Abstract
Purpose
The development of photoreceptor replacement therapy for retinal degenerative disorders requires the identification of the optimal cell source and immunosuppressive regimen in a large animal model. Allotransplants are not acutely rejected in swine subretinal space, although it is not known if survival can be improved with immunosuppression. Here we investigated the survival and integration of expanded pig retinal progenitor cells (pRPCs) in normal recipients with and without transient anti-inflammatory suppression.
Methods
pRPCs were derived from the neural retina of E60 GFP transgenic pigs, expanded for six passages, characterized, and transplanted into the subretinal space of 12 pigs. Six recipients received a single intravitreal injection of rapamycin and dexamethasone.
Results
pRPCs expressed the photoreceptor development genes Sox2, Pax6, Lhx2, Crx, Nrl, and Recoverin in vitro. Transplanted cells were identified in 9 out of 12 recipients 4 weeks after the injection. pRPCs integrated primarily into the photoreceptor inner segment layer and outer nuclear layer with single cells present in the inner nuclear layer. Donor cells remained recoverin-positive and acquired rhodopsin. We did not observe any signs of graft proliferation. The immunosuppression did not affect the survival or distribution of grafts. No macrophage infiltration or loss of retinal structure was observed in either group.
Conclusions
Local immunosuppression with rapamycin and dexamethasone does not improve the outcome of pRPC allotransplantation into the subretinal space.
Translational Relevance
Survival and integration of pRPC together with the lack of graft proliferation suggests that allogeneic RPC transplantation without transient immunosuppression is a favorable approach for photoreceptor cell replacement.
Keywords: retina, photoreceptors, retinal progenitor cells, cell therapy, rapamycin
Introduction
The success of cell therapies in retinal degenerative diseases in which cell replacement is a prerequisite depends in part on a combination of three factors: survival, integration, and differentiation. In order to positively influence these factors, conjoint treatments with anti-inflammatory and immunosuppressive agents are being considered. Cells are often administered directly into the affected subretinal anatomical site. Due to the surgical nature of targeted delivery of cells into the subretinal space, procedure-related inflammation is likely. This can be controlled for by the use of either systemically or locally administered anti-inflammatory drugs in the immediate days after surgical intervention. The choice of anti-inflammatory agents, the duration, and the route of administration are often guided by previous experience and published work. However, the major factor to consider and overcome in all cell therapies is rejection of the transplanted cells. In the nonclinical setting, this factor is compounded by the xenograft nature of preclinical investigations of human cells intended for therapeutic application. Xenograft transplantation studies often use immunosuppressive agents to control for rejection or deploy genetically engineered immunocompromised species. Previously we and others have shown that retinal progenitor cells/photoreceptor precursors integrate into the host retina and differentiate into mature photoreceptors after transplantation into the subretinal space.1–4 While there are no current clinical studies on photoreceptor replacement, retinal pigment epithelium cells5 and neural progenitor cells6 have recently been transplanted to patients as allografts with a comprehensive immunosuppressive regimen.
The necessity of immunosuppression, however, remains an open question and success can be variable even with similar treatment regimes in allograft or syngeneic studies.7
The healthy eye is considered an immune-privileged organ with a blood–tissue barrier, immunosuppressive microenvironment, and ability to induce a systemic form of a tolerance.8 However, in retinal degenerative disease, these passive and active barriers are compromised, and transplantation itself leads to the infiltration of the retina with macrophages/neutrophils. The potential negative effect of the immunosuppressive drugs on the retina and transplanted cells also has to be considered.
Although subretinal injections are always accompanied with glia activation and gliosis, mouse-to-mouse subretinal syngeneic transplants do not cause distinctive immune response, leading to good survival of cells. The immune component of photoreceptor precursor allotransplantation has not been studied in small rodent models, although RPE9 and pancreatic islets10 survived for several months as allografts. Cyclosporine treatment does not improve the long-term survival in a rabbit model.11
We have shown that pRPC allotransplants are well tolerated in wild-type12 and retinal degenerative pigs,13 while other groups have demonstrated this for freshly isolated4 and iPSc-derived14 photoreceptor precursors.
The most important information comes from clinical observations15 showing that without immunosuppression the rejection rate remains high, possibly due to major histocompatibility complex (MHC) II expression.16 This led to the inclusion of the comprehensive immunosuppressive regimen (tacrolimus and mycophenolate mofetil) into the ongoing clinical trials of subretinal RPE transplants for retinal degenerative disorders.5 Retinal progenitor cells do not express MHC II in vitro in the culture conditions described below, although 100% of cells express MHC I.17
Due to all the above information, the necessity and choice of potential local and systemic immunosuppressive and anti-inflammatory therapy for RPC allotransplantation remain an open question with several options available.18 In the current allograft study, we evaluated the effect on survival and engraftment of a single intravitreal injection of rapamycin (sirolimus)19 and dexamethasone20 from several local regimens that have been tested previously: fujimycin (tacrolimus),21 triamcinolone,22 cyclosporine,23 and amethopterin (methotrexate).24 Cyclosporine A and other calcineurin inhibitors prevent the subsequent activation of T-cells by reducing interleukin (IL)-2 production. Rapamycin prevents the activation of immunocompetent cells by IL-2 through mTORC1 inhibition. It has been used as a systemic and local immunosuppressive agent including intravitreal injection as a route of delivery. Rapamycin and dexamethasone combination was chosen for this study due to their reported minimal side effects and retinal toxicity compared with the other listed agents.
Materials and Methods
Pig Retinal Progenitor Cell (pRPC) Isolation and Culture
pRPC were isolated as previously described for human and pRPCs.25,26 Eyecups from E60 embryos of EGF-transgenic pigs27 were collected, dissected with neuroretinas peeled from retinal pigment epithelium, minced, and digested in Collagenase IV (Sigma-Aldrich, St. Louis, MO) with gentle stirring. Pooled cells and clumps were transferred to fibronectin-coated (Akron, Boca Raton, FL) flasks and cultured in Ultraculture medium, supplemented with 10 ng/mL rh bFGF (Peprotech, Rock Hills, NJ) and 20 ng/mL rh EGF at 3% O2, 5% CO2, and 37°C. Cells were passaged upon reaching 80% confluency using recombinant trypsin (Trypzean, Sigma-Aldrich), defined trypsin inhibitor (DTI, Gibco, Grand Island, NY), and benzonase (EMD Chemicals, Billerica, MA). pRPCs were replated at a density of 10,000 cells/cm2 in the medium described above. For passages, two to five cells were passaged every 48 hours, which was sufficient to reach the required 75% to 85% confluency. At passage 5, pRPCs were frozen in cryomedium: 10% dimethylsufoxide (DMSO) in Ultraculture medium.
Cell Preparation for Transplantation
Frozen pRPCs were thawed and plated at a density of 10,000 viable cells/cm2 in the medium described above and incubated in 20% oxygen incubator. Two days later, cells were collected using Trypzean, DTI, and benzonase, centrifuged at 300 g, counted, washed once with Hanks Balacned Salt Solution (HBSS), and formulated as a suspension of 50,000 cells/μL in HBSS-N-acetyl-L-cysteine (HBSS-NAC). We have previously described the effectiveness of NAC for maintaining human RPC viability in suspension on ice, and it has been shown as a potent protector against reactive-oxygen species induced cell death.28 To test the efficacy of NAC for pRPC formulation, we have compared the viability of pRPC, formulated in HBSS versus HBSS-NAC by Trypan blue. All the injections were performed within 6 hours of cell formulation.
Animals, Cell Transplantation, and Immunosuppressive Therapy
Animal work was performed according to ARVO recommendations for animal research and has been approved by University of Goias animal care committee. For transplantation all animals were preanesthetized with intramuscular injections of 15 mg midazolam and a composition consisting of zolazepam 11.9 mg/mL and tiletamine 11.9 mg/mL mixed with xylazine 12.38 mg/mL, ketamine 14.29 mg/mL (Intervet, Madison, NJ), and methadone 2.38 mg/mL. The pigs underwent endotracheal intubation and were artificially ventilated with 2% to 3% isoflurane in combination with oxygen. Stroke volume (300 mL/stroke) and respiratory frequency (12/minute) were kept constant throughout. In each case, the left eye was treated with topical drops consisting of a combination of 0.4% oxybuprocaine, 10% Metaoxedrin, 0.5% Mydriacyl, 1% atropine, and 5% povidone–iodine. At surgery, the central and posterior vitreous was removed together with the posterior hyaloid membrane using a three-port pars plana 23G vitrectomy.29 One hundred microliters of cell suspension in HBSS-NAC were injected through a 38-gauge needle connected to a 1-mL Hamilton syringe close to the visual streak 4- to 5-mm supero-medial to optic nerve head, avoiding vessels of the arcade. The rate of injection was approximately 200 μL/minute, with an air bubble at the end to seal the retinotomy.
Following the subretinal injection, 250 μg of rapamycin and 400 μg of dexamethasone were administered as a single intravitreal injection in six animals. This dose of rapamycin has been shown to form a depot in the vitreous in a rabbit model and provides a therapeutic concentration for at least 1 month.30 The half-life of dexamethasone is much shorter: It is cleared in less than 4 hours after intravitreal administration.31
Immunocytochemistry and Reverse Transcription–Polymerase Chain Reaction (RT-PCR)
For immunocytochemical analysis, frozen pRPC at passage 5 were revived and plated on poly-D-lysine (Sigma-Aldrich) and fibronectin-coated 16-well glass chamber slide. Twenty-four hours later, slides with cells were fixed in Davidson solution for 15 minutes, washed with phosphate-buffered saline (PBS)-T, blocked with 10% goat serum (GeneTex) for 1 hour, and then incubated with primary antibodies (Supplementary Figure 2) at 4°C. Next day slides were washed three times with PBS-T and incubated with secondary Cy-3 conjugated goat secondaries (Jackson Immunoresearch) at 1:400 dilution for 1 hour at room temperature. After triple washed nuclei were counterstained with DAPI (400 ng/mL), the slides were mounted.
For RT-PCR analysis, total mRNA from pRPC was isolated using purelink mRNA kit (Life Technologies). cDNA was synthetized using SuperScript first-strand kit (Life Technologies) with Oligo(dT) utilized as primers. PCR reaction was performed for 32 cycles using Platinum Taq polymerase (Life Technologies). Products were separated using 2% agarose gel and stained by SYBR Safe.
Histology
Four weeks after the injection, animals were euthanized by IV injection of pentobarbital. Eyes were enucleated and placed in 10% buffered neutral formalin. Whole eyes bisected in the sagittal plane were processed to paraffin blocks. Five-micrometer serial sections collected throughout the region of interest centered on the site of transplantation were stained with hematoxylin and eosin (H&E) or subjected to fluorescence immunohistochemistry (IHC) to determine pRPC survival, proliferation, and photoreceptor differentiation. Dual-label IHC was performed using a cocktail of anti-GFP (Abcam, ab13970 1:500) to detect GFP pRPCs and anti-Ki-67 (Vector, VP-K452 1:100; proliferation), anti-recoverin (Millipore, AB5585 1:500; cones), or mouse anti-rhodopsin (Millipore, MABN15 1:500; rods) antibodies. For infiltrating leukocytes/macrophages, we used anti-CD45 antibody (Abcam, 1:200). Primary antibodies were detected with species specific Alexa Fluor conjugates using Alexa 488 (green; Molecular Probes, A11039 1:300) for the GFP Alexa 568 (red; Molecular Probes, A11031, A11011 1:2000) to detect proliferation and differentiation markers. All tissue sections were counterstained with Hoechst 1 μg/mL in 0.1 M PBS.
Microscopic assessment of H&E stained sections was performed by light microscopy for histopathology. Fluorescently labeled sections were analyzed using epi-fluorescent optics. pRPC survival was quantified using a crude scoring system based on the presence (1, 2, or 3) or absence (0) of GFP pRPC. Based on the size of a bleb, we estimated that score 1 corresponds to less than 1000, score 2: 1000 to 10,000, and score 3: more than 10,000 GFP-positive cells.
Results
pRPC Isolation and Culture
In this study, we have observed that pig PRCs, isolated at E60, can be expanded in vitro without a decrease in proliferation for at least six passages. Cells in culture grow as a monolayer of spindle- or triangle-like cells (Fig. 1a).
Figure 1.
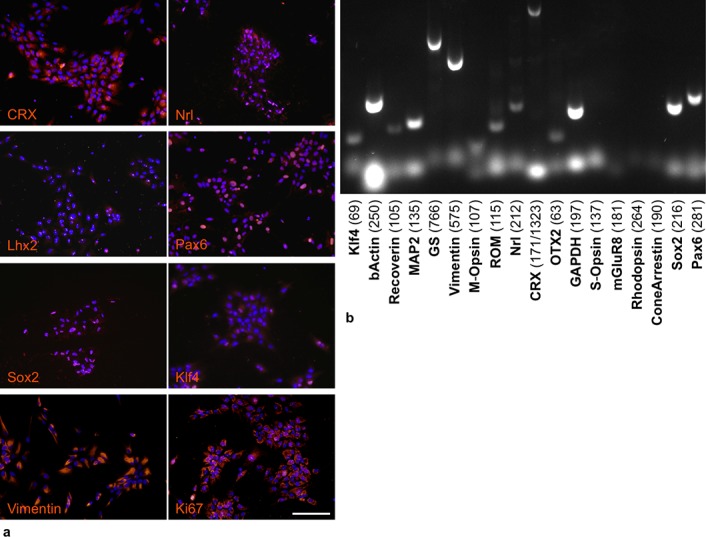
Characteristics of pRPC at passage 6 in vitro. By immunocytochemistry (a) we observed positive nuclear staining for development transcription factors Sox2, Klf4, Pax6, Lhx2, cone-rod homeobox (Crx), rod-specific Neural retina-specific leucine zipper (Nrl), proliferation marker Ki-67, and intermediate filament Vimentin. Nuclei are counterstained with DAPI. Scale bar, 100 μm. RT-PCR analysis (b) confirmed the expression of these factors and absence of mature visual pigments (Rhodopsin, S-Opsin, M-Opsin), although Recoverin and rod outer membrane (ROM1) mRNA was expressed. We also have observed the expression GS.
Immunocytochemistry and RT-PCR
Immunocytochemical analysis (Fig. 1a) showed the presence of proliferative marker Ki-67 (nuclear dots), early transcriptional factors such as Sox2 (nuclear) and Klf4 (nuclear), eye-specific Pax6 (nuclear) and Lhx2 (nuclear dots), and photoreceptor-specific Crx (nuclear, perinuclear) and Nrl (nuclear). We did not observe mature photoreceptor markers such as rhodopsin, Pde6b, or ROM1 in culture of undifferentiated cells, and neither did we see the mature glial cell marker CRAL-BP. The majority of the pRPC population was vimentin-positive. RT-PCR analysis (Fig. 1b) confirmed the results of immunocytochemistry and also showed the presence of neural and glial markers microtubule-associated protein 2 (MAP2) and glutamine synthetase (GS).
Cell Preparation for Transplantation
The viability of cells after formulation was >90% in both groups, and pRPC maintained 80% viability for the first 9 hours after formulation and more than 65% for 48 hours (Supplementary Fig. S1). There was no effect of either NAC or temperature (+0°C vs. 4°C) on pRPC viability in the suspension.
Histopathological Analysis
The retina of recipient animals, apart from the presence of grafted cells, was unremarkable. Lamination was normal, and retinal thickness was not different from controls (data not shown). Grafted pRPCs were found in the inner segment region and in the outer nuclear layer (ONL) in 9 out of 12 retinas: five in nonimmunosuppressed group and four in immunosuppressed group (Fig. 2). A few retinas also displayed small bipolar-like cells and cell processes in the ONL. In two transplanted animals that were investigated in more detail, transplanted pRPCs did not express Ki-67; host tissue demonstrated a low level of proliferation with very small numbers of Ki-67 cells scattered throughout the retinal layers. GFAP, recoverin, and rhodopsin were shown to co-localize with GFP-labeled RPCs (Fig. 3). Histopathological analyses did not show any infiltration of macrophages or inflammatory cells (Fig. 4). We did not find any effect of local transient immunosuppression on overall pRPC survival (Fig. 5).
Figure 2.
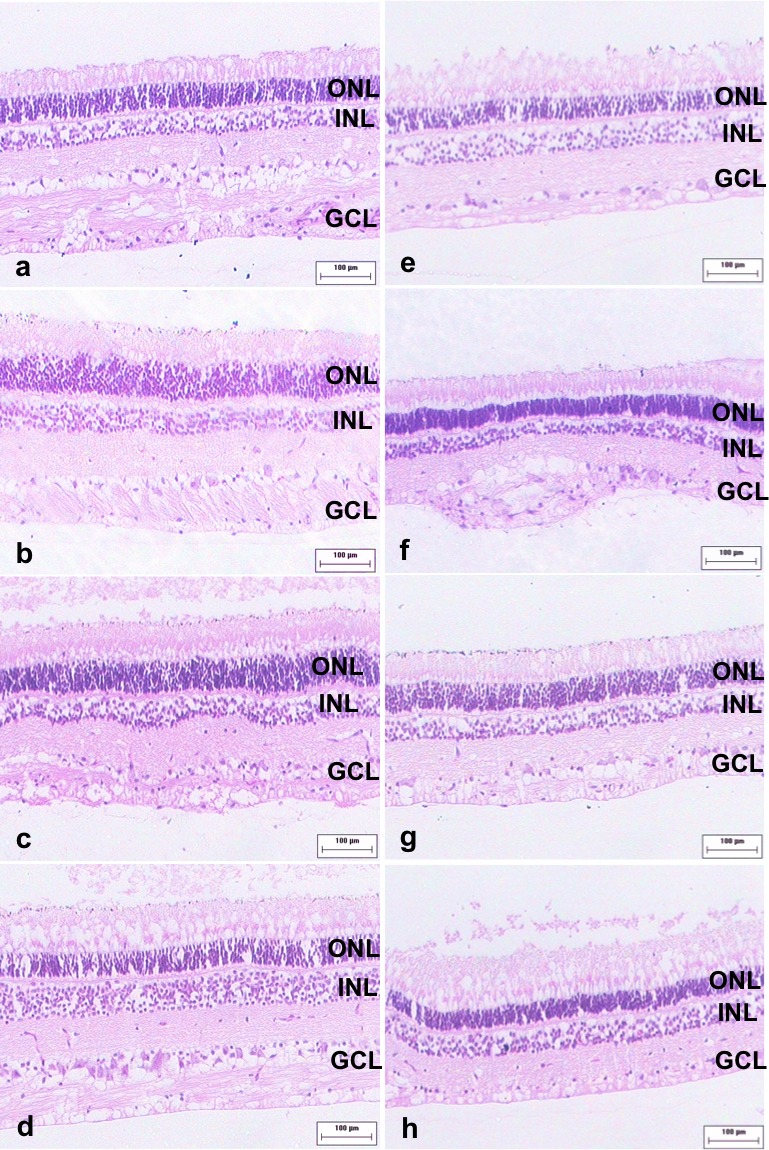
Morphology of the retina at the transplantation site. Four weeks after transplantation, we did not observe any evidence of immune response or disruption of retinal laminar structure in either group. Here we show representative images from each animal in nonimmunosuppressed (a through d) and immunosuppressed (e through h) groups.
Figure 3.
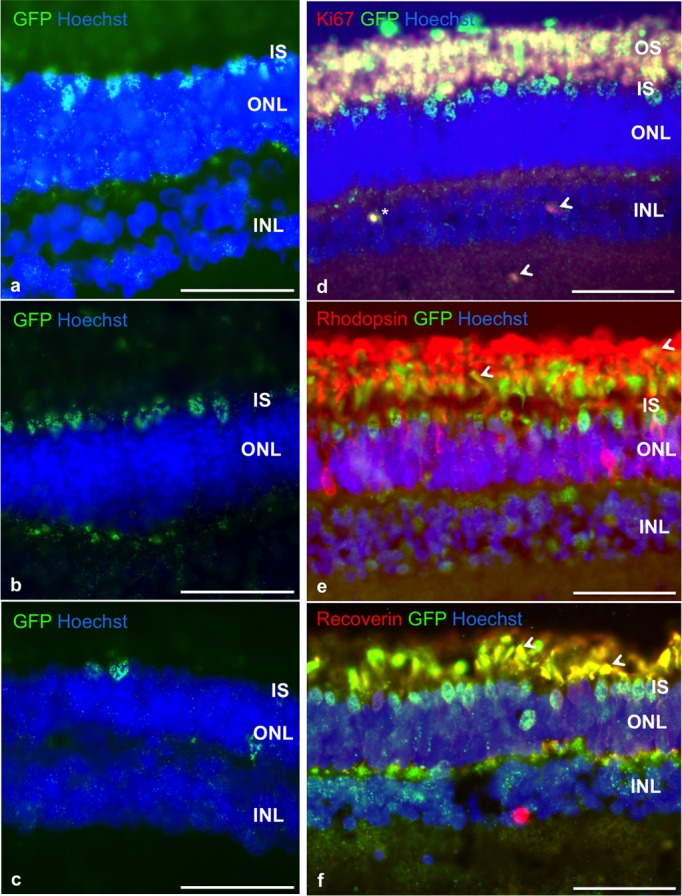
Survival and differentiation of pRPCs after subretinal transplantation to healthy recipients. Four weeks after surgery, we observed striking survival and integration of cells, with most of cells migrating into photoreceptor inner segment layer (IS; GFP, green). The bodies of several cells were found in the ONL (a, b, c) and inner nuclear layer (INL; b, c). We did not observe any signs of graft proliferation in any of the animals with only a single donor cell found to be Ki-67 positive (d, *), while host tissue showed some Ki-67 reactivity in INL (d, arrows). Most of the transplanted cells remained recoverin-positive (f), as can be seen by co-localization (yellow, arrows) of recoverin (red) and GFP (green). The proportion of cells obtaining rhodopsin expression (e) was significantly lower, with co-localization (yellow, arrows) observed in photoreceptor outer and inner segment layer. Nuclei are counterstained with Hoechst. Scale bar: 50 μm on all images.
Figure 4.
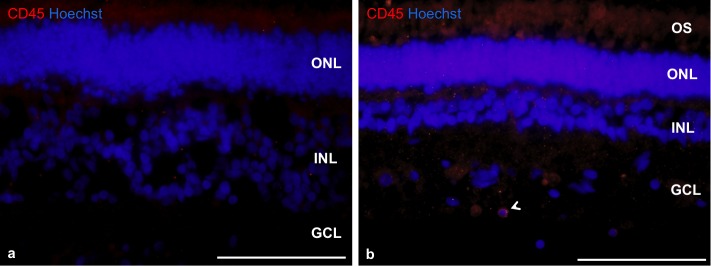
The absence of immune response to transplant in nonimmunosuppressed (a) and immunosuppressed (b) animals 4 weeks after cell delivery. We have observed only single CD45 cells (arrow, b) in the inner retina with no difference between groups. Nuclei are counterstained with Hoechst. Scale bar: 50 μm.
Figure 5.
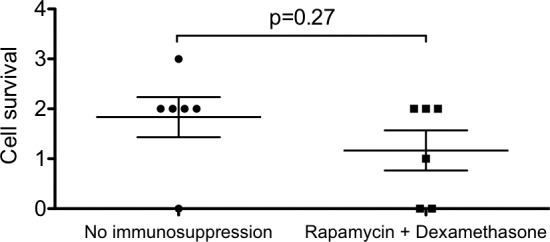
Quantification of graft survival. Donor cell survival was assessed by counting GFP+ cells on five sections for each eye (mean ± SEM). Overall graft survival was graded on a scale from 0 (no cells) to 3. The t-test analysis shows no difference in graft survival in injected versus noninjected group.
Discussion
Due to its anatomical similarity to the human eye, pigs have been extensively used as a valuable model for retinal surgery to study pathophysiology32,33 and to investigate therapeutic approaches for retinal disease, such as gene therapy, sustained growth factor delivery, or cell transplantation.
All these approaches have been shown to protect photoreceptors to some extent, although photoreceptor cell replacement is the only applicable option in advanced stages of retinal degeneration. The source of photoreceptors for such therapy, though, remains an open question. Full thickness adult or fetal34 retinal transplants and freshly isolated photoreceptor precursors14 provide a potential source for cell therapy but can not be considered for clinical application.
Two viable options include retinal progenitor and embryonic and induced pluripotent stem cell (ES/iPSC)-derived photoreceptor transplantation. The advances in differentiation of photoreceptors from iPSC, including enrichment in CD73 and CD24+ photoreceptor precursors35 and depletion of SSEA1+ undifferentiated cells, made transplants more effective and safe.2
Retinal progenitor cells can be isolated from developing mouse,36 human,37,38 and pig retina12,39 propagated in culture and transplanted. It has been shown that serum-free growth factor–rich (EGF, bFGF) conditions support the maintenance of RPC in vitro. Two main approaches have been tested for expansion: floating neurospheres39 and adherent culture.12 While both of these maintained Ki-67 and pax6 expression, adherent culture can be standardized for scale-up expansion and is less prone to spontaneous differentiation, providing more homogenous populations. We have demonstrated for human40 RPC that cells propagated as adherent population in 3% oxygen26 continue to express recoverin, Crx, Nrl, and NeuroD1.
Here we show that pRPCs, expanded in 3% oxygen up to passage 6 (similar to human cells), express early eye field transcription factors Sox2, Klf4, and Lhx2, and also photoreceptor-specific Nrl and Crx. We did not observe any mature photoreceptor markers, such as rhodopsin, mGluR8, or cone arrestin. Surprisingly, rod outer membrane mRNA was expressed, while the protein was not detected before transplantation and differentiation.
The transplantation outcome confirmed the ability of propagated pRPC to form rod photoreceptors in vivo, contradictory to previous reports of RPC's incapability to form photoreceptors after expansion.41 Morphological analysis showed remarkable survival of donor GFP-positive cells 4 weeks after transplantation with the majority of the donor cells remaining recoverin-positive, losing Ki-67 expression, and a few acquiring rhodopsin expression.
Observations of survival, integration, and differentiation of expanded retinal progenitor cells suggests that this approach is a viable option for allogeneic cell replacement even in the absence of local transient immunosuppressive treatment.
Rejection of allografts is a result of several processes, involving both innate and adaptive immune system with T cells central to this process,42 as they recognize unique donor antigens via direct or indirect pathways. In case of direct recognition, T cells respond to “MHC class I – peptide” complex expressed on donor cells. Indirect recognition involves the presentation of antigens by the MHC class II of recipient antigen presenting cells, such as RPE and migrating macrophages. Retinal progenitor cells of different species, including mice, rat, pig, and human, all express MHC complex I antigens after expansion in culture, which allows recognition by host immune system.
In this study, we did not observe any signs of macrophage infiltration 4 weeks after cell delivery, which correlates with previous transplantation studies, where freshly isolated cells were used. We did not observe any difference in cell survival in rapamycin and nonimmunosuppressed groups, which suggests that rapamycin does not have toxic effect on pRPC in vivo and that local transient immunosuppression is not required for allotransplantation of retinal progenitor cells.
Conclusions
Local transient rapamycin/dexamethasone immunosuppressive treatment had no significant impact on allogeneic survival and engraftment of pRPCs transplanted into normal wild-type pigs with healthy retina. Grafted cells maintained the ability to differentiate into photoreceptors after 4 weeks in vivo. No abnormal pathology was identified in any of the engrafted cells found in the retina. There was no evidence of immune response, hypercellularity, or autofluorescent macrophages, regardless of the local rapamycin immunosuppression treatment. Taken together, survival of expanded donor pRPC in vivo, integration of donor cells, lack of graft proliferation, and absence of immune response at 4 weeks postimplantation suggests allogeneic RPC cell transplantation is a viable strategy for photoreceptor replacement even in the absence of immunosuppression. Longer term studies and diseased hosts are needed to draw conclusions on the donor cell function. In the clinical setting, the omission of conjoint therapies would confer a clear surgical advantage in a single site delivery of treatment alone and also remove the confounding effects of the known negative influence of immunosuppressive agents, in particular rapamycin,43 on the differentiation potential of retinal progenitor cells.
Longer time points and transplantation into hosts with retinal degeneration are important future studies to consider.
Supplementary Material
Supplement 1
Acknowledgments
The authors thank the veterinary staff members of University of Goias for their work.
This work was supported by ReNeuron and University of Goias.
Disclosure: M. Abud, None; P. Baranov, None; C. Hicks, None; S. Patel, None; B. Lieppman, None; C. Regatieri, None; J. Sinden, None; D. Isaac, None; M. Avila, None; M. Young, None
References
- 1. MacLaren RE Pearson RA, MacNeil A,, et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006; 444: 203–207. [DOI] [PubMed] [Google Scholar]
- 2. Tucker BA, Park IH, Qi SD,, et al. Transplantation of adult mouse iPS cell-derived photoreceptor precursors restores retinal structure and function in degenerative mice. PLoS One. 2011; 6: e18992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aftab U Jiang C, Tucker B,, et al. Growth kinetics and transplantation of human retinal progenitor cells. Exp Eye Res. 2009; 89: 301– 310. [DOI] [PubMed] [Google Scholar]
- 4. Wang W, Zhou L, Lee SJ,, et al. Swine cone and rod precursors arise sequentially and display sequential and transient integration and differentiation potential following transplantation. Invest Ophthalmol Vis Sci. 2014; 55: 301– 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schwartz SD Hubschman JP, Heilwell G,, et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012; 379: 713– 720. [DOI] [PubMed] [Google Scholar]
- 6. Rentsch C, Schneiders W, Hess R,, et al. Healing properties of surface-coated polycaprolactone-co-lactide scaffolds: a pilot study in sheep. J Biomater Appl. 2014; 28: 654– 666. [DOI] [PubMed] [Google Scholar]
- 7. Jiang LQ,, Jorquera M,, Streilein JW,, Ishioka M. Unconventional rejection of neural retinal allografts implanted into the immunologically privileged site of the eye. Transplantation. 1995; 59: 1201– 1207. [PubMed] [Google Scholar]
- 8. Taylor AW. Ocular immune privilege. Eye (Lond). 2009; 23: 1885– 1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crafoord S,, Algvere PV,, Seregard S,, Kopp ED. Long-term outcome of RPE allografts to the subretinal space of rabbits. Acta Ophthalmol Scand. 1999; 77: 247– 254. [DOI] [PubMed] [Google Scholar]
- 10. Inoue M,, Maeno T,, Hatchell DL. Survival of allografted pancreatic islets in the subretinal space in rats. Ophthalmic Res. 2003; 35: 48– 53. [DOI] [PubMed] [Google Scholar]
- 11. Crafoord S,, Algvere PV,, Kopp ED,, Seregard S. Cyclosporine treatment of RPE allografts in the rabbit subretinal space. Acta Ophthalmol Scand. 2000; 78: 122– 129. [DOI] [PubMed] [Google Scholar]
- 12. Klassen H Kiilgaard JF, Zahir T,, et al. Progenitor cells from the porcine neural retina express photoreceptor markers after transplantation to the subretinal space of allorecipients. Stem Cells. 2007; 25: 1222– 1230. [DOI] [PubMed] [Google Scholar]
- 13. Klassen H, Kiilgaard JF, Warfvinge K,, et al. Photoreceptor differentiation following transplantation of allogeneic retinal progenitor cells to the dystrophic rhodopsin Pro347Leu transgenic pig. Stem Cells Int. 2012: 939801. [DOI] [PMC free article] [PubMed]
- 14. Zhou L Wang W, Liu Y,, et al. Differentiation of induced pluripotent stem cells of swine into rod photoreceptors and their integration into the retina. Stem Cells. 2011; 29: 972– 980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Algvere PV,, Gouras P, Dafgard Kopp E. Long-term outcome of RPE allografts in non-immunosuppressed patients with AMD. Eur J Ophthalmol. 1999; 9: 217– 230. [DOI] [PubMed] [Google Scholar]
- 16. Osusky R,, Dorio RJ,, Arora YK, Ryan SJ, Walker SM. MHC class II positive retinal pigment epithelial (RPE) cells can function as antigen-presenting cells for microbial superantigen. Ocul Immunol Inflamm. 1997; 5: 43– 50. [DOI] [PubMed] [Google Scholar]
- 17. Klassen H. Transplantation of cultured progenitor cells to the mammalian retina. Expert Opin Biol Ther. 2006; 6: 443– 451. [DOI] [PubMed] [Google Scholar]
- 18. Dunn JP. Review of immunosuppressive drug therapy in uveitis. Curr Opin Ophthalmol. 2004; 15: 293. [DOI] [PubMed] [Google Scholar]
- 19. Nguyen QD Ibrahim MA, Watters A,, et al. Ocular tolerability and efficacy of intravitreal and subconjunctival injections of sirolimus in patients with non-infectious uveitis: primary 6-month results of the SAVE Study. J Ophthalmic Inflamm Infect. 2013; 3: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Francis PJ, Wang S, Zhang Y,, et al. Subretinal transplantation of forebrain progenitor cells in nonhuman primates: survival and intact retinal function. Invest Ophthalmol Vis Sci. 2009; 50: 3425– 3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ishikawa T,, Hokama H,, Katagiri Y,, Goto H,, Usui M. Effects of intravitreal injection of tacrolimus (FK506) in experimental uveitis. Curr Eye Res. 2009; 30: 93– 101. [DOI] [PubMed] [Google Scholar]
- 22. Young S,, Larkin G,, Branley M,, Lightman S. Safety and efficacy of intravitreal triamcinolone for cystoid macular oedema in uveitis. Clin Exper Ophthalmol. 2001; 29: 2– 6. [DOI] [PubMed] [Google Scholar]
- 23. Lai CC,, Gouras P,, Doi K,, et al. Local immunosuppression prolongs survival of RPE xenografts labeled by retroviral gene transfer. Invest Ophthalmol Vis Sci. 2000; 41: 3134– 3141. [PubMed] [Google Scholar]
- 24. Taylor SR, Banker A, Schlaen A,, et al. Intraocular methotrexate can induce extended remission in some patients in noninfectious uveitis. Retina. 2013; 33: 2149– 2154. [DOI] [PubMed] [Google Scholar]
- 25. Klassen H Warfvinge K, Schwartz PH,, et al. Isolation of progenitor cells from GFP-transgenic pigs and transplantation to the retina of allorecipients. Cloning Stem Cells. 2008; 10: 391– 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baranov PY,, Tucker BA,, Young MJ. Low-oxygen culture conditions extend the multipotent properties of human retinal progenitor cells. Tissue Eng Part A. 2014; 20: 1465−1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lai L Park KW, Cheong HT,, et al. Transgenic pig expressing the enhanced green fluorescent protein produced by nuclear transfer using colchicine-treated fibroblasts as donor cells. Mol Reprod Dev. 2002; 62: 300– 306. [DOI] [PubMed] [Google Scholar]
- 28. Mayer M,, Noble M. N-acetyl-L-cysteine is a pluripotent protector against cell death and enhancer of trophic factor-mediated cell survival in vitro. Proc Natl Acad Sci U S A. 1994; 91: 7496– 7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Warfvinge K Kiilgaard JF, Lavik EB,, et al. Retinal progenitor cell xenografts to the pig retina: morphologic integration and cytochemical differentiation. Arch Ophthalmol. 2005; 123: 1385– 1393. [DOI] [PubMed] [Google Scholar]
- 30. Mudumba S, Bezwada P, Takanaga H,, et al. Tolerability and pharmacokinetics of intravitreal sirolimus. J Ocul Pharmacol Ther. 2012; 28: 507– 514. [DOI] [PubMed] [Google Scholar]
- 31. Kwak HW,, D'Amico DJ. Evaluation of the retinal toxicity and pharmacokinetics of dexamethasone after intravitreal injection. Arch Ophthalmol. 1992; 110: 259– 266. [DOI] [PubMed] [Google Scholar]
- 32. Kyhn MV Kiilgaard JF, Lopez AG,, et al. Functional implications of short-term retinal detachment in porcine eyes: study by multifocal electroretinography. Acta Ophthalmol. 2008; 86: 18– 25. [DOI] [PubMed] [Google Scholar]
- 33. Diederen RM,, La Heij EC,, Lemmens MA, Kijlstra A, de Vente J, Hendrikse F. Cyclic GMP in the pig vitreous and retina after experimental retinal detachment. Mol Vis. 2008; 14: 255– 261 [PMC free article] [PubMed] [Google Scholar]
- 34. Ghosh F,, Arnér K. Transplantation of full-thickness retina in the normal porcine eye: surgical and morphologic aspects. Retina. 2002; 22: 478– 486. [DOI] [PubMed] [Google Scholar]
- 35. Lakowski J Han YT, Pearson RA,, et al. Effective transplantation of photoreceptor precursor cells selected via cell surface antigen expression. Stem Cells. 2011; 29: 1391– 1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jensen AM,, Raff MC. Continuous observation of multipotential retinal progenitor cells in clonal density culture. Dev Biol. 1997; 188: 267– 279. [DOI] [PubMed] [Google Scholar]
- 37. Kelley MW,, Turner JK,, Reh TA. Regulation of proliferation and photoreceptor differentiation in fetal human retinal cell cultures. Invest Ophthalmol Vis Sci. 1995; 36: 1280– 1289. [PubMed] [Google Scholar]
- 38. Klassen H,, Ziaeian B,, Kirov II,, Young MJ,, Schwartz PH. Isolation of retinal progenitor cells from post-mortem human tissue and comparison with autologous brain progenitors. J Neurosci Res. 2004; 77: 334– 343. [DOI] [PubMed] [Google Scholar]
- 39. Gu P Harwood LJ, Zhang X,, et al. Isolation of retinal progenitor and stem cells from the porcine eye. Mol Vis. 2007; 13: 1045– 1057. [PMC free article] [PubMed] [Google Scholar]
- 40. Luo J, Baranov P, Patel S,, et al. Human retinal progenitor cell transplantation preserves vision. J Biol Chem. 2014; doi:http://dx.doi.org/10.1074/jbc.M113.513713. [DOI] [PMC free article] [PubMed]
- 41. Mansergh FC Vawda R, Millington-Ward S,, et al. Loss of photoreceptor potential from retinal progenitor cell cultures, despite improvements in survival. Exp Eye Res. 2010; 91: 500– 512. [DOI] [PubMed] [Google Scholar]
- 42. Ingulli E. Mechanism of cellular rejection in transplantation. Pediatr Nephrol. 2010; 25: 61– 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Love NK,, Keshavan N,, Lewis R,, Harris WA,, Agathocleous M. A nutrient-sensitive restriction point is active during retinal progenitor cell differentiation. Development. 2014; 141: 697– 706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement 1


