the infection by targeting the HBV infected hepatocytes either through cytolytic killing or non-cytolytic mechanisms or both [19, 56].
Finding new treatment modalities using novel therapeutic approaches and enhancing existing therapies by combining the appropriate NAs to overcome the emergence of antiviral resistance will lead to better response to treatment for chronic HBV infection. Combination therapies have been shown to be more successful than the monotherapies for a number of infectious diseases such as tuberculosis and AIDS [56, 57].
Ongoing and future developments on CHB therapy
In the recent past DNA based treatments using nucleic acid polymers (NAP) have shown excellent anti-viral activity at preclinical levels [58, 59]. Results of a preliminary investigation show that two CHB patients that received weekly treatment of a particular NAP lost HBsAg and developed sustained virological response including anti-HBs response in some patients [60, 61]. This data is very promising as HBsAg loss and sero-conversion to anti-HBs antibodies are only rarely achieved with current NA monotherapy regimens even after 2 years of therapy in >90 % patients. Further studies in other CHB patients are underway.
NAPs also have future clinical applications in preventing rebound of HBV infection following liver transplantation. NAPs target the amphipathic domain(s) that is/are structurally conserved in enveloped viruses, independent of their sequence relatedness. This feature may make NAPs an attractive broad spectrum antiviral against many viruses including HBV, HCV, HIV and herpes viruses. NAPs appeared to interact with sequence independent but structurally conserved targets which would make the multiple mutations that occur during the emergence of antiviral resistance less likely. Hence, they would minimise the problems associated with antiviral resistance.
Promising results of a combination regimen of TFV and FTC against persistent duck HBV infection provides support for this approach in treating CHB patients, as suggested by studies in which this combination was used to treat HIV/HBV co-infected patients. More studies are needed to test the ability of TFV–FTC combination therapy to induce cccDNA clearance and prevent the rebound that commonly occurs following the cessation of NA monotherapy [62].
Another novel approach to treat CHB patients will be combining NAPs with conventional NA therapy. This will help to target multiple stages of HBV replication and can overcome the problems of antiviral resistance with better treatment outcomes in CHB patients. For example, such a combination antiviral therapy might be used in transplant recipients to effectively eliminate the risk of escape mutants and thus terminate HBV replication.
Concluding remarks
Despite the availability of a vaccine, consequences of the chronic HBV infection is still a major public health issue in South and Southeast Asian and African countries. HBV infection in the majority of adults results in an acute infection which even with a clinical hepatitis resolves. However, in children majority of the infections become mild but progress to a persistent infection resulting in continuous virus replication and hepatocellular damage due to immune mediated mechanisms. How some clear the virus from a HBV infection and others end up having persistent or chronic infection is also not clear. A few treatment options including IFN-α and NAs such as 3TC, AFV and ETV are currently available for CHB. However, permanent loss of HBV DNA including cccDNA which is the critical factor for viral persistence in chronic HBV infection is not removed by the current treatment modalities. A rare minority of the treated patients responds to treatment and has improvement in necro-inflammation of the liver with sero-conversion to anti-HBs. More recent data suggest that loss of HBsAg gives a better SVR than the loss of HBV DNA among patients who respond to pegIFN-α therapy. Novel treatment strategies including the DNA based antiviral agents as mono or combination therapies that target the cccDNA will be handy in achieving the removal of viral persistence and thus a resolution of infection in individuals with CHB.
References
- 1.McMahon BJ. Epidemiology and natural history of hepatitis B. Semin Liver Dis. 2005;25(S1):3–8. doi: 10.1055/s-2005-915644. [DOI] [PubMed] [Google Scholar]
- 2.Lin CL, Kao JH. Hepatitis B viral factors and clinical outcomes of chronic hepatitis B. J Biomed Sci. 2008;15(2):137–145. doi: 10.1007/s11373-007-9225-8. [DOI] [PubMed] [Google Scholar]
- 3.Ocama P, Opio CK, Lee WM. Hepatitis B virus infection: current status. Am J Med. 2005;118(12):1413. doi: 10.1016/j.amjmed.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 4.Mast EE, Margolis HS, Fiore AE, Brink EW, Goldstein ST, Wang SA, Moyer LA, Bell BP, Alter MJ. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep. 2005;54(RR-16):1–31. [PubMed] [Google Scholar]
- 5.Osiowy C, Gordon D, Borlang J, Giles E, Villeneuve JP. Hepatitis B virus genotype G epidemiology and co-infection with genotype A in Canada. J Gen Virol. 2008;89(12):3009–3015. doi: 10.1099/vir.0.2008/005124-0. [DOI] [PubMed] [Google Scholar]
- 6.Blumberg BS, Alter HJ, Visnich S. A new antigen in leukemia sera. JAMA. 1965;191:541–546. doi: 10.1001/jama.1965.03080070025007. [DOI] [PubMed] [Google Scholar]
- 7.Blumberg BS. Australia antigen and the biology of hepatitis B. Science. 1977;197(4298):17–25. doi: 10.1126/science.325649. [DOI] [PubMed] [Google Scholar]
- 8.Brunello F, Emanuelli G, Camussi G. Markers of type B viral hepatitis. Minerva Med. 1979;70(41):2791–2800. [PubMed] [Google Scholar]
- 9.Alberti A, Diana S, Scullard GH, Eddleston WF, Williams R. Full and empty Dane particles in chronic hepatitis B virus infection: relation to hepatitis B e antigen and presence of liver damage. Gastroenterology. 1978;75(5):869–874. [PubMed] [Google Scholar]
- 10.He BG, Melnick JL, Siddiqui A, Robinson WS, Law SW, Lai EC. Molecular cloning and characterization of the cDNA coding for hepatitis B virus surface antigen. Sci Sin [B] 1985;28(1):49–59. [PubMed] [Google Scholar]
- 11.Dejean A, Brechot C, Tiollais P, Wain-Hobson S. Characterization of integrated hepatitis B viral DNA cloned from a human hepatoma and the hepatoma-derived cell line PLC/PRF/5. Proc Natl Acad Sci. 1983;80(9):2505–2509. doi: 10.1073/pnas.80.9.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi M, Koike K. Complete nucleotide sequence of hepatitis B virus DNA of subtype adr and its conserved gene organization. Gene. 1984;30(1–3):227–232. doi: 10.1016/0378-1119(84)90124-0. [DOI] [PubMed] [Google Scholar]
- 13.Kramvis A, Kew M, François G. Hepatitis B virus genotypes. Vaccine. 2005;23(19):2409–2423. doi: 10.1016/j.vaccine.2004.10.045. [DOI] [PubMed] [Google Scholar]
- 14.Norder H, Couroucé AM, Coursaget P, Echevarria JM, Lee SD, Mushahwar IK, Robertson BH, Locarnini S, Magnius LO. Genetic diversity of hepatitis B virus strains derived worldwide: genotypes, subgenotypes, and HBsAg subtypes. Intervirology. 2004;47(6):289–309. doi: 10.1159/000080872. [DOI] [PubMed] [Google Scholar]
- 15.Neurath AR, Kent SB, Strick N, Parker K. Identification and chemical synthesis of a host cell receptor binding site on hepatitis B virus. Cell. 1986;46(3):429–436. doi: 10.1016/0092-8674(86)90663-X. [DOI] [PubMed] [Google Scholar]
- 16.Glebe D, Urban S. Viral and cellular determinants involved in hepadnaviral entry. World J Gastroenterol. 2007;13(1):22–38. doi: 10.3748/wjg.v13.i1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jilbert AR, Mason WS, Kann M. Hepatitis B Virus Replication. In: Locarnini S, Lai C-L, editors. Hepatitis B Virus. London, Atlanta: International Medical Press; 2008. pp. 4.1–4.13. [Google Scholar]
- 18.Kim DH, Ni Y, Lee SH, Urban S, Han KH. An anti-viral peptide derived from the pre-S1 surface protein of hepatitis B virus. BMB Rep. 2008;41(9):640–644. doi: 10.5483/BMBRep.2008.41.9.640. [DOI] [PubMed] [Google Scholar]
- 19.Zoulim F. Combination of nucleoside analogues in the treatment of chronic hepatitis B virus infection: lesson from experimental models. J Antimicrob Chemother. 2005;55(5):608–611. doi: 10.1093/jac/dki095. [DOI] [PubMed] [Google Scholar]
- 20.Bartenschlager R, Schaller H. Hepadnaviral assembly is initiated by polymerase binding to the encapsidation signal in the viral RNA genome. EMBO J. 2008;11(9):3413–3420. doi: 10.1002/j.1460-2075.1992.tb05420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newbold JE, Xin H, Tencza M, Sherman G, Dean J, Bowden S, Locarnini S. The covalently closed duplex form of the hepadnavirus genome exists in situ as a heterogeneous population of viral minichromosomes. J Virol. 1995;69(6):3350–3357. doi: 10.1128/jvi.69.6.3350-3357.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Block TM, Guo H, Guo JT. Molecular virology of hepatitis B virus for clinicians. Clin Liver Dis. 2007;11(4):685–706. doi: 10.1016/j.cld.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruss V. Hepatitis B virus morphogenesis. World J Gastroenterol. 2007;13(1):65–73. doi: 10.3748/wjg.v13.i1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mhamdi M, Funk A, Hohenberg H, Will H, Sirma H. Assembly and budding of a hepatitis B virus is mediated by a novel type of intracellular vesicles. Hepatology. 2007;46(1):95–106. doi: 10.1002/hep.21666. [DOI] [PubMed] [Google Scholar]
- 25.Funk A, Mhamdi M, Hohenberg H, Heeren J, Reimer R, Lambert C, Prange R, Sirma H. Duck hepatitis B virus requires cholesterol for endosomal escape during virus entry. J Virol. 2008;82(21):10532–10542. doi: 10.1128/JVI.00422-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sprengers D, Janssen HL. Immunomodulatory therapy for chronic hepatitis B virus infection in children. Fundam Clin Pharmacol. 2005;19(4):447. doi: 10.1111/j.1472-8206.2005.00362.x. [DOI] [PubMed] [Google Scholar]
- 27.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2001;34(6):1225–1241. doi: 10.1053/jhep.2001.29401. [DOI] [PubMed] [Google Scholar]
- 28.Kara IH, Yilmaz ME, Suner A, Kadiroglu AK, Isikoglu B. The evaluation of immune responses that occur after HBV infection and HBV vaccination in hemodialysis patients. Vaccine. 2004;22(29–30):3963–3967. doi: 10.1016/j.vaccine.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Akbar SM, Abe M, Masumoto T, Horiike N, Onji M. Mechanism of action of vaccine therapy in murine hepatitis B virus carriers: vaccine-induced activation of antigen presenting dendritic cells. J Hepatol. 1999;30(5):755–764. doi: 10.1016/S0168-8278(99)80125-1. [DOI] [PubMed] [Google Scholar]
- 30.Guidotti LG, Rochford R, Chung J, Shapiro M, Purcell R, Chisari FV. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284(5415):825–829. doi: 10.1126/science.284.5415.825. [DOI] [PubMed] [Google Scholar]
- 31.Weber F, Kochs G, Haller O. Inverse interference: how viruses fight the interferon system. Viral Immunol. 2004;17(4):498–515. doi: 10.1089/vim.2004.17.498. [DOI] [PubMed] [Google Scholar]
- 32.Scully LJ, Brown D, Lloyd C, Shein R, Thomas HC. Immunological studies before and during interferon therapy in chronic HBV infection: identification of factors predicting response. Hepatology. 1990;12(5):1111–1117. doi: 10.1002/hep.1840120506. [DOI] [PubMed] [Google Scholar]
- 33.Dandri M, Petersen J. Hepatitis B virus cccDNA clearance: killing for curing? Hepatology. 2005;42(6):1453–1455. doi: 10.1002/hep.20976. [DOI] [PubMed] [Google Scholar]
- 34.Rosenthal KL. “Tweaking innate immunity: the promise of innate immunologicals as anti-infectives. Can J Infect Dis Med Microbiol. 2006;17(5):307–314. doi: 10.1155/2006/195957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Visvanathan K, Skinner NA, Thompson AJ, Riordan SM, Sozzi V, Edwards R, Rodgers S, Kurtovic J, Chang J, Lewin S, Desmond P, Locarnini S. Regulation of Toll-like receptor-2 expression in chronic hepatitis B by the precore protein. Hepatology. 2007;45(1):102–110. doi: 10.1002/hep.21482. [DOI] [PubMed] [Google Scholar]
- 36.Valsamakis A. Molecular testing in the diagnosis and management of chronic hepatitis B. Clin Microbiol Rev. 2007;20(3):426–439. doi: 10.1128/CMR.00009-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rehermann B. Intrahepatic T cells in hepatitis B: viral control versus liver cell injury. J Exp Med. 2000;191(8):1263–1268. doi: 10.1084/jem.191.8.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48(2):335–352. doi: 10.1016/j.jhep.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 39.Yuen MF, Lai CL. In: The natural history of chronic hepatitis B. Locarnini S, Lai CL, editors. London, Atlanta: International Medical Press; 2008. pp. 12.1–12.11. [Google Scholar]
- 40.Villeneuve JP. The natural history of chronic hepatitis B virus infection. J Clin Virol. 2005;34(1):S139–S142. doi: 10.1016/S1386-6532(05)80024-1. [DOI] [PubMed] [Google Scholar]
- 41.Dai CY, Chuang WL, Huang JF, Yu ML. Hepatitis B e antigen-negative patients with persistently normal alanine aminotransferase levels and hepatitis B virus DNA >2000 IU/mL. Hepatology. 2009;49(2):705–706. doi: 10.1002/hep.22723. [DOI] [PubMed] [Google Scholar]
- 42.Fabrizi F, Martin P, Dixit V, Bunnapradist S, Dulai G. Meta-analysis: the effect of age on immunological response to hepatitis B vaccine in end-stage renal disease. Aliment Pharmacol Ther. 2004;20(10):1053–1062. doi: 10.1111/j.1365-2036.2004.02264.x. [DOI] [PubMed] [Google Scholar]
- 43.Mancini-Bourgine M, Fontaine H, Scott-Algara D, Pol S, Brechot C, Michel ML. Induction or expansion of T-cell responses by a hepatitis B DNA vaccine administered to chronic HBV carriers. Hepatology. 2004;40(4):874–882. doi: 10.1002/hep.20408. [DOI] [PubMed] [Google Scholar]
- 44.Harley EJ, Kellner A. A controlled clinical trial of the efficacy of the hepatitis B vaccine (Heptavax B) Hepatology. 1981;1(5):377–385. doi: 10.1002/hep.1840010502. [DOI] [PubMed] [Google Scholar]
- 45.Zuckerman J. Immune response to a new hepatitis B vaccine in healthcare workers who had not responded to standard vaccine: randomised double blind dose-response study. BMJ. 1997;314(7077):329–333. doi: 10.1136/bmj.314.7077.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McMahon BJ. Antibody levels and protection after hepatitis B vaccine: results of a 22- year follow-up study and response to a booster dose. J Infect Dis. 2009;200:1390–1396. doi: 10.1086/606119. [DOI] [PubMed] [Google Scholar]
- 47.Chathuranga LS, Noordeen F, Abeykoon AM. Immune response to hepatitis B vaccine in a group of health care workers in Sri Lanka. Int J Infect Dis. 2013;17(11):e1078–e1079. doi: 10.1016/j.ijid.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 48.Leuridan E, Van Damme P. Hepatitis B and the need for a booster dose. CID. 2011;53:68–75. doi: 10.1093/cid/cir270. [DOI] [PubMed] [Google Scholar]
- 49.Hadziyannis SJ, Papatheodoridis GV, Vassilopoulos D. Treatment of HBeAg-negative chronic hepatitis B. Semin Liver Dis. 2003;23(1):81–88. doi: 10.1055/s-2003-37584. [DOI] [PubMed] [Google Scholar]
- 50.Younger HM, Bathgate AJ, Hayes PC. Review article: nucleoside analogues for the treatment of chronic hepatitis B. Aliment Pharmacol Ther. 2004;20(11–12):1211–1230. doi: 10.1111/j.1365-2036.2004.02211.x. [DOI] [PubMed] [Google Scholar]
- 51.Fournier C, Zoulim F. Combination therapy in chronic hepatitis B. Gastroenterol Clin Biol. 2008;32(1 Pt 2):S42–S49. doi: 10.1016/S0399-8320(08)73264-0. [DOI] [PubMed] [Google Scholar]
- 52.Marcellin P, Asselah T, Boyer N. Treatment of chronic hepatitis B. J Viral Hepat. 2005;12(4):333–345. doi: 10.1111/j.1365-2893.2005.00599.x. [DOI] [PubMed] [Google Scholar]
- 53.Rapti I, Dimou E, Mitsoula P, Hadziyannis SJ. Adding-on versus switching-to adefovir therapy in lamivudine-resistant HBeAg-negative chronic hepatitis B. Hepatology. 2007;45(2):307–313. doi: 10.1002/hep.21534. [DOI] [PubMed] [Google Scholar]
- 54.Moucari R, Mackiewicz V, Lada O, Ripault MP, Castelnau C, Martinot-Peignoux M, Dauvergne A, Asselah T, Boyer N, Bedossa P, Valla D, Vidaud M, Nicolas-Chanoine MH, Marcellin P. Early serum HBsAg drop: a strong predictor of sustained virological response to pegylated interferon alfa-2a in HBeAg-negative patients. Hepatology. 2009;49(4):1151–1157. doi: 10.1002/hep.22744. [DOI] [PubMed] [Google Scholar]
- 55.Locarnini S, Bathholomeusz A. Emergence and patterns of drug resistance. London, Atlanta: International Medical Press; 2008. pp. 12.1–12.11. [Google Scholar]
- 56.Liaw YF. Therapy of chronic hepatitis B: current challenges and opportunities. J Viral Hepat. 2002;9(6):393–399. doi: 10.1046/j.1365-2893.2002.00388.x. [DOI] [PubMed] [Google Scholar]
- 57.Zoulim F. Antiviral therapy of chronic hepatitis B: can we clear the virus and prevent drug resistance? Antivir Chem Chemother. 2004;15(6):299–305. doi: 10.1177/095632020401500602. [DOI] [PubMed] [Google Scholar]
- 58.Noordeen F, Vaillant A, Jilbert AR. Nucleic acid polymers inhibit duck hepatitis B virus infection in vitro. Antimicrob Agents Chemother. 2013;57(11):5291–5298. doi: 10.1128/AAC.01003-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Noordeen F, Vaillant A, Jilbert AR. Nucleic acid polymers prevent the establishment of duck hepatitis B virus infection in vivo. Antimicrob Agents Chemother. 2013;57(11):5299–5306. doi: 10.1128/AAC.01005-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Al-Mahtab M, Bazinet M, Vaillant A. Preliminary evidence of rapid HBsAg seroconversion in patients with chronic hepatitis B (CHB) treated with a DNA-based amphipathic polymer. Antivir Res. 2010;86(1):A28. doi: 10.1016/j.antiviral.2010.02.358. [DOI] [Google Scholar]
- 61.Al-Mahtab M, Bazinet M, Vaillant A. REP 9AC is a potent HBsAg release inhibitor which clears serum HBSAg and elicits SVR in patients with chronic hepatitis B. J Hepatol. 2011;54(1):S34. doi: 10.1016/S0168-8278(11)60077-9. [DOI] [Google Scholar]
- 62.Noordeen F. Development of antiviral therapies for chronic hepatitis B virus infection. Digital research theses collection of University of Adelaide, 09PH N818. 2010. http://library.adelaide.edu.au/item/1523542.